Nanobubble and nanodroplet template growth of particle nanorings versus nanoholes in drying nanofluids and polymer films†
S.
Darwich
a,
K.
Mougin
*a,
L.
Vidal
a,
E.
Gnecco
b and
H.
Haidara
*a
aInstitut de Science des Matériaux de Mulhouse, IS2M-CNRS, 15 Rue Jean Starcky, 68057, Mulhouse, France. Fax: +33 389608799; E-mail: karine.mougin@uha.fr; hamidou.haidara@uha.fr
bInstitute of Physics, University of Basel, and NCCR “Nanoscale Science”, Klingelbergstrasse 82, 4056, Basel, Switzerland
First published on 24th January 2011
Abstract
Here we demonstrate how confined nanobubbles and nanodroplets, which can either form spontaneously at the suspension/substrate interface, or can more interestingly be purposely introduced in the system, allow assembly of nanoparticles (NPs) into nanoring-like structures with a flexible control of both the size and distribution. As with most wetting-mediated nanopatterning methods, this approach provides an alternative to direct replication from templates. The formation of two-dimensional ring-shaped nanostructures was obtained by drying a nanocolloidal gold (Au) suspension drop confining nanobubbles (or nanodroplets) that are settled at a solid substrate. AFM investigation of the dry nanostructures showed the formation of isolated Au NPs rings having diameters ranging from 200 nm to 500 nm along the dewetting–drying path of the suspension drop. The flexibility of these wetting processes for the variation of the spatial features of the nanoring (size and shape resolution) essentially depends on physical parameters such as the nanobubble/nanodroplet size and concentration, the wettability, and the evaporation rate of the nanofluid drop on the substrate. Furthermore, we show that the underpinning mechanism of this evaporation-assisted assembly of Au NPs into supported functional nanoring patterns is fairly similar to that at work in the spontaneous formation of nanoholes in drying polymer thin films. Finally, the method proves to be a simple and flexible nanofabrication tool to be extended to various nanosize objects, towards specific optical and sensing applications.'
Introduction
Patterns of nanosize rings have recently attracted intense research interest, driven by their promising nanotechnological applications1 which are related to the functional properties of those objects that are highly sensitive to the arrangement of the individual nanoring blocks. Among these functional arrangements, nanoring patterns can serve, for instance, as nanometer-scale sensors, resonators and transducers, or provide a unique platform for biological and pollutants analysis at small scales.2The first particle nanorings, obtained by a spontaneous process (self-coiling of nanobelts), was zinc oxide nanorings discovered by researchers at Georgia Institute of Technology3 less than 10 years ago. Since this work, which described some of the underlying mechanisms of the spontaneous self-assembly and growth of particle nanorings, other techniques have been developed based on different processes, especially on template-assisted techniques and nanolithography. For instance, F. Q. Zhu et al. have proposed a method, referred to as “natural lithography”, which uses nanospheres as a template to create nanoring patterns. The diameter, area, density, thickness and composition of nanorings can be easily controlled by this method. The symmetry of nanorings can also be engineered to obtain asymmetric nanorings.4,5 In a similar way, F. J. García de Abajo et al. have prepared ring-shaped gold nanoparticles using colloidal lithography.6 A while ago, Savinova et al. have described an in situ approach to the fabrication of metal nanoring arrays using an assembly of microbeads7 as a template. This method differs essentially from the other microbeads template-based techniques in that it relies on the in situ synthesis of the NPs, whereas the others use NP suspensions. Tripp et al.8 have found a way to create assembly of Co nanoparticles into rings which can be formed by two different mechanisms: dipole-directed self-assembly (typically 5–12 particles, ring diameters between 50 and 100 nm), and evaporation-driven hole formation in viscous wetting layers (ring diameters ranging from 0.5 to 10 μm). As a result of that intense activity toward nanorings, a number of metal particle nanoring structures have been created either with nanolithography (such as e-beam,9,10capillary force lithography11,12) and template-assisted techniques (μ-contact printing13 and porous membranes14,15).
However, new alternative approaches relying on self-assembly processes, and especially drying-mediated techniques, are still being developed for the fabrication of nanoparticle rings. For instance, Govor et al.16,17 have proposed an approach based on a combination of phase separation in a thin film of a binary (polymer and nanoparticles) mixture, leading to a bilayer structure, subsequent decomposition of the top layer into droplets, and finally the deposition of the CoPt3 nanoparticles at the edges of the evaporating droplets. Liu et al.18 have used pre-patterned surface edges of the evaporating droplets prepared by phase separation of a polymer film to direct the assembly of nanoparticles. Other methods using evaporation-assisted assembly have also been studied. J. Heath et al.19 have proposed a method based on the dewetting of a thin suspension film from a substrate, which leads to the formation of a peripheral particle accumulation (coffee ring), around opening holes. This approach often leads to the formation of micrometer sized rings. The work developed by Pileni's group in this field instead uses Bénard–Marangoni instabilities20,21 and related convection cells in the drying suspension drop to self-assemble the nanoparticles into nanorings. Finally, E. Zubarev et al.22,23 have succeeded in using condensed water droplets on a nonpolar solvent as template to self-assemble dense arrays of polystyrene coated gold nanorods on solid supports. Thus, it appears from these studies that drying-mediated techniques represent a simple and major route toward the self-organization of nanoparticles into large scale nanoring arrays.24
Here, we have developed a system that uses confined nanodroplets and/or nanobubbles, which can either form spontaneously at the suspension/substrate interface, or can more interestingly be introduced purposely into the system, allowing flexible control of both the size and distribution of the resulting nanoring patterns. In that sense, the approach we propose is a template-assisted method that essentially benefits from simple interfacial phenomena, namely the confinement of naturally occurring or purposely prepared nanobubbles or nanodroplets, embedded at the interface between a hydrophobic substrate and a immiscible liquid drop. In contrast to the drying-mediated assembly and nanoring formation described above, especially the closer methodological approach of Zubarev et al.,23 the method we here report has the following distinctive feature: it allows more flexible control of the size and spatial distribution of the nanorings, as well as the organization of the NPs within the ring, through the introduction of a calibrated nanobubble (-droplet) template, and confinement prevents that template from direct evaporation-induced destabilization. In addition, this topic of nanobubble-related interface phenomena has appeared recently to have strong relevance to many current technological issues (wall slippage in microfluidic devices, hydrophobic interaction, stability of colloidal systems,25 nanoholes in drying of thin organic films26).
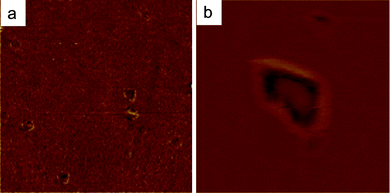
A representative illustration of these nanobubble-related interface phenomena is given in Fig. 1, which shows the spontaneous nucleation of nanohole defects, observed by Atomic Force Microscopy in a thin wetting film of sodium alginate solution (0.2 wt% in water ∼19.2 μmol L−1) deposited by drop-casting, and dried on a cleaned silicon wafer. These often undesired but observed nanoring defects in organic thin films26 can also be changed into an opportunity to form well-defined and organized nanohole (nanoporous) structures. We here describe an unexplored use and application of these confined nanobubbles and -drops, as well as the underlying mechanisms involved in their formation in drying nanofluids.
 | ||
| Fig. 1 Height AFM images of a dry wetting film of sodium alginate solution of concentration C = 0.2 wt% in water (19.2 μmol L−1), deposited by dropcasting on a cleaned silicon wafer. (a) Randomly dispersed nanoholes in the thin wetting film, (b) 3D-zoom on nanoholes at the surface. Frame sizes: 5 μm and 2 μm respectively. | ||
Nanobubbles (nanodroplets) supported by hydrophobic surfaces and surrounded by an immiscible NP suspension (solvent) represent an ideal platform to self-assemble chemically coated nanoparticles. As the NPs adsorb at the nanobubble/solution interface, the drying of the surrounding suspension drop, and the subsequent rupture of the NP-coated nanobubble, leads to the formation of a peripheral ring of particles on the substrate. The explosion of the confined nanobubbles (evaporation of nanodroplets) can be controlled to a large extent by the evaporation of the surrounding nanofluid drop. This method requires two interfacial conditions for the particle nanoring patterns to form: i) a specific contact angle between the suspension drop and the hydrophobic surface to favour slippage on the substrate and, ii) a short evaporation time of the suspension drop to ensure a rapid retraction of the contact line under non-pinning conditions.
Experimental
The hydrophobic surfaces were self-assembled molecular films of methyl-terminated n-hexadecyltrichlorosilane Cl3Si(CH2)15CH3 (referred to as CH3), formed on silicon wafers bearing their native oxide layer (SiO2). These self-assembled molecular films (SAM) were prepared according to a standard path of organosilanes grafting from dilute millimolar silane solution onto clean, hydroxylated SiO2 substrates.27 The advancing and receding contact angles and the related hysteresis of water on these CH3 surfaces were θa = 110 ± 1° and θr = 105 ± 1°. The ellipsometric thickness of these close packed hydrophobic SAMs was 2.3 ± 0.1 nm.The nanocolloidal suspension was an aqueous solution of gold nanoparticles (Au NPs) that was synthesized from tetrachloroauric(III)acid hydrate ([HAuCl4]·H2O), and stabilized with citric acid trisodium. The concentrations of [HAuCl4]·H2O and citric acid tri-sodium were, respectively, 0.03 and 0.02 wt.% in water (Milli-Q water), for a pH of 6.5. By reducing HAuCl4 with trisodium citrate, stabilized Au nanoparticles bearing the negative charge of the citrate ions are obtained.28 The average size of these particles, as determined from Transmission Electronic Microscopy (Fig. 2), was 25 ± 5 nm, and its volume fraction determined by differential weighing between different volumes of nanofluid (drops) and the resulting dry NPs residue was ca. 2.8 × 10−4. In order to make these as-synthesized citrate stabilized Au NPs soluble (dispersible) in organic solvents, the NPs were hydrophobized by grafting methyl-terminated alkylthiol molecules onto them. The alkyl-thiol (1-hexadecanthiol) was from Sigma- Aldrich and used as received. Methyl-thiol-stabilized Au NPs were prepared according to a modified route of two common standard methods. The raw NP solution20,21 was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 20 min to decant and pellet the NPs, which were then resuspended in tetrahydrofuran (THF) for nanobubble-assisted nanoring formation, or cyclohexane for nanodroplet-assisted nanoring formation. Then, alkyl-thiol was added to these organic NP solutions (volume fraction of 10−3) which were then sonicated for approximately 2 h to allow the thiol grafting reaction to reach completion. The volume fraction of Au NPs in the final organic suspension (THF or cyclohexane) was about 7.8 x10−5.
000 rpm for 20 min to decant and pellet the NPs, which were then resuspended in tetrahydrofuran (THF) for nanobubble-assisted nanoring formation, or cyclohexane for nanodroplet-assisted nanoring formation. Then, alkyl-thiol was added to these organic NP solutions (volume fraction of 10−3) which were then sonicated for approximately 2 h to allow the thiol grafting reaction to reach completion. The volume fraction of Au NPs in the final organic suspension (THF or cyclohexane) was about 7.8 x10−5.
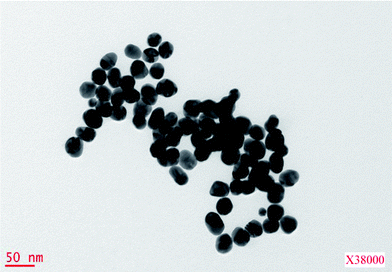 | ||
| Fig. 2 400 nm × 400 nm TEM image of 25 nm diameter gold nanoparticles. | ||
The mechanism of the nanoparticle ring formation on hydrophobic surfaces relies on the non-miscibility of two fluids: either polar nanodroplets, or nanobubbles dispersed in an organic NPs suspension. These nanodroplet and nanobubble dispersions were prepared as follows.
For nanodroplets, a water-ethanol mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 in volume) was added to the NP suspension in cyclohexane. This solution was sonicated with an electrode at 20 kHz during 10 s (1 pulse/s), using a sonicator from Bioblock Scientific (VIBRA-CELL, MOD. 72441), operated at a nominal output power of 300 W. Nanodroplets were formed within a few seconds, with both sizes and distribution remaining stable for up to ∼30 min. The average nanodroplet size in the suspension, characterized by light backscattering measurements using a Turbiscan device was ca. 90 nm.
4 in volume) was added to the NP suspension in cyclohexane. This solution was sonicated with an electrode at 20 kHz during 10 s (1 pulse/s), using a sonicator from Bioblock Scientific (VIBRA-CELL, MOD. 72441), operated at a nominal output power of 300 W. Nanodroplets were formed within a few seconds, with both sizes and distribution remaining stable for up to ∼30 min. The average nanodroplet size in the suspension, characterized by light backscattering measurements using a Turbiscan device was ca. 90 nm.
For the formation of nanobubbles, the NP suspension in THF was simply sonicated under identical conditions. The average size of the nanobubbles produced in this way was ca. 250 nm. In contrast to the nanodroplets, the stability of nanobubble dispersions could not be reliably monitored through their size and density as this nano-emulsion was less stable than the previous one.
After all the suspensions were prepared (dispersions of nanodroplets and nanobubbles in organic NP suspensions), a 20 μL drop of the suspension was deposited onto the hydrophobic CH3 surface, and allowed to dry in ambient conditions (33% RH, 22 °C) for the suspension drop embedding nanodroplets, and at 75 °C for the suspension drop embedding nanobubbles (Scheme 1).
 | ||
| Scheme 1 Scheme of a nanocolloid suspension drop confining immiscible ethanol/water nanodroplets, or nanobubbles, that are settled at a solid substrate. | ||
Results and discussion
For our particular systems (volatile organic solvents) and operating conditions, the high evaporation rate does not allow a strong pinning of the contact line from the convective transport and accumulation of nanobubbles (nanodroplets) or nanoparticles in the wedge of the drop. Indeed, by using a low boiling point solvent such as cyclohexane (boiling point at 80.74 °C) and tetrahydrofuran (boiling point at 66 °C), the evaporation rate (90 s for the drying of the cyclohexane suspension drop and a few seconds for the THF one) is increased and the timescale to reach the receding contact angle is shortened before peripheral particle accumulation definitively impedes the retraction. In addition, a short evaporation timescale before contact line retraction reduces the density of sedimented nanodroplets/nanobubbles, preventing their collapse under the shear pressure.At the early stage of drying, NP-coated nanobubbles of small size (diameters <250 nm)/nanodroplets (diameters <150 nm) are entrained at the periphery of the suspension drop, whereas bigger ones mainly settle slowly in the center due to the predominance of gravitational forces. In contrast to the large nanodroplets, the drainage of the small ones inside the wedge is favored by both the convective outward flow generated by the evaporation gradient along the drop,29 and the low resisting hydrodynamic force proportional to the size nanodroplets.
The dewetting dynamics of the evaporating suspension drops were then investigated for the formation of the nanobubble (nanodroplet) arrays as a function of both the solvent (THF, cyclohexane) and the receding velocity of the retracting drop. These two parameters determine the competition between, (i) the adsorption of the NPs to the surface, versus their retention in the bulk solvent and, (ii) the magnitude of the shear stress that nanobubbles (nanodroplets) experience during the receding of the contact line. The balance between these two forces determines either the detachment or drainage of the nanodroplets, or their morphological distortion under the shear stress exerted by the receding wedge of the evaporating drop. The magnitude of the shear stress within the wedge of thickness h, receding with an average velocity U, is in the first order proportional to the viscosity η of the nanofluid flowing past the wall, and given by, σ ∼ η(U/h), for small enough thickness h, and assuming no-slip at the interface. That no-slip boundary condition is satisfied here by the low interaction forces between NPs and the CH3 coated substrate, which mainly involves van der Waals interactions.
For our particular systems, U is of the order of 25 μm s−1 for cyclohexane and three times more for THF drop, with a receding contact angle θr of 11 ± 1° for both THF and cyclohexane suspension drops (comparable wedge thickness h). Since the viscosities of these two solvents are comparable, one can assume from these data that the shear stress experienced by nanobubbles in the wedge of the THF drop is about three times larger than for nanodroplets in cyclohexane.
For the nanodroplets to remain attached to the substrate when the wedge of the suspension drop crosses them, their adhesion and the related threshold static frictional force required to detach them should be higher than the above shear force developed within the retracting wedge. Otherwise, the nanodroplets are detached and collected within the receding drop.
The mode of destabilization and rupture of the confined nanobubbles/nanodroplets seems to result from two distinct phenomena, depending on whether nanobubbles or nanodroplets are involved, as depicted in Schemes 2 and 3. The evaporation of the drop first leads to a decrease of the overall thickness of the drop and further confinement of the NP coated nanodroplets/nanobubbles in the wedge. For nanobubbles, when the thickness of the THF film covering the nanobubble at its apex becomes comparable to the length-scale of long-range (L-R) van der Waals forces, the air encapsulated in the bubble, and that in the surrounding atmosphere, couple and attract each other, creating an extra-compressive pressure across the suspension (solvent) film. This extra pressure, which acts to conjoin the air phases by further thinning the film until it ruptures (destabilization), has a magnitude which is related to the Hamaker constant (A) of the (air/suspension film/air) interface (A > 0), and the thickness h of the suspension film, defined as Π ∼ −A/6πh3. It is worth mentioning at this point that we have implicitly assumed that the apex of the nanobubble (nanodroplet) was no longer covered by the nanoparticles under the above confinement condition, leaving an air bubble/solvent/air configuration of the system. Indeed, as the suspension film thins down, the local confinement (and fluctuations) at the apex pushes the nanoparticles downward along the nanobubble interface. Typically, the configuration of the receding wedge, where such confinement and destabilization processes can develop, is that for which the size of the wedge (both thickness and width) is of the order of the diameter of the nanobubble. The variation of the Laplace pressure inside the nanobubble, induced by the rupture of the thin solvent film, drives the explosion of the nanobubble. Finally, the rupture of the suspension film at the apex and the collapse of the nanobubble provoke the downward drainage of the nanoparticles along the nanobubble/suspension interface, leading to their peripheral assembly (nanoring) as shown in Scheme 2C. In Fig. 3, the average size of the nanorings is 250 nm, with an average distance of 700 nm.
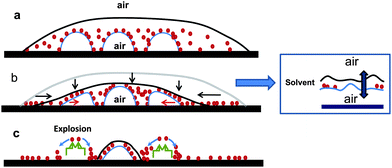 | ||
| Scheme 2 Scheme depicting the three main steps of the evaporation-drying process of a nanocolloid suspension confining immiscible nanobubbles and leading to the formation of isolated Au NPs rings. (a) Initial nanocolloid suspension drop confining immiscible nanobubbles, (b) decrease of the whole volume of the drop due to the evaporation of the solvent and confinement of the nanobubbles coated with the Au NPs; inset: zoom in on the thickening of the apex of the nanobubbles at (nanobubble\suspension\air) interface. (c) Rupture (explosion) of the nanobubble and formation of a peripheral particles nanoring. | ||
 | ||
| Scheme 3 Scheme depicting the three main steps of the evaporation-drying process of a nanocolloid suspension confining immiscible nanodroplets and leading to the formation of isolated Au NPs rings: (a) Initial nanocolloid suspension drop confining immiscible nanodroplets, (b) decrease of the whole volume of the drop due to the evaporation of the solvent and confinement of the nanodroplets coated with the Au NPs. Inset: zoom in on the nanodroplet\suspension\air interface at the apex of a nanodroplet. (c) Collapse of nanodroplets and sliding of the Au NPs along their interface, leading to the formation of a peripheral particles nanoring. | ||
 | ||
| Fig. 3 Height AFM images of 25 nm diameter gold nanoparticles self-assembled into ring-like structures after drying of confined nanobubbles embedded at a hydrophobic surface. (a) Random organisation of ∼200 nm size Au NPs nanorings structures, (b) 3D-zoom-in on a single Au NP nanoring. Frame sizes: 1 μm and 300 nm respectively. | ||
As stated previously, the confinement of the ethanol/water (E/W) nanodroplets in the wedge of the receding cyclohexane suspension drop, and the shear force they experience from the contact line, induce their local morphological distortion. But the L-R van der Walls forces act to stabilize the cyclohexane suspension film against rupture due to the polar nature of the ethanol/water mixture, which now invert the sign of the Hamaker constant in this system (A ≈ −0.5 × 10−20J < 0),30 leading to a disjoining pressure (−A/6πh3) > 0 that tends to maintain the nanodroplet/suspension film, and suspension film/surrounding air interfaces.
As previously noted, we still consider the assumption that no NPs remain locally at the apex under the confinement condition and right before the complete destabilization of the nanodroplet. At the same time, the evaporation, which physically reduces the thickness of the suspension film at the apex of the nanodroplet, leads to a local distortion,31 although the nanodroplet would prefer, due to the above interaction free energy condition (sign of A), to be covered by a suspension film of finite thickness. To circumvent this ineluctable evaporation-driven reduction of the suspension film thickness against the disjunction pressure, the nanodroplet proceeds with, through the unique freedom that characterizes soft matters, the local and small deformations of the interface which for these systems has a low energy cost. This local deformation, which takes place at the apex of the nanodroplet/suspension film interface toward the centre of the nanodroplet (depression), physically amounts to a local thickening of the solvent film that stabilizes the latter against rupture, until the wedge of the drop crosses the nanodroplet, as shown in Scheme 3b.
As the wedge of the drop crosses the nanodroplet, this latter starts to relax and dry on the hydrophobic substrate (Fig. 4) as it comes into contact with the surrounding environment (air). The “unconfined” nanodrop then goes through a relaxation, before it instantaneously evaporates in the mode of an “explosion”, which leads to its complete drying. It is during this last evaporation-drying step of the nanodroplet that the NPs adsorbed at its interface move downward along the nanodroplet/substrate periphery, driven by their low interaction with the aqueous nanodroplet (see Scheme 3c). The result of this whole process is the formation of a ring-like pattern of gold nanoparticles, which are randomly dispersed along the drying path of the suspension drop. The average size of the nanorings is 150 nm, with an average distance of 400 nm (Fig. 5).
 | ||
| Fig. 4 Optical microscope images: the same area was imaged several times (a–f) during the drying of a cyclohexane suspension drop onto a hydrophobic substrate. The sequential images depict the receding of the drop contact line and its local distortion (c–e) due to the presence of ethanol\water nanodroplet embedded at the surface. In images b) and c), the nanodroplet displays local and small deformations at its apex with the suspension film toward the centre (depression). The local thickening of the solvent film resulting from that local depression stabilizes this latter against rupture, until the wedge of the drop crosses the nanodroplet. Frame size: 100 μm. | ||
 | ||
| Fig. 5 Height AFM images of 25 nm diameter gold nanoparticles self-assembled into ring-like structures due to the confinement of nanodroplets embedded at a hydrophobic surface. (a) Random organisation of Au NP nanoring structures, (b) 3D zoom-in on Au NP nanoring. Frame sizes: 10 μm and 2 μm respectively. | ||
As observed on the macroscopic scale, the density of large nanorings (diameter > 400 nm) in the center of the suspension drop is higher than in the periphery, and this number density decreases exponentially along the lateral profile, as displayed in Chart 1. As already mentioned above, this arises from the balance between the gravitation and the outward convective flow during the drying of the suspension drop which generates a fractionation between large and small coated NP nanodroplets/nanobubbles, as well as a depletion of small ones in the center of the drop. As a result, structures made of small nanorings are highly concentrated in the vicinity of the contact line. This high nanoparticle (nanoring) concentration can even create a complete line pinning and confinement of the residual dispersion film in the latter stages of drop evaporation.
 | ||
| Chart 1 Evolution of Au NP nanoring size (s), vs.cyclohexane suspension drop radius (R). The red curve corresponds to the fit of the experimental variation (●) of the NPs ring size vs. drop radius, which follows a first order exponential decay of equation, s = 3921exp(−R/1808)−258, with a regression coefficient r2 = 0.99. On the same graph, the columns correspond to the evolution of nanoring density (number/μm2), vs. the radius of the cyclohexane suspension drop. | ||
Finally, we showed that the spherical nanoparticles could be replaced by Au nanorods which were found to also spontaneously self-assemble into ring-like superstructures, as depicted in Fig. 6.
 | ||
| Fig. 6 Height AFM images of gold nanorods self-assembled into ring-like structures after drying of confined nanodroplets embedded at a hydrophobic surface. (a) Random organisation of Au NPs nanorings structures, (b) zoom in on Au nanorods assembled into a ring pattern. Frame sizes: 4 μm, 800 nm respectively. | ||
As compared to existing nanofabrication techniques relying mostly on templates and lithography, our method undoubtedly presents the advantage of being capable of producing in a few seconds a large amount of well-defined nanorings of adjustable size which can be organized in various shapes and spatial distributions (arrays). These nanorings in general, and those prepared here by self-assembly, can find potential and direct applications in nanotechnological areas such as highly sensitive optical sensors, or superlenses. Indeed, our preliminary optical characterization shows that the nanorings have an amplification of the plasmonic signal (characterized by the hot spots in nano-Raman spectroscopy), which is around ten orders stronger than that of the randomly adsorbed nanoparticles clusters of comparable size. Beyond this useful tuning of nanobubbles towards the creation of functional metal nanoring patterns, this study also contributes understanding of the undesired growth of nanohole defects from dissolved or spontaneously nucleated bubbles in coatings that are prepared from polymers or suspension solutions.26
Conclusion
The formation of nanoring-like patterns based on a self-assembly of gold nanoparticles from confined nanobubbules and nanodroplets in drying nanofluids has been studied. Compared to previous techniques, this drying mediated method avoids direct replication from pre-fabricated templates, taking advantage of naturally occurring interfacial phenomena such as the formation of nanobubbles embedded at hydrophobic surfaces.During drying of the suspension drop, nanodroplets and nanobubbles bearing adsorbed nanoparticles at their interface settled at the hydrophobic surface. The formation of the nanorings was well accounted for by the confinement and destabilization of the nanobubbles (nanodroplets) within the receding wedge as the contact line of the evaporating suspension drop crosses them, and ultimately the migration of the NPs along the nanobubbles (nanodroplets) to their periphery with the substrates. Beyond predictable parameters like the evaporation rate, the receding velocity of the suspension drop or the adhesion of the nanobubbles to the substrate, we showed that the destabilization (rupture, evaporation) of the nanobubbles/nanodroplets was controlled by the disjoining pressure in the suspension film at their apex. Furthermore, our system and mechanistic description reveals information beyond spontaneous nanoring formation, accounting also for the often observed (and unwanted) formation of nanoholes in drying polymer thin films.
Acknowledgements
This work was supported by the Centre National de la Recherche Scientifique (CNRS). The authors are grateful to Dr C. Labbe for her help with the Turbiscan measurements.References
- J.-J. Miao, R.-L. Fu, J.-M. Zhu, K. Xu, J.-J. Zhu and H.-Y. Chen, Chem. Commun., 2006, 3013 RSC.
- Y. Liu, T. Murao, Y. Nakano, M. Naito and M. Fujiki, Soft Matter, 2008, 4, 2396 RSC.
- Z. L. Wang, Mater. Today, 2004, 7(6), 26 CrossRef.
- F. Q. Zhu, D. L. Fan, X. C. Zhu, J. G. Zhu, R. C. Cammarata and C. L. Chien, Adv. Mater., 2004, 16, 2155 CrossRef CAS.
- F. Q. Zhu, G. W. Chern, O. Tchernyshyov, X. C. Zhu, J. G. Zhu and C. L. Chien, Phys. Rev. Lett., 2006, 96, 27205 CrossRef CAS.
- J. Aizpurua, P. Hanarp, D. S. Sutherland, M. Käll, W. Bryant and F. J. García de Abajo, Phys. Rev. Lett., 2003, 5, 057401 CrossRef.
- M. Bayati, P. Patoka, M. Giersig and E. R. Savinova, Langmuir, 2010 Search PubMed , webrelease..
- S. L. Tripp, S. V. Pusztay, A. E. Ribbe and A. Wei, J. Am. Chem. Soc., 2002, 124, 7914 CrossRef CAS.
- J. Rothman, M. Kleaui, L. Lopez-Diaz, C. A. F. Vaz, A. Bleloch, J. A. C. Bland and Z. Cui, Phys. Rev. Lett., 2001, 86, 1098 CrossRef CAS.
- R. J. Warburton, C. Schaeflein, D. Haft, F. Bickel, A. Lorke, K. Karrai, J. M. Garcia, W. Schoenfeld and P. M. Petroff, Nature, 2000, 405, 926 CrossRef CAS.
- K. Y. Suh and S. Jon, Langmuir, 2005, 21, 6836 CrossRef CAS.
- S. Y. Lee, J.-R. Jeong, S.-H. Kim, S. Kim and S.-M. Yang, Langmuir, 2009, 25, 12535 CrossRef CAS.
- S. Lee, J. R. Jeong, S. H. Kim, S. Kim and S. M. Yang, Langmuir, 2009, 25, 12535 CrossRef CAS.
- F. Yan and W. A. Goedel, Nano Lett., 2004, 4, 1193 CrossRef CAS.
- K. L. Hobbs, P. R. Larson, G. D. Lian, J. C. Keay and M. B. Johnson, Nano Lett., 2004, 4, 167 CrossRef CAS.
- L. V. Govor, G. H. Bauer, G. Reiter, E. Shevchenko, H. Weller and J. Parisi, Langmuir, 2003, 19, 9573 CrossRef CAS.
- L. V. Govor, J. Parisi and G. H. Bauer, Z. Naturforsch., 2003, 58a, 392 Search PubMed.
- Z. Liu and R. Levicky, Nanotechnology, 2004, 15, 1483 CrossRef CAS.
- P. C. Ohara, J. R. Heath and W. M. Gelbart, Angew. Chem., 1078, 36, 10 Search PubMed.
- M. Maillard, L. Motte, A. T. Ngo and M. P. Pileni, J. Phys. Chem., 2000, 104, 11871 Search PubMed.
- M. Maillard, L. Motte and M. P. Pileni, Adv. Mater., 2001, 13, 200 CrossRef CAS.
- W.-S. Chang, L. S. Slaugther, B. P. Khanal, E. R. Zubarev and S. Link, Nano Lett., 2009, 9, 1152 CrossRef CAS.
- Bishnu P. Khanal and Eugene R. Zubarev, Angew. Chem., Int. Ed., 2007, 46, 2195 CrossRef CAS.
- S. Stannard, H. Alhummiany, E. Pauliac-Vaujour, J. S. Sharp and P. Moriarty, Langmuir, 2010, 26, 13892 CrossRef.
- K. Werner, B. Stöckelhuber, A. Radoev, Wenger and H. J. Schulze, Langmuir, 2004, 20, 164 CrossRef.
- Z. Wu, X. Zhang, X. Zhang, J. Sun, Y. Dong and J. Hu, Chin. Sci. Bull., 2007, 52(14), 1913 CrossRef CAS.
- H. Haidara, K. Mougin and J. Schultz, Langmuir, 2000, 16, 7773 CrossRef CAS.
- K. Mougin, H. Haidara and G. Castelein, Colloids Surf., A, 2001, 193, 231 CrossRef CAS.
- R. Deegan and D. Capillary, Nature, 1997, 389, 827 CrossRef CAS.
- J. Israelachvili, Intermolecular & Surface Forces, Academic Press, San Diego, CA, 1992 Search PubMed.
- K. Mougin, H. Haidara and J. Schultz, Langmuir, 2001, 17, 5952 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c0nr00750a |
| This journal is © The Royal Society of Chemistry 2011 |
