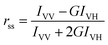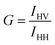Multiple prototropism of fisetin in sodium cholate and related bile salt media†
Susithra
Selvam
and
Ashok K.
Mishra
*
Department of Chemistry, Indian Institute of Technology Madras, Chennai, 600 036, Tamilnadu, India. E-mail: mishra@iitm.ac.in; Fax: +91 2257 4202; Tel: +91 2257 4207
First published on 26th October 2010
Abstract
Fisetin, a bioflavonoid, has important biological relevance. It exhibits intramolecular excited state proton transfer (ESIPT), analogous to the structurally similar flavonoids. The presence of multiple prototropic forms of fisetin was observed at various concentrations of different bile salt molecules. The presence of ground state fisetin anion (FA)GS (λex 418 nm; λem 490 nm) in alcohols and bile salt micellar media is a novel observation. The interaction of fisetin with sodium cholate (NaC) and some other bile salts has been studied in detail, using the intrinsic fluorescence of different prototropic forms of fisetin: neutral form (FN, λex 369 nm, λem ∼ 400 nm), ground state anion form ((FA)GS, λex 418 nm, λem 490 nm) and phototautomer (FT, λex 369 nm, λem 540 nm). The hypsochromic shift of (FA*)ES emission and bathochromic shift of FT emission with increasing bile salt concentration suggests the progressive reduction of polarity of the bile salt media, which could be resulting from the neutralization of bile salt molecules as their concentration increases.
Introduction
Fisetin (Chart 1A) belongs to a class of bioflavonoids, which are polyphenolic compounds present in common plant-based food items, such as onions, apples, tea and beverages, like red wine.1–3 The biological activities of fisetin are various. It has antioxidant property (analogous to vitamin E), inhibits protein kinase C, is used in the treatment of AIDS, anti-inflammatory activity in brain microglia, enhances long-term memory and it is present in dietary supplements.4 The optical absorption and fluorescence properties of fisetin are quite interesting and challenging. It exhibits intramolecular excited state proton transfer (ESIPT) and shows two emission peaks in organized media like liposome and cyclodextrins; one peak is at 400 nm due to S1 → S0 emission and the other peak at a red-shifted wavelength, 540 nm due to phototautomerization of the neutral molecule under excitation.5–11 Recently the presence of anionic species of fisetin was observed in alkaline pH.6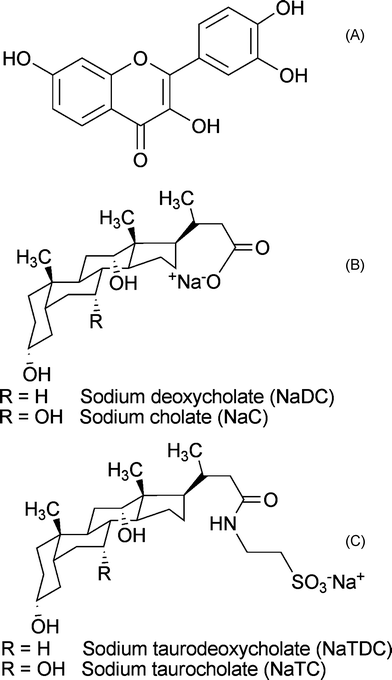 | ||
| Chart 1 Chemical structure of (A) 3,4,3′,4′-tetrahydroxyflavone (fisetin), (B) and (C) bile salts. | ||
Bile salts are biologically important molecules, synthesized from cholesterol within the liver. Bile salts have a hydrophobic steroidal backbone with one to three hydroxyl groups and a carboxyl side chain lying along in the same plane of hydroxyl groups (Chart 1B and Chart 1C). This structure leads to a unique aggregation pattern, accounting for their solubilization of both hydrophobic and hydrophilic solutes.12,13 Consequently bile salts have received much attention as drug delivery media.13 The micellization of bile salts is a multi-step process; initially dimeric primary aggregates are formed by hydrophobic interaction between steroidal domains and as the concentration of these bile salts is increased they lead to the formation of larger secondary aggregates, which are formed primarily due to the hydrogen bonding between hydroxyl and carboxyl groups of different dimeric bile salt units.14–16 The secondary bile salts are derived from the primary bile salts (cholates and chenodeoxycholates) and conjugated to either taurine or glycine amino acids through a peptide linkage.12 For the present work, four bile salts, namely sodium cholate (NaC), sodium deoxycholate (NaDC), sodium taurocholate (NaTC) and sodium taurodeoxychoalte (NaTDC), are used. Among these four bile salts, NaC and NaDC are primary (unconjugated) bile salts, whereas NaTC and NaTDC are secondary (conjugated) bile salts. The bile salt aggregates are considered as pseudo-micellar species, since they do not have a sharp monomer to aggregate transition, which is characterized by well defined critical micellar concentrations (cmc). Instead they have a cmc range, viz. for NaC and NaTC of 12–16 mM, and for NaDC and NaTDC of 4–6 mM.16
The absorption and fluorescence properties of fisetin show a simultaneous presence of more than one prototropic equilibria in different media,5,6 which implies simultaneous presence of more than two prototropic forms. This phenomenon has been referred to as ‘multiple prototropism’ in the present discussions. An understanding of the interaction between bile salts (anionic surfactants) and fisetin, exhibiting various prototropic forms is important. The study will help in establishing bile salts as possible drug delivery media for other pharmaceutically important flavonoids. The objective of this work is to study the interaction between fisetin and all the four bile salts by analyzing the intrinsic photophysical properties of different prototropic forms of fisetin.
Experimental
Materials
Fisetin, sodium taurodeoxycholate (NaTDC) and sodium taurocholate (NaTC) were purchased from Sigma Chemical Company (USA) and were used without any further purification. Sodium deoxycholate (NaDC) and sodium cholate (NaC) were purchased from S. D. Fine Chemicals, India. Spectroscopy grade solvents were obtained from Sisco Research Laboratories, India. Analytical grade ethanol was obtained from Merck, USA. The stock solutions of fisetin for the homogeneous media study were prepared by dissolving it in the required solvents separately.Measurements
The absorption spectra were recorded using Jasco −V550 UV-Visible spectrophotometer and fluorescence spectra using Hitachi F4500 model spectrofluorimeter. The excitation and emission slits were set to a bandwidth of 10 nm. Temperature control was attained by using a double walled cuvette holder; connected to an INSREF thermostat with an accuracy of ±0.1 °C. For steady-state fluorescence anisotropy measurements, polacoat grating polarisers and Glan-Thompson polarisers were used. The steady state fluorescence anisotropy is defined as:17where, IVV and IVH are the fluorescence intensities and the subscript indicates the vertical (V) and horizontal (H) orientations of the excitation and emission polarizer. G is the instrumental correction factor,
The fluorescence decays are collected using Horiba Jobin Yvon TCSPC Lifetime system with FluoroHub single-photon counting controller module and equipped with the TBX single-photon detection module, typically of 180 ps FWHM. The NanoLED-03 nanosecond source with UV output at 370 nm, typical optical pulse duration of 1.3 ns FWHM and a repetition rate of 1 MHz was used. The decay curves are further analysed using the DAS6 analysis software. A fitted value with 0.99 ≤ χ2 ≤ 1.2 was accepted. The emission spectra of fisetin in different bile salts are deconvoluted using a multi-peak fitting of Gaussian spectrum in Origin 6.0 software and the correlation factor (r) is maintained at ∼1.0
Preparation of fisetin–bile salt solutions
Fisetin was first dissolved in ethanol and further diluted with bile salt solutions having concentrations above their cmc (20.0 mM for NaDC and NaTDC; 48.0 mM for NaC and NaTC). In these solutions, the final fisetin concentration is maintained constant at 10 μM and varying bile salts concentrations were prepared by appropriate addition of bile salt solution and the concentration of ethanol was kept at 2%. To maintain the physiological condition, pH was kept constant at 7.4 with 50 mM sodium phosphate buffer for all bile salt experiments.Results and discussion
Photophysical behavior of fisetin in sodium cholate aggregates
 | ||
| Fig. 1 (A) Absorption spectra of fisetin in varying concentration of NaC (0–43.2 mM). (B) Emission spectra of FN (λex 369 nm). (C) Emission spectra of FA (λex 418 nm). (T = 25 °C, pH = 7.4.) | ||
Fig. 1A shows an intense absorption peak at 369 nm and a slightly enhanced absorption at 418 nm. The peak at 369 nm increases with NaC concentration and correspondingly the absorbance at 418 nm decreases, with an isosbestic point observable at ca. 395 nm. Thus the enhanced absorption at 418 nm can be considered a weak band. These spectral features of fisetin in NaC are comparable with those in homogeneous medium (Fig. S1, ESI†). The intense peak at 369 nm corresponds to FN (fisetin neutral), similar to those observed in non-polar solvents. The interesting observation of a weak band at 418 nm (Fig. 1A) is analogous to that observed in presence of alcoholic solvents (Fig. S1) and to the anion peak of the parent flavonoid, 3-hydroxyflavone (3-HF).18,19 Hence the peak at 418 nm in the absorption spectra of fisetin can be attributed to its ground state anionic species ((FA)GS).
The fluorescence spectra (Fig. 1B and Fig. 1C) of fisetin in NaC aggregates are measured at two excitation wavelengths; λex 369 nm corresponding to the FN species (Fig. 1B) and λex 418 nm corresponding to the (FA)GS species (Fig. 1C). The emission spectra of FN species (λex 369 nm) show that it exhibits ESIPT, leading to the intense FT (fisetin tautomer) emission (λem 521–540 nm), comparable to the emission spectra of fisetin in non-polar solvents (Fig. S2) 5–11 and a less intense peak of (FA*)ES (excited state fisetin anion) at 490 nm along with the FN emission having a weak band at around 400 nm. FT emission increases gradually with increase in the NaC concentration, whereas the intensity of (FA*)ES decreases simultaneously and a minor increase with the FN emission is also noted. The emission spectra of (FA)GS with an excitation at 418 nm (Fig. 1C), show a prominent peak at 490 nm and presence of another peak overlaid along with the other peak. The second peak shifts from 521–542 nm with increasing concentration of NaC. The emission at 490 nm decreases along the NaC concentration and a small increase in the second peak is observed. The 490 nm peak is similar to that observed in alcoholic solvents (Fig. S2) and also comparable with that of the parent flavonoid, 3-HF.18,19 So far, there has been just one report of emission peak at this region and it is assigned to the anion form of fisetin in the homogeneous medium.6 Further evidence was obtained from a methanolic solution of fisetin in 0.1 M sodium methoxide, showing excitation peak at 414 nm and corresponding emission peak at 490 nm. Thus the peak observed at 490 nm (λex 418 nm) in NaC is due to the emission of (FA)GS. It is to be noted that the presence of the ground state anion in the anionic pesudo-micellar media of NaC can not be explained just by the pH of the medium; possibly the bile salt anions play the role of proton acceptor. The red shifted peak present in the emission spectra (Fig. 1C) is attributed to the FT species by comparing with the other spectra, Fig. 1B. The presence of FT emission with an excitation at 418 nm is an indication of the substantial presence of FN along with the (FA)GS in the ground state and thereby contributing to the characteristic FT emission. A normalized emission spectra (Fig. 1C) of (FA)GS indicates that as the NaC concentration increases, there is a decrease in the emission of (FA*)GS along with the increase in FT emission. The excitation spectra of FN and (FA)GS for their emission at 540 nm and 490 nm respectively are given in Fig. S3. Correlation of the absorption and emission measurements of fisetin in NaC aggregates to that of fisetin in homogeneous medium, suggests that the photophysical behavior of fisetin in NaC micellar media follows the processes involving ESIPT and ground state prototropic equlibria, provided in Scheme 1.
 | ||
| Scheme 1 Mechanism of ground state and excited state proton transfer reactions of fisetin in water (FN – fisetin neutral, FN* – excited state fisetin neutral, (FA)GS – ground state fisetin anion, (FA*)GS – excited species of ground state fisetin anion, (FA*)ES – excited state fisetin anion and FT – fisetin phototautomer). | ||
According to Scheme 1, there exists an equilibrium between FN and (FA)GS even in the ground state. The structure of fisetin anion has been assigned so as to show the first deprotonation at the hydroxyl group in the position ortho to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in the fisetin molecule, by comparing it with the ground state anion structure of the parent 3-hydroxyflavone.19 Moreover the excited state phototautomer does form by intramolecular proton transfer from this hydroxyl group.6 Upon excitation at their characteristic wavelengths, FN species (λex 369 nm) gives rise to FT, (FA*)ES and FN emission species, whereas the (FA)GS (λex 418 nm) gives rise to (FA*)GS and FT emission. Scheme 1 also describes the prototropic equilibria of the various forms in the excited state, which leads to the highly overlaid emission spectra of FN and (FA)GS and hence there is no clear description of the emission peaks to each of the prototropic forms. However, the assignment of the emission peaks are rationalised by drawing analogy with the characteristic spectral features of the parent flavonoid, 3-HF.18,19
O in the fisetin molecule, by comparing it with the ground state anion structure of the parent 3-hydroxyflavone.19 Moreover the excited state phototautomer does form by intramolecular proton transfer from this hydroxyl group.6 Upon excitation at their characteristic wavelengths, FN species (λex 369 nm) gives rise to FT, (FA*)ES and FN emission species, whereas the (FA)GS (λex 418 nm) gives rise to (FA*)GS and FT emission. Scheme 1 also describes the prototropic equilibria of the various forms in the excited state, which leads to the highly overlaid emission spectra of FN and (FA)GS and hence there is no clear description of the emission peaks to each of the prototropic forms. However, the assignment of the emission peaks are rationalised by drawing analogy with the characteristic spectral features of the parent flavonoid, 3-HF.18,19
The nature of shift associated with (FA*)ES and FT emission peaks, the emission spectra of FN (λex 369 nm) in different NaC concentrations (Fig. 1B) were analyzed by deconvolution of these spectra (Fig. S4). The spectra contain a broad spectrum with large FWHM at the initial concentrations of NaC and as the NaC concentration increases, the subsequent spectra resolve into two distinct peaks. The emission spectra of fisetin in buffer medium (pH 7.4) were deconvoluted into two peaks corresponding to the well defined (FA*)ES emission peak at 478 nm in the blue-violet region and FT emission peak at 521 nm in the green region (Fig. S2). However, when fisetin is in NaC aggregates ([NaC] = 43.2 mM), the (FA*)ES peak shifts to 460 nm and the FT peak shifts to 544 nm. Collectively, the deconvoluted spectra show a blue shift associated with (FA*)ES emission peak and a red shift associated with FT along with the progressive increase in NaC concentration. These shifts in the emission maxima of (FA*)ES and FT in NaC aggregates can be correlated to those observed in the homogeneous medium (Fig. S2).
Owing to the charge on both the forms of fisetin molecules and also on NaC, the interaction between them is also expected to be dependent on the charge and polarity surrounding the fluorophore. However, the persistence of (FA)GS form even in the highest concentration of NaC (43.2 mM) used here, suggests that the anion formation is not retarded by the negative charge on NaC aggregates. Thus the spectral features indicate that polarity of the medium affects the molecular association of fisetin with NaC aggregates. Correlating these absorption and emission measurements to that of fisetin in homogeneous medium suggests that the photophysical behavior of fisetin in NaC micellar media follows the processes involving ESIPT and ground state prototropic equilibria, provided in Scheme 1. It is to be noted that absorption and emission spectra of ground and excited state prototropic forms do overlap, and sometimes extensively. Thus exclusive excitation of a particular prototropic species is sometimes as difficult as getting the excitation spectrum of a particular prototropic species.
From these results, it appears that the fisetin molecule senses an increasing non-polar environment as the concentration of NaC increases. The shifts in the emission peak for both the forms of fisetin are due to the dipolar relaxation of the NaC molecules located at the interface. The interesting shifts in the emission maxima and the variations of emission intensity are conveniently represented by excitation emission matrix fluorescence (EEMF) (Fig. S5). The contour plots of fisetin in buffer medium (pH 7.4) is predominantly that of the (FA)GS form. As the concentration of NaC is increased, the contours shift gradually to the higher wavelength leading to a contour of FT fluorescence.
The fluorescence lifetime measurements at various emission wavelengths (λem = 480 to 560 nm) with a light source of 370 nm LED were carried out to evaluate the prototropism of fisetin in NaC aggregates (Table S1).
These fluorescence decays are fitted with a bi-exponential function and these decays depend on the emission wavelength. Following the assignment by Funes et al., 6 the longer lifetime component can be attributed to (FA*)ES form and the shorter to the FT species. The fluorescence lifetimes of both (FA*)ES and FT species increase with the emission wavelengths. When an excited-state reaction or relaxation process is present and new species are created which emit at a different emission wavelength, the fluorescence decay dynamics will vary across the emission spectrum.17 Thus the changes provided in Table S1, give another evidence for the prototropism of fisetin in NaC aggregates. These increasing lifetimes across the emission spectrum could also be interpreted in terms of dipolar relaxation of the micellar medium around the excited-state fluorophore.
![(A) Dependence of (FA*)ES and FT emission frequencies on NaC concentration. (B) and (C) Dependence of (FA*)ES and FT emission intensities ([NaC] = 0 to 43.2 mM).](/image/article/2011/PP/c0pp00120a/c0pp00120a-f2.gif) | ||
| Fig. 2 (A) Dependence of (FA*)ES and FT emission frequencies on NaC concentration. (B) and (C) Dependence of (FA*)ES and FT emission intensities ([NaC] = 0 to 43.2 mM). | ||
The plot in Fig. 2A shows a gradual blue shift in (FA)GS emission and a red shift in FT emission as NaC concentration increases. The results obtained here (Fig. 2A) along with earlier results (Fig. 1B) indicate that the fisetin molecule reports the change in NaC micellar medium to become non-polar as the NaC concentration increases. The change in the relative population of (FA*)ES and FT is further analyzed by correlating the emission intensity of (FA*)ES (λem 490 nm; λex 369 nm) and FT (λem 540 nm; λex 369 nm) with increasing concentrations of NaC (Fig. 2B and C). The fluorescence intensity corresponding to a particular emitting species depends upon the population, the molar extinction coefficient (ε) and the fluorescence quantum yield (φf). In the present case, the light absorbing species at λex 369 nm is the neutral form, thus the fluorescence intensity changes do not arise from ε. An indication of φf changes can come from the fluorescence lifetime (τ) values (Table S1) which are nearly constant for both (FA*)ES and FT emissions. Thus bile salt induced population changes appear to be the primary reason for the fluorescence intensity changes. The observation of more intense FT emission at the expense of (FA*)ES emission appears to be because of increased hydrophobicity of the bile salt aggregates that causes higher fraction of FN converting to FT, viaFN* (Scheme 1, Fig. 2C). Even at the highest concentration of NaC used here ([NaC] = 43.2 mM), there is no complete conversion to the tautomer form. The cross-over point ([NaC] = 23 mM), given in Fig. 2C quantifies the concentration of NaC, where equal amounts of (FA*)ES and FT are present within the NaC aggregates. The progressive hypsochromic shift of (FA*)ES and bathochromic shift of FT cannot be explained if the environment of fisetin is purely aqueous. The only rational explanation can be provided in terms of increased hydrophobicity of the environment within the micellar aggregates. Thus, the plots provided here indicate that the multiple prototropism of fisetin closely follows the progressive micellization of NaC molecules.
At low concentrations of bile salts, they behave like an electrolyte and pH of the bile salt solution increases steadily as the bile salt micellization progresses. The role of bile salt aggregates in providing environment for the presence of multiple prototropic forms of fisetin, can be examined by taking different bile salts. The bile salts chosen for the present work are NaTC, NaDC and NaTDC. These three bile salts along with NaC are structurally different from one another. The structural variations of these bile salts are depicted in Fig. 1. In comparison to NaC, NaDC has one –OH group less on its steroidal moiety, NaTC has a side chain conjugation with taurine, an amino acid, and NaTDC has one –OH group less on its steroidal moiety along with a side chain conjugation. Therefore, the nature of the three bile salts with respect to their polarity differs from NaC as follows: NaDC in facial polarity, NaTC in terminal polarity and NaTDC in both facial and terminal polarity. Thus the deoxycholates (NaDC and NaTDC) are less polar than the cholates (NaC and NaTC). The alterations in their structure affect their micellization and solubilising properties. The micellization of bile salts are largely affected by their facial polarity and the presence of side-chain conjugation is very minimal. This is reflected in the cmc range of the bile salts; NaC = NaTC = 12–16 mM and NaDC = NaTDC = 4–6 mM.16
Photophysical behavior of different prototropic forms of fisetin in different bile salt media
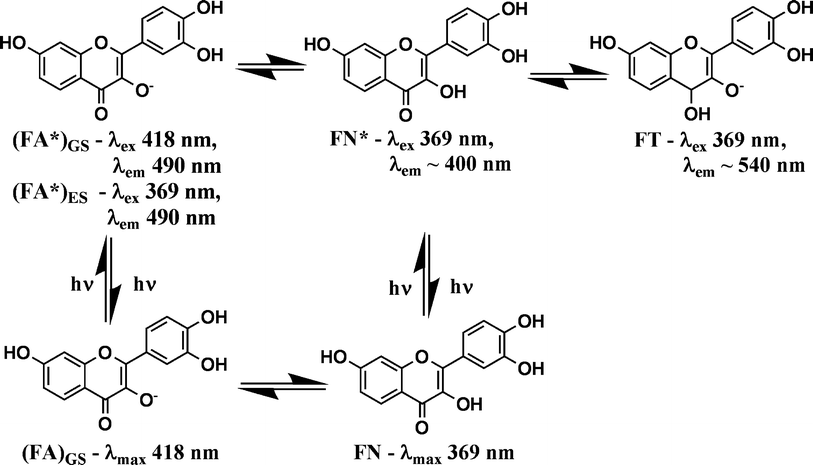
The photophysical studies of fisetin in three bile salts (NaDC, NaTC and NaTDC) are carried out using the same procedure described earlier for fisetin in NaC aggregates. The absorption spectra (Fig. S6) and fluorescence spectra (Fig. S7) of fisetin in all these three bile salts are identical with those obtained in NaC molecules. The results obtained from these studies indicate that the presence of the (FA)GS species in the ground state, its spectral behavior and the unique shifts associated with the (FA*)ES and FT emissions within the different bile salt media is true in case of all four bile salts including those observed in presence of NaC (Fig. 1). Small variations in presence of side-chain conjugated bile salts are observed and hence it can indicate minor effect due to these bile salts.The intensity of (FA*)ES emission increases in presence of the conjugated bile salts (NaTC and NaTDC). These results suggests that the FA species is more stabilized in presence of conjugated bile salts, since the –SO3−group is less polar than the –CO2−group, which is present at the side chain of bile salts. The normalized fluorescence intensity at emission peak maximum (λex 369 nm) of both the species of fisetin in bile salts aggregates is correlated with the increasing concentrations of bile salts (Fig. 3). A cross-over point for each bile salt is obtained, viz.NaDC = 4.8 mM, NaTC = 29 mM and NaTDC = 9.8 mM. The higher cross-over concentration of the conjugated bile salts compared to that of the corresponding unconjugated bile salts clearly indicates that the side-chain of these bile salts does play a role in their association with fisetin.
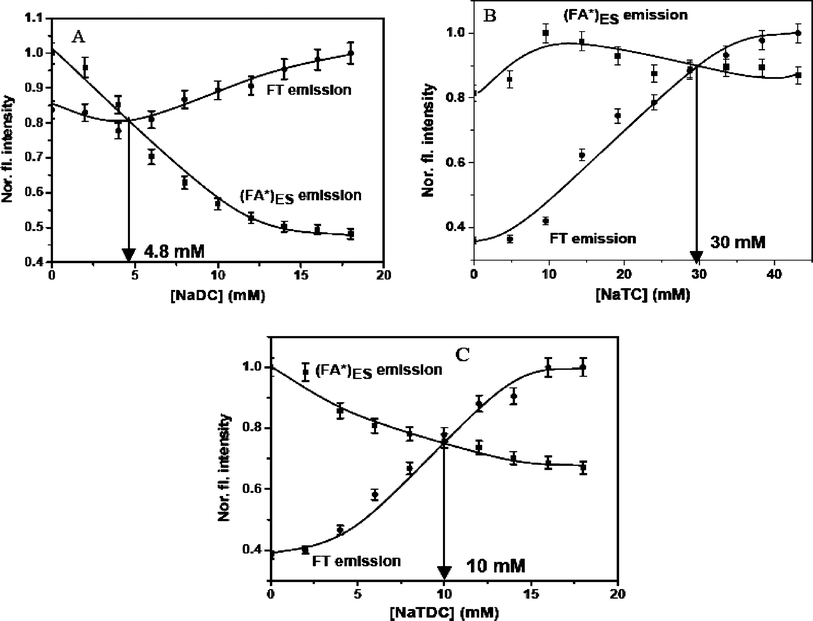 | ||
| Fig. 3 Dependence of (FA*)ES and FT normalized fluorescence intensity at emission peak maximum on bile salt concentration at λex 369 nm, showing the crossover point for each bile salt. (A) NaDC (0–18 mM), (B) NaTC (0–43.2 mM) and (C) NaTDC (0–18 mM). | ||
These studies give evidence for the hydrophobic steroidal face of the bile salts being involved in their interaction with both forms of fisetin, despite the possible electrostatic interaction between bile salts and both FA and FT species of fisetin. The results obtained from the absorption and emission spectral measurements of different prototropic forms of fisetin in various bile salt micelles, also support the hypothesis that the bile salt anion undergoes partial neutralization as their concentration is increased and results in increased hydrophobicity. The association of fisetin with all four bile salts are further studied with the steady state fluorescence anisotropy and fluorescence lifetime measurements.
Steady state fluorescence anisotropy and lifetime measurements
The steady state fluorescence anisotropy (rss) values for (FA)GS (λex 418 nm, λem 490 nm) and FT (λex 369 nm, λem 540 nm) forms of fisetin in buffer medium (pH 7.4) and different bile salt aggregates ([NaDC] = [NaTDC] = 18.0 mM and [NaC] = [NaTC] = 43.2 mM) are given in Table 1. Both (FA*)GS and FT emissions have lower rss values at pH 7.4 and gradually increase as the concentration of bile salt increases (Table 1).This suggests that both forms of fisetin are conveniently located within the rigid micellar environments of bile salts, where the rotational motion of the fluorophore is restricted. The equilibrium between (FA)GS and FN exists within a same environment of bile salt aggregates, which can be interpreted from the near similar values of rss for both the forms of fisetin. The fluorescence lifetime measurements of fisetin in different bile salts are obtained by using an excitation source of 370 nm LED for both (FA*)ES emission (λem 490 nm) and FT emission (λem 540 nm). The fluorescence decays are fitted with a bi-exponential function and measured for different concentrations of all four bile salts. The fluorescence lifetimes along with their individual components are given in Table 2 to show the change in the fraction of (FA)GS and FT forms of fisetin.
| Micellar system | [Bile salt]/mM | Anion (λem 490 nm) | Phototautomer (λem 540 nm) | ||
|---|---|---|---|---|---|
| τ 1 (α1) | τ 2 (α2) | τ 1 (α1) | τ 2 (α2) | ||
| Fisetin-buffer (pH 7.4) | — | 0.7 (0.83) | 2.6 (0.16) | 0.9 (0.94) | 3.3 (0.05) |
| Fisetin-NaC | 4.8 | 0.7 (0.81) | 2.7 (0.19) | 1.0 (0.87) | 3.2 (0.12) |
| 16.0 | 0.7 (0.79) | 3.0 (0.20) | 1.0 (0.51) | 3.2 (0.48) | |
| 43.2 | 0.7 (0.73) | 3.3 (0.26) | 1.0 (0.45) | 3.3 (0.54) | |
| Fisetin-NaDC | 2.0 | 0.7 (0.83) | 2.7 (0.17) | 0.9 (0.93) | 2.9 (0.07) |
| 6.0 | 0.7 (0.82) | 2.7 (0.18) | 0.9 (0.62) | 3.1 (0.38) | |
| 18.0 | 0.7 (0.80) | 2.7 (0.20) | 1.0 (0.56) | 3.2 (0.44) | |
| Fisetin-NaTC | 4.8 | 0.8 (0.79) | 2.7 (0.21) | 1.1 (0.82) | 3.2 (0.18) |
| 16.0 | 0.8 (0.75) | 3.2 (0.24) | 1.5 (0.58) | 3.3 (0.42) | |
| 43.2 | 0.8 (0.69) | 3.5 (0.30) | 1.5 (0.47) | 3.9 (0.53) | |
| Fisetin-NaTDC | 2.0 | 0.9 (0.81) | 2.8 (0.19) | 1.0 (0.53) | 3.0 (0.47) |
| 6.0 | 0.9 (0.73) | 3.0 (0.26) | 1.2 (0.51) | 3.9 (0.49) | |
| 18.0 | 0.9 (0.64) | 3.2 (0.36) | 1.4 (0.41) | 4.0 (0.59) | |
The emission decay profile of both species measured in their respective emission peak shows enhancement of the FT emission and also persistence of (FA)GS emission. The increase in τ1 of (FA)GS emission is seen only in conjugated bile salts, whereas τ2 increases in all four bile salts. Both τ1 and τ2 of FT emission increases with the increasing concentrations of all four bile salts and the magnitude of the increase in lifetimes is more in conjugated bile salt aggregates. Even though the change in all the lifetimes are minor, the change in the individual contribution of each lifetime shows that there is a certain equilibrium between the anionic and tautomer fisetin species within the bile salt aggregates. The microheterogeneity around the fluorophore is expected to be rather distributed. Hence, fluorescence lifetime data analysis involving a few component fit may be approximate in describing the physical situation. Thus the lifetime data reported can only be considered as supporting data.
Effect of temperature
The effect of temperature on the interaction between fisetin and bile salts are carried out within the temperature range 15–45 °C.20,21 The photophysical parameters such as fluorescence emission intensities (Fig. 4A, Fig. 4B and Fig. S8) and steady state fluorescence anisotropy (Fig. 4C, Fig. 4D and Fig. S9) of (FA)GS (λex 418 nm, λem 490 nm) and FT (λex 418 nm, λem 490 nm) are utilized to probe the molecular association of fisetin along with the four bile salts at different temperatures.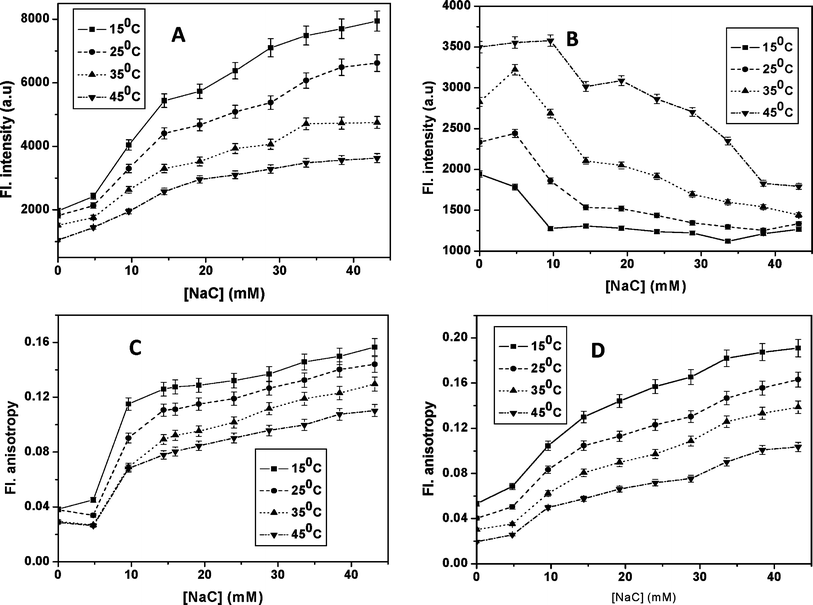 | ||
| Fig. 4 Dependence of (A) FT emission intensity (λex 369 nm and λem 540 nm) and (B) (FA)GS emission intensity (λex 418 nm, λem 490 nm), (C) FT fluorescence anisotropy (λex 369 nm and λem 540 nm) and (D) (FA)GSfluorescence anisotropy (λex 418 nm, λem 490 nm) on NaC concentrations at different temperatures. | ||
With increasing temperature, the fluorescence intensity of the FT emission decreases in presence of all four bile salts, even in a narrow temperature range of 15–45 °C. Such a strong dependence of prototropism is usually not expected in homogeneous media. Hence the observed decrease in the FT emission could be due to temperature induced bile salt structural changes, like micellar disaggregation, which can lead to an increase in the polarity of the bile salt media. The fluorescence anisotropy values of both the species in all the four bile salts decreases with decreasing concentration of bile salts. Thus these results strongly suggest that both the forms of fisetin experiences restrictive rotation within the micellar core of bile salt media. The almost similar values of the both fisetin species are observed here and this indicates that they are located within a similar micro-heterogeneous environment.
The retardation of FT and enhancement of (FA)GS emission intensities follow the progressive micellization behavior of bile salts, through an initial formation of dimeric primary aggregates and then larger secondary aggregates of bile salts, in all the temperatures used here. Temperature dependence of the progressive micellization of bile salts suggests that the dimeric aggregation is unaffected, while the larger aggregates are further grown with increasing temperature.21 As the temperature increases, there is decrease in secondary aggregate formation, i.e. lowering of micellization due to the hydrogen bond distortions. Thus at higher temperatures, the polarity of bile salt media increases, leading to an unfavorable condition for the FT formation and hence assists the formation of (FA)GS by participating in intermolecular hydrogen bonding with the fisetin molecules. This observation is conveniently visualized in the EEMF spectra of fisetin in bile salt aggregates at higher temperature of 45 °C (Fig. S10). At the highest concentration of NaC, the contour of FT emission is blurred at this temperature, as compared to its characteristic contour observed at 25° C (Fig. S5) and the proportion of FA is seen to be more. The results suggest that the fisetin molecule senses the disaggregation of bile salts aggregates as temperature increases. The results obtained from the fluorescence intensity and fluorescence anisotropy of two forms of fisetin at different temperatures provides corroborating evidence for the hydrophobic nature of interaction between fisetin and bile salts.
Conclusions
This study reports the observation of ground state fisetin anion ((FA)GS) in alcohols and bile salts for the first time. The hypsochromic shift of (FA*)ES emission and the bathochromic shift of FT emission provides a convenient way of monitoring the progressive increase of medium nonpolarity when the bile salt concentration is increased. The spectral features of these prototropic forms give a valuable evidence for the neutralization of the bile salt anions as their concentration is increased. The absorption and fluorescence spectra of fisetin in varying concentration of NaC show that not only multiple prototropic forms, namely FN, (FA)GS and FT are present within bile salt aggregates, but also that their relative concentrations change with change of bile salt concentration.Acknowledgements
The authors wish to acknowledge the Department of Biotechnology, Govt. of India for financial support. The fluorescence lifetime data were recorded by an instrument obtained through a project funded by the Department of Science and Technology, New Delhi, India.References
- N. Rouge, P. Buri and E. Doelker, Drug absorption sites in the gastrointestinal tract and dosage forms for site-specific delivery, Int. J. Pharm., 1996, 136, 117–139 CrossRef CAS.
- D. Horter and J. B. Dressman, Influence of physicochemical properties on dissolution of drugs in the gastrointestinal tract, Adv. Drug Delivery Rev., 2001, 46, 75–87 CrossRef CAS.
- B. de Castro, P. Gameiro, C. Guimaräes, J. L. F. C. Lima and S. Reis, Fluorimetry and solubility studies of nadolol and atenolol in SDS micelles, J. Pharm. Biomed. Anal., 1998, 18, 573–577 CrossRef CAS.
- M. Miyake, T. Minami, M. Hirota, H. Toguchi, M. Odomi, K. Ogawara, K. Higaki and T. Kimura, Novel oral formulation safely improving intestinal absorption of poorly absorbable drugs: utilization of polyamines and bile acids, J. Controlled Release, 2006, 111, 27–34 CrossRef CAS.
- J. Guharay, S. M. Dennison and P. K. Sengupta, Influence of different environments on the excited-state proton transfer and dual fluorescence of fisetin, Spectrochim. Acta, Part A, 1999, 55, 1091–1099 CrossRef.
- M. Funes, M. A. Biasutti, N. M. Correa and J. J. Silber, Comparative study of the photophysical behavior of fisetin in homogeneous media and in anionic and cationic reverse micelles media, Photochem. Photobiol., 2007, 83, 486–493 CrossRef CAS.
- A. Sytnik, D. Gormin and M. Kasha, Interplay between excited-state intramolecular proton transfer and charge transfer in flavonols and their use as protein-binding-site fluorescence probes, Proc. Natl. Acad. Sci. U. S. A., 1994, 91, 11968–11972 CAS.
- V. V. Sentchouk and E. V. Bondaryuk, Fluorescent analysis of interaction of flavonols with hemoglobin and bovine serum albumin, J. Appl. Spectrosc., 2007, 74, 659–664 CrossRef.
- A. Zhu, B. Wang, J. O. White and H. G. Drickamer, The effect of pressure on the excited-state intramolecular proton transfer of polyhydroxyflavone, J. Phys. Chem. B, 2004, 108, 895–898 CrossRef CAS.
- S. Chaudhuri, A. Banerjee, K. Basu, B. Sengupta and P. K. Sengupta, Interaction of flavonoids with red blood cell membrane lipids and proteins: antioxidant and antihemolytic effects, Int. J. Biol. Macromol., 2007, 41, 42–48 CrossRef CAS.
- B. Sengupta, A. Banerjee and P. K. Sengupta, Investigations on the binding and antioxidant properties of the plant flavonoid fisetin in model biomembranes, FEBS Lett., 2004, 570, 77–81 CrossRef CAS.
- C. O'Connor and R. G. Wallace, Physico-chemical behavior of bile salts, Adv. Colloid Interface Sci., 1985, 22, 1–111 CrossRef CAS.
- S. Mukhopadhyay and U. Maitra, Chemistry and biology of bile acids, Curr. Sci., 2004, 87, 1666–1683.
- D. M. Small, S. A. Penkett and D. Chapman, Studies on simple and mixed bile salt micelles by nuclear magnetic resonance spectroscopy, Biochim. Biophys. Acta, Lipids Lipid Metab., 1969, 176, 178–189 Search PubMed.
- C. I. O'Connor, B. T. Ch'ng and R. G. Wallace, Studies in bile salt solutions: 1. Surface tension evidence for a stepwise aggregation model, J. Colloid Interface Sci., 1983, 95, 410–419 CrossRef CAS.
- S. Reis, C. G. Moutinho, C. Matosa, B. de Castro, P. Gameiroc and J. L. F. C. Lima, Noninvasive methods to determine the critical micelle concentration of some bile acid salts, Anal. Biochem., 2004, 334, 117–126 CrossRef CAS.
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Edition 3, Springer Publishers, New York, 2006 Search PubMed.
- P. K. Mandal and A. Samanta, Evidence of ground-state proton-transfer reaction of 3-hydroxyflavone in neutral alcoholic solvents, J. Phys. Chem. A, 2003, 107, 6334–6339 CrossRef CAS.
- T. Shyamala and A. K. Mishra, Ground and excited state proton transfer reaction of 3-hydroxyflavone in dimyristoylphosphatidylcholine liposome membrane, Photochem. Photobiol., 2004, 80, 309–315 CrossRef CAS.
- G. Sugihara, K. Yamakawa, Y. Murata and M. Tanaka, Effects of pH, pNa and temperature on micelle formation and solubilization of cholesterol in aqueous solutions of bile salts, J. Phys. Chem., 1982, 86, 2784–2788 CrossRef CAS.
- H. Sugioka, K. Matsuoka and Y. Moroi, Temperature effect on formation of sodium cholate micelles, J. Colloid Interface Sci., 2003, 259, 156–162 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1 (absorption spectra of fisetin in different solvents); Fig. S2 (fluorescence spectra of fisetin in different solvents); Fig. S3 (excitation spectra of fisetin in different NaC concentrations); Fig. S4 (deconvoluted emission spectra of FN (λex 369 nm) in buffer and NaC); Fig. S5 (EEMF spectra of fisetin in buffer medium and NaC at 25 °C); Fig. S6 (absorption spectra of fisetin in different bile salts); Fig. S7 (emission spectra of FT and (FA)GS in different bile salts); Fig. S8 (plot of fluorescence intensity of FT emission and (FA)GS emission against different bile salt concentrations at various T); Fig. S9 (plot of fluorescence anisotropy of FT emission and (FA)GS emission against different bile salt concentrations at various T) and Fig. S10 (EEMF spectra of fisetin in buffer medium and NaC at 45 °C). See DOI: 10.1039/c0pp00120a |
| This journal is © The Royal Society of Chemistry and Owner Societies 2011 |