Stabilisation of SWNTs by alkyl-sulfate chitosan derivatives of different molecular weight: towards the preparation of hybrids with anticoagulant properties†
Dimitrios G.
Fatouros
a,
Kieron
Power
a,
Omar
Kadir
a,
Imre
Dékány
b,
Spyros N.
Yannopoulos
c,
Nikolaos
Bouropoulos
cd,
Aristides
Bakandritsos
d,
Milan D.
Antonijevic
e,
George D.
Zouganelis
f and
Marta
Roldo
*a
aSchool of Pharmacy and Biomedical Sciences, University of Portsmouth, St. Michael's Building, White Swan Road, Portsmouth, PO1 2DT, UK
bDepartment of Physical Chemistry and Material Science, University of Szeged, Aradi Vt. 1, Szeged, 6720, Hungary
cFoundation for Research and Technology, Hellas-Institute of Chemical Engineering and High Temperature Chemical Processes—FORTH/ICE-HT, P.O. Box 1414, GR-26504, Patras, Greece
dDepartment of Materials Science, University of Patras, 26504, Rio Patras, Greece
eSchool of Science, University of Greenwich, Medway Campus, Central Avenue, Chatham Maritime, ME4 4TB, Kent, UK
fSchool of Biological Sciences, University of Portsmouth, King Henry I Street, King Henry I Building, Portsmouth, PO1 2DY, UK
First published on 24th January 2011
Abstract
We have previously demonstrated that chitosan derivative N-octyl-O-sulfate chitosan (NOSC), which presents important pharmacological properties, can suspend single walled carbon nanotubes (SWNTs) up to 20 times more effectively than other chitosan derivatives in an aqueous environment. In an attempt to further investigate the impact of different molecular weights of chitosan to the solubilization and anticoagulant properties of these hybrids an array of NOSC derivatives varying their molecular weight (low, medium and high respectively) was synthesised and characterised by means of FT-IR spectroscopy, NMR spectroscopy and thermal gravimetric analysis (TGA). Microwave and nitric acid purified SWNTs, characterised by FT-IR spectroscopy, transmission electron microscopy (TEM) and Raman spectroscopy, were colloidally stabilised by these polymers and their anticoagulant activity was assessed. The results revealed that the low molecular weight NOSC coated SWNTs exhibit the highest activity when 0.5 mg mL−1NOSC solutions are used, activity which is similar to that of the free polymer. Preliminary studies by exposure of these hybrids to Brine Shrimp (Artemia) cysts revealed no effect on the viability of sub-adult Artemia. Our findings suggest the possibility of tailoring these nanomaterials to bear the required properties for application as biocompatible building blocks for nanodevices including biosensors and biomaterials.
Introduction
Carbon nanotubes have attracted considerable attention for several types of biomedical applications such as biosensors or tissue scaffolding materials. Nevertheless the development of these materials remains attractive with the proviso that these systems are biocompatible.Covalent and non-covalent stabilization of aqueous suspensions of carbon nanotubes (CNTs) have been recently attempted using chitosan and its derivatives.1,2 Non-covalent methods which could preserve the nanotube structure and thus retain their excellent electronic properties are preferable. Most studies report mixing pristine CNTs with 0.5 to 1% w/v solutions of chitosan or its derivatives, followed by prolonged sonication (up to 5 h) to assist dispersion and centrifugation to further eliminate any large bundles still present in suspension.3,4 Chitosan thermodynamically stabilises CNTs by disrupting the intertubular attractions and reducing the hydrophobicity of the carbon surface in contact with water.5 Additionally the molecular weight of the polymer used seems to affect the loading capacity: for example, it has been reported that by increasing the molecular weight of chitosan, from 20 to 200 kDa, the suspension efficiency was significantly improved from ∼36% to ∼47%, respectively.3 Furthermore, Long et al.6 reported that when using a water soluble chitosan derivative, carboxymethylated chitosan, of increasing molecular weight, multi-walled carbon nanotubes (MWNTs) were obtained with increasing thickness of coating. These data are in good agreement with the thickness of coating recently reported in our laboratory using different chitosan derivatives, namely trimethyl chitosan chloride (TMC)7 and N-octyl-O-sulfate chitosan (NOSC).8 Moreover, chitosan has been shown not only to separate CNTs from amorphous carbonaceous impurities4 but also to specifically segregate CNTs according to their diameter and chilarity,9,10 property also demonstrated by its derivative TMC.7 These properties are promising for several nanotechnology and biotechnology applications, such as the development of biosensors.1,11Carbon nanotubes/chitosan complexes have also shown enhanced DNA condensation properties compared to chitosan alone, which in turn could be useful in the development of gene delivery systems.12 Furthermore, metal binding properties of chitosan, combined with absorption properties of carbon nanotubes, give scope for further investigation into environmentally friendly nanocomposites.13
The presence of functional groups such as primary and secondary hydroxyl groups and amino groups along the polymeric backbone of chitosan has allowed for easy modification of the polysaccharide and lead to the synthesis of derivatives with new and improved properties.14,15 An example of these derivatives is sulfated chitosans that present novel pharmacological activities15 including anticoagulant,16,17 antimicrobial,18antioxidant,19 anti HIV-120 and anti-inflammatory.21
In a recent study we have demonstrated that the anticoagulant properties of complexes of NOSC and single-walled carbon nanotubes (SWNTs) exhibit similar activity to the polymer alone. Such properties would eliminate the risk for SWNTs to induce coagulation as a host reaction process when used in vivo.8
In order to further our investigation into the properties of NOSC–SWNT hybrids, in the current study we synthesised an array of chitosan derivatives with different molecular weights and investigated their anticoagulant activity before and after condensation with purified SWNTs. Finally preliminary results on the in vivo biomodification of these hybrids by Brine Shrimp (Artemia) cysts are presented.
Materials and methods
Materials
Low, medium and high viscosity chitosan, pyrene, chlorosulfonic acid, hydrochloric acid (HCl), nitric acid (HNO3) and sodium hydroxide (NaOH) were purchased from Fluka Biochemika, UK. Methanol (MeOH), dimethylformamide (DMF), acetone, diethyl ether and sodium chloride (NaCl) were provided by Fisher Scientific, UK. Octaldehyde was from Sigma-Aldrich, UK whilst butyraldehyde, palmitoyl chloride and sodium borohydride (NaBH4) were purchased from Acros Organics, UK. Single walled carbon nanotubes (SWNTs) were obtained from Carbon Nanotechnologies Inc., Houston, US. The Amax heparin accucolor kit was purchased from Trinity Biologicals, Dublin, Ireland.Synthesis of chitosan derivatives
In an attempt to investigate the effect of molecular weight of chitosan on the properties of chitosan derivatives N-octyl chitosan (NOC) and N-octyl-O-sulfate chitosan (NOSC) derivatives (Fig. 1) were synthesised as previously described,22 using, as starting material, chitosan of different molecular weights (L, low; M, medium and H, high) (see ESI†).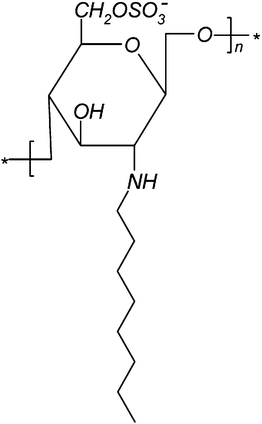 | ||
| Fig. 1 Chemical structure of NOSC. | ||
Characterization of chitosan derivatives
Chitosan derivatives were characterised by means of 1H NMR, FTIR, elemental analysis and thermal gravimetric analysis (TGA). 1H NMR spectra were obtained on a JEOL 400 MHz spectrometer operating at 400 MHz. The samples were dissolved in D2O and TMS was used as a standard. ATR spectra were recorded on a Tensor 27 FTIR spectrophotometer. Thermal gravimetric analysis (TGA) of chitosan and its derivatives was performed with a HighRes TGA 2950 (TA Instruments) between 20 and 500 °C with a heating rate of 10 °C min−1 under a N2 atmosphere (60 mL min−1).Critical micelle concentration (cmc) determination
The cmc of N-octyl-O-sulfate chitosan was determined using pyrene as a hydrophobic probe.23Purification and characterisation of SWNTs
Pristine nanotubes were purified using two different methods, namely: microwave assisted purification24 and purification by oxidation in nitric acid (see ESI†).25Transmission electron microscopy (TEM)
Samples for TEM were prepared by casting a droplet of a dilute suspension of the SWNTs on copper grids coated by Formvar film (SPI Supplies, USA). Micrographs were obtained by a CM-10 electron microscope (operating at 100 kV accelerating voltage) equipped with a side-mounted CCD camera “Mega View II” (Soft Imaging System Co.).Preparation and characterisation of NOSC–SWNT aqueous dispersions
Stable dispersions of SWNTs were obtained by mixing either pristine or purified material (final concentration 0.1 mg mL−1) and NOSC (10 mL, 0.1, 0.3 and 0.5 mg mL−1) and subjecting the mixture to sonication in an ultrasonic bath for 2 h. As a control, SWNTs were dispersed in distilled water (0.1 mg mL−1) and subjected to sonication under identical conditions to the sample. The dispersions were then centrifuged at 6000 rpm for 3 min to eliminate any bundles still present in suspension and the O.D. of the suspensions obtained was read at 500 nm. The apparent absorption coefficient of SWNTs dispersed in NOSC solutions was determined as described previously.8Raman spectroscopy measurements were performed using an Ar+ ion laser (Spectra Physics) operating at 514.5 nm. A metallurgical microscope (Olympus BHSM-BH2) was used for the delivery of the excitation beam and for the collection of scattered light.Anticoagulant activity
The potential anticoagulant activity of NOSC in solution and wrapped onto SWNTs was determined using the Amax heparin accucolor kit, according to the manufacturer's instructions and as previously described.8 Solutions of the polymer ranging from 0.001 to 5 mg mL−1 were tested to obtain a dose–response curve for NOSC. Samples of NOSC–SWNTs, prepared as described above, were also tested.Results and discussion
Synthesis and characterization of NOSC
Chitosan of three different molecular weights (ca. 150 kDa, low; 400 kDa, medium and 600 kDa, high) was modified by reductive amination of the free amino group in position C2 with octaldehyde. The product was successfully obtained in the form of an off-white powder (L-NOC, 1.38 ± 0.18 g per gram of initial chitosan) or flakes (M-NOC and H-NOC, 1.30 ± 0.03 g and 1.34 ± 0.05 g, respectively). The highest degree of alkylation was obtained for H-NOC (0.84, Table 1); which could be explained by the fact that H-chitosan exhibited the highest degree of deacetylation (83%) compared to the L- and M-chitosan (80%), respectively (the degree of deacetylation was calculated by 1H NMR).26| Polymer | %C | %N | %S | DA | DS | CMC/mg mL−1# |
|---|---|---|---|---|---|---|
| a One-way Anova: #p < 0.05 and Tukey–Kramer post-hoc test: *p < 0.05 compared to H-NOSC. | ||||||
| L-NOC | 53.96 | 5.67 | — | 0.79 | — | |
| L-NOSC | 30.88 | 3.44 | 10.14 | 0.75 | 1.29 | 0.042 ± 0.024* |
| M-NOC | 50.13 | 5.59 | — | 0.75 | — | |
| M-NOSC | 25.34 | 3.11 | 6.06 | 0.68 | 0.85 | 0.084 ± 0.012 |
| H-NOC | 54.72 | 5.46 | — | 0.84 | — | |
| H-NOSC | 20.35 | 5.52 | 7.00 | 0.31 | 0.55 | 0.101 ± 0.012 |
The second step of the reaction involved the sulfation of NOC in chlorosulfonic acid and DMF (see ESI for mechanism of reaction†). L-NOSC was obtained as an off-white light material (0.17 ± 0.04 g per gram of L-NOC employed), as well as M- and H-NOSC (0.21 ± 0.07 g and 0.28 ± 0.07 g, respectively). The successful synthesis of NOSC was confirmed by ATR and 1H-NMR data (see ESI†) which are in good agreement with our previous report.22 Further thermal analysis of the polymers was carried out and is reported in the ESI†. The cmc values (Table 1) increased with the molecular weight of the polymer (p < 0.05, one-way Anova), meaning that higher molecular weight decreases the stability of the colloidal system. A direct correlation between the degree of alkylation and sulfation and the value of the cmc were also established. The polymer with the lowest cmc value was L-NOSC (0.042 ± 0.024 mg mL−1), which was also the polymer with the highest degree of alkylation and sulfation, therefore this is the polymer with the most accentuated amphiphilic character.
Characterization of purified carbon nanotubes
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching and at 1350 cm−1 assigned to O–H bending.2
O stretching and at 1350 cm−1 assigned to O–H bending.2
 | ||
| Fig. 2 ATR spectra of (A) pristine, (B) microwave and (C) HNO3−-purified SWNTs, respectively. | ||
 | ||
| Fig. 3 Transmission electron microscopy image of (A) pristine, (B) HNO3− and (C) microwave-purified SWNTs, respectively. | ||
 | ||
| Fig. 4 Stokes-side Raman spectra of the radial breathing modes for the pristine (a) and microwave purified (b) SWNT powders and SWNT dispersions in 0.5 mg mL−1 of L-NOSC. | ||
On the other hand the blue-shift of the RBM frequencies has frequently been attributed either to sample morphology (the increase or decrease of bundle size has been considered) or to an increase of the “molecular pressure” exerted by the functionalizing agent on the atomically thin graphene layer that constitutes the SWNTs.28 However, there are cases where the blue-shift is considered as apparent, owing to the change of resonance enhancement conditions. For example, one particular chirality of SWNTs could fall out of resonance and another one could come into resonance.28 The fact that all RBMs in the whole frequency range of the Raman spectra of dispersions (Fig. 4) exhibit comparable frequency shifts in comparison to the values obtained for the powders seems to support the idea that debundling effects and molecular pressure underlie the observed effects. The tangential modes (G bands) of pristine, microwaved and NOSC coated nanotubes were further investigated. These bands provide a means for the assessment of the metallic character of such materials (see ESI†).29
Based on the results obtained from ATR, TEM and Raman studies, a decision was made to use microwave treated nanotubes for the formation of the hybrids and successive studies.
Characterization of SWNTs suspension in aqueous solutions of NOSC
The capacity of aqueous solutions of NOSC to stabilize suspensions of SWNTs was investigated. The nanotubes previously characterised were tested in solutions of L-NOSC at different concentrations. Fig. 5A shows that the amount of SWNTs loaded into L-NOSC solutions was dependent on the treatment of SWNTs received before loading (p < 0.05, one-way ANOVA). In general the concentration of nitric acid purified SWNTs was found to be the highest. However, it was further observed that values above 100% were obtained when the nanotubes were suspended in L-NOSC 0.1 mg mL−1, an indication of bundles present in the dispersion; the latter was confirmed by the fact that after centrifugation at higher speed no SWNTs were left in suspension (Fig. 5B). Even though microwave purified SWNTs gave the lowest concentration in solution these were selected for further studies for two reasons: firstly for their superior purity as discussed above, secondly because it was shown that their concentration in suspension is not dependent on polymer concentration (p > 0.05, one-way ANOVA). Fig. 6 presents the loading data for microwave purified SWNTs in solutions of polymers with different molecular weights. For both low and medium molecular weight NOSC, the concentration of the polymer did not significantly affect the concentration of SWNTs in suspension (p > 0.05). However, H-NOSC exhibited a higher amount of SWNTs suspended when the concentration was increased from 0.1 to 0.5 mg mL−1 (Tukey–Kramer post-hoc test p < 0.05) reaching a maximum loading of 74.41 ± 4.69%. | ||
Fig. 5 Pristine (black), HNO3 purified (white) and microwave purified (grey) SWNTs loading into L-NOSC solutions at different concentrations after centrifugation at (A) 6000 rpm × 3 min and (B) 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm × 30 min. Data are reported as mean ± SD (n = 3). Tukey–Kramer post-hoc test: #p < 0.05; ##p < 0.01 when comparing with the polymer solution of concentration 0.1 mg mL−1; *p < 0.05, **p < 0.01 and ***p < 0.001. 000 rpm × 30 min. Data are reported as mean ± SD (n = 3). Tukey–Kramer post-hoc test: #p < 0.05; ##p < 0.01 when comparing with the polymer solution of concentration 0.1 mg mL−1; *p < 0.05, **p < 0.01 and ***p < 0.001. | ||
 | ||
| Fig. 6 SWNTs loading into L-NOSC (black), M-NOSC (white) and H-NOSC (grey) solutions at different concentrations. Data are reported as mean ± SD (n = 3). Tukey–Kramer post-hoc test: *p < 0.05 and **p < 0.01. | ||
As we have previously demonstrated, optimum stabilisation of SWNTs is obtained when the polymer molecules are aligned along the length of the nanotube as this maximises the van der Waals interaction between the polymeric chain and the carbonaceous surface.30 Longer chains provide higher flexibility and better coverage of the hydrophobic surface translating into better stabilisation.
Anticoagulant activity
Fig. 7 illustrates the heparin dose–response curve obtained with the Amax heparin accucolor kit, in the heparin concentration range 0–1 µg mL−1; results are expressed as the percentage residual activity of factor Xa after inhibition with heparin. The activity of heparin increased exponentially with increasing concentrations (y = 96.76e − 2.57x, R2 = 0.989) and the maximum activity was reached at a concentration of 0.8 µg mL−1. The effect of the molecular weight of the polysaccharide backbone on the anticoagulant activity of the three NOSC polymers was further investigated (Fig. 8). L-NOSC exhibited significantly different anticoagulant activities between 0.05 and 0.3 mg mL−1 (75.04 ± 4.38%, p < 0.01 Tukey–Kramer post-hoc test compared to the control), the latter concentration can be considered as the ceiling since no significant changes were observed above this value (p > 0.05), noteworthy, the maximum activity was observed at 3 mg mL−1 (4.23 ± 1.93%). M-NOSC exhibited similar behaviour with increasing activity between 0.05 mg ml−1 (88.42 ± 4.60%, p < 0.05 Tukey–Kramer post-hoc test compared to the control) and 0.5 mg mL−1, above which no more statistically significant changes were observed (p > 0.05). | ||
| Fig. 7 Heparin dose–effect curve obtained with Amax heparin accucolor kit (Trinity Biologicals, IRE). Data are reported as mean ± SD (n = 6). One-way Anova analysis of variance, p < 0.0001. | ||
 | ||
| Fig. 8 Anticoagulant activity of N-octyl-O-sulfate chitosan of low (black square), medium (empty circle) and high (empty triangle) molecular weights. Data are given as mean ± SD (n = 3). | ||
The maximum activity for M-NOSC was obtained at 5 mg mL−1 (6.74 ± 2.23%). H-NOSC showed the lowest level of anticoagulant activity; significant activity (59.37 ± 8.80%, p < 0.05 compared to the control) was obtained only at 1 mg mL−1, maximum activity was 14.38 ± 4.70%. Two-way ANOVA analysis of variance showed a statistically significant difference in anticoagulant activity dependent on the polymers molecular weight (p < 0.001) and polymers concentration (p < 0.001). In order to verify whether the mechanism of action of NOSC polymers is similar to that of heparin, the anticoagulant activity of the most potent polymer sample (L-NOSC 0.5 mg mL−1) was measured in the absence of antithrombin III. The absence of activity in these conditions illustrated that the polymer has a similar mechanism of action to that of heparin, in accordance with previous reports about sulfated chitosan.16,31,17Heparin activity is known to be affected by molecular weight; non fractionated heparin (5–25 kDa) acts by inhibiting two factors involved in the coagulation process, IIa and Xa, however, the selectivity towards inhibition of factor Xa can be increased by the use of low molecular weight heparin (5–7 kDa).17
For this reason the molecular weight of the NOSC polymers was considered to be a major factor that could affect the activity. L-NOSC and M-NOSC were prepared from chitosan of 150 and 400 kDa respectively; therefore the resulting polymers not only have a different chemical structure and three dimensional conformation but also have a molecular weight which is higher than that of heparin, these factors contribute to a decreased affinity for antithrombin III, explaining why these polymers are active only at concentration 1000 times higher than that of heparin. Our findings are in accordance to those of Vikhoreva et al.17 who found that in order for sulfated polymers to possess potent anticoagulant activity, they must have a molecular weight of 19–71 kDa, and by Vongchan et al.32 who noticed that a decrease in Mw of sulfated chitosan from 66 to 35 kDa resulted in increased anticoagulant activity.
Fig. 9A shows the anticoagulant activity of the NOSC polymers wrapped around SWNTs after centrifugation of the suspensions at 6000 rpm. SWNTs wrapped in L-NOSC showed the highest activity, significantly higher than those of M-NOSC and H-NOSC at 0.3 mg mL−1 (p < 0.05 and p < 0.01, respectively) and significantly higher than that of H-NOSC at 0.5 mg mL−1 (p < 0.05). The highest activity was observed for SWNTs wrapped in L-NOSC at 0.5 mg mL−1, the activity that was similar to that of the free polymer (p > 0.05, Fig. 9B). In all cases the presence of SWNTs in suspension did not affect the activity of the polymers.
 | ||
| Fig. 9 (A) Anticoagulant activity of SWNTs suspended in N-octyl-O-sulfate chitosan of low (black), medium (white) and high (grey) molecular weight solutions. Data are given as mean ± SD (n = 3). One-way Anova, to determine the effect of concentration on the activity, was significant only for L-NOSC (p < 0.0001); Tukey–Kramer post-hoc test ***p < 0.001. One-way Anova, to determine the effect of molecular weight on the activity, was significant only at concentrations of 0.3 and 0.5 mg mL−1 (p < 0.05); Tukey–Kramer post-hoc test, #p < 0.05, ##p < 0.01. (B) Comparison of the anticoagulant activity of L-NOSC in solution (grey) and wrapped on SWNTs (black), no statistical difference was found (p > 0.05, one-way Anova). | ||
Cytotoxicity studies
Cytotoxicity studies on Caco-2 cells (MTT assay, see ESI† for method) indicate the viability of 96.8 ± 7.0% when M-NOSC is used in a concentration of 0.1 mg mL−1 (Fig. 10). Furthermore, some preliminary ecotoxicity studies performed on Artemia larvae demonstrated the safe profile of these hybrids (see ESI†). | ||
| Fig. 10 Cytotoxicity of M-NOSC on Caco-2 cells. Results are given as mean ± SD 9 (n = 3). One-way Anova, p < 0.0001; Tukey–Kramer post-hoc test ***p < 0.001. **p < 0.01 compared to media; ###p < 0.001 compared to SDS. | ||
Conclusion
The current results clearly demonstrate that NOSC can be successfully used in the preparation of stable SWNT suspensions and that its molecular weight has a significant effect on the loading efficacy of the hybrids.The polymeric coating confers anticoagulant properties to the hybrid that suggest they might have good blood compatibility for in vivo applications. Since SWNTs are chemically inert and exhibit high mechanical strength they might be potential materials for biomedical applications.
Furthermore, preliminary studies conducted with Artemia demonstrate that NOSC–SWNTs can be considered as a biocompatible and/or biomodifiable building component for biomaterials. Finally, manipulation of the polymer properties can alter the activity of these hybrids underpinning their potential as important candidates for in vivo applications.
References
- Y.-T. Shieh and Y.-F. Yang, Eur. Polym. J., 2006, 42, 3162–3170 CrossRef CAS.
- Z. Wu, W. Feng, Y. Feng, Q. Liu, X. Xu, T. Sekino, A. Fujii and M. Ozaki, Carbon, 2007, 45, 1212–1218 CrossRef CAS.
- R. Haggenmueller, S. Rahatekar, J. Fagan, J. Chun, M. Becker, R. Naik, T. Krauss, L. Carlson, J. Kadla, P. Trulove, D. Fox, H. DeLong, Z. Fang, S. Kelley and J. Gilman, Langmuir, 2008, 24, 5070–5078 CrossRef CAS.
- M. Zhang, A. Smith and W. Gorski, Anal. Chem., 2004, 76, 5045–5050 CrossRef CAS.
- M. J. O'Connell, P. Boul, L. M. Ericson, C. Huffman, Y. Wang, E. Haroz, C. Kuper, J. Tour, K. D. Ausman and R. E. Smalley, Chem. Phys. Lett., 2001, 342, 265–271 CrossRef CAS.
- D. Long, G. Wu and G. Zhu, Int. J. Mol. Sci., 2008, 9, 120–130 Search PubMed.
- A. Wise, J. Smith, N. Bouropoulos, S. Yannopoulos, S. van der Merwe and D. Fatouros, J. Biomed. Nanotechnol., 2008, 4, 67–72 Search PubMed.
- M. Roldo, K. Power, J. R. Smith, P. A. Cox, K. Papagelis, N. Bouropoulos and D. G. Fatouros, Nanoscale, 2009, 1, 366–373 RSC.
- T. Takahashi, C. Luculescu, K. Uchida, T. Ishii and H. Yajima, Chem. Lett., 2005, 34, 1516–1517 CrossRef CAS.
- H. Yang, S. Wang, P. Mercier and D. Akins, Chem. Commun., 2006, 1425–1427 RSC.
- C. Lau, M. J. Cooney and P. Atanassov, Langmuir, 2008, 24, 7004–7010 CrossRef CAS.
- Y. Liu, Z.-L. Yu, Y.-M. Zhang, D.-S. Guo and Y.-P. Liu, J. Am. Chem. Soc., 2008, 130, 10431–10439 CrossRef CAS.
- G. Ke, W. Guan, C. Tang, W. Guan, D. Zeng and F. Deng, Biomacromolecules, 2007, 8, 322–326 CrossRef CAS.
- N. M. Alves and J. F. Mano, Int. J. Biol. Macromol., 2008, 43, 401–414 CrossRef CAS.
- R. Jayakumar, N. Nwe, S. Tokura and H. Tamura, Int. J. Biol. Macromol., 2007, 40, 175–181 CrossRef CAS.
- P. Vongchan, W. Sajomsang, D. Subyen and P. Kongtawelert, Carbohydr. Res., 2002, 337, 1239–1242 CrossRef CAS.
- G. Vikhoreva, G. Bannikova, P. Stolbushkina, A. Panov, N. Drozd, V. Makarov, V. Varlamov and L. Gal'braikh, Carbohydr. Polym., 2005, 62, 327–332 CrossRef CAS.
- R. Huang, Y. Du, L. Zheng, H. Liu and L. Fan, React. Funct. Polym., 2004, 59, 41–51 CrossRef CAS.
- R. Xing, H. Yu, S. Liu, W. Zhang, Q. Zhang, Z. Li and P. Li, Bioorg. Med. Chem., 2005, 13, 1387–1392 CrossRef CAS.
- S.-I. Nishimura, H. Kai, K. Shinada, T. Yoshida, S. Tokura, K. Kurita, H. Nakashima, N. Yamamoto and T. Uryu, Carbohydr. Res., 1998, 306, 427–433 CrossRef CAS.
- J. L. Jordan, S. Henderson, C. M. Elson, J. Zhou, A. Kydonieus, J. Downie and T. D. G. Lee, Urology, 2007, 70, 1014–1018 CrossRef.
- S. Green, M. Roldo, D. Douroumis, N. Bouropoulos, D. Lamprou and D. G. Fatouros, Carbohydr. Res., 2009, 344, 901–907 CrossRef CAS.
- C. Zhang, P. Qineng and H. Zhang, Colloids Surf., B, 2004, 39, 69–75 CrossRef CAS.
- E. Vázquez, V. Georgakilas and M. Prato, Chem. Commun., 2002, 2308–2309 RSC.
- V. Datsyuk, M. Kalyva, K. Papagelis, J. Parthenios, D. Tasis, A. Siokou, I. Kallitsis and C. Galiotis, Carbon, 2008, 46, 833–840 CrossRef CAS.
- M. Lavertu, S. Méthot, N. Tran-Khanh and M. D. Buschmann, Biomaterials, 2006, 27, 4815–4824 CrossRef CAS.
- M. S. Dresselhaus, G. Dresselhaus, R. Saito and A. Jorio, Phys. Rep., 2005, 409, 47–99 CrossRef.
- D. A. Britz and A. N. Khlobystov, Chem. Soc. Rev., 2006, 35, 637–659 RSC.
- S. N. Yannopoulos, G. D. Zouganelis, S. Nurmohamed, J. R. Smith, N. Bouropoulos, G. Calabrese, D. G. Fatouros and J. Tsibouklis, Nanotechnology, 2010, 21, 85101 CrossRef CAS.
- S. Piovesan, P. A. Cox, J. R. Smith, D. G. Fatouros and M. Roldo, Phys. Chem. Chem. Phys., 2010, 12, 15636–15643 RSC.
- P. Vongchan, W. Sajomsang, W. Kasinrerk, D. Subyen and P. Kongtawelert, ScienceAsia, 2003, 29, 115–120 CrossRef CAS.
- P. Vongchan, W. Sajomsang, D. Subyen and P. Kangtawelert, Carbohydr. Res., 2002, 337, 1239–1442 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis of NOC and NOSC; SWNT purification methods; MTT assay; mechanism of sulfation; ATR and NMR data; and further Raman analysis, in vivo biomodification assay. See DOI: 10.1039/c0nr00952k |
| This journal is © The Royal Society of Chemistry 2011 |
