New integrated elemental and molecular strategies as a diagnostic tool for the quality of water soluble quantum dots and their bioconjugates†
Laura
Trapiella-Alfonso‡
,
Antonio R.
Montoro Bustos‡
,
Jorge Ruiz
Encinar
,
Jose M.
Costa-Fernández
,
Rosario
Pereiro
and
Alfredo
Sanz-Medel
*
Department of Physical and Analytical Chemistry, University of Oviedo, Julián Clavería, 8, 33006, Oviedo, Spain. E-mail: asm@uniovi.es; Tel: +34 985103474
First published on 14th January 2011
Abstract
Herein, we demonstrate that both qualitative molecular and quantitative elemental data obtained from size exclusion chromatography coupled online for the first time to both molecular fluorescence and elemental mass spectrometry, respectively, turned out to be critical to evaluate the quality of coatings of quantum dots. Moreover, such an instrumental approach also allowed us to study quantitatively the appropriated bioconjugation of quantum dots to antibodies, a critical step for QDs future use in quantitative fluorescence immunoassays.
One of the fastest moving research fields in nanoscience is the use of quantum dots (QDs) for bioanalytical applications. The unique optical properties of those nanoparticles and the ability to bioconjugate them to specific biomolecules, such as DNA, aptamers, enzymes or antibodies, make QDs appealing as high-value fluorescent labels in a wide variety of bioanalytical investigations, where traditional organic fluorophores fall short of providing long-term stability as simultaneous detection of multiple signals. Despite recent progress, there is still an urgent need for effective procedures for QD bioconjugates purification and characterization, in order to further facilitate reliable quantitative bioassays.1–3
Spectroscopy, such as photoluminescence4 and transmission electron microscopy (TEM),5 is a commonly used technique for such pursued purposes. Alternative strategies for purification and characterization of the QD bioconjugates including repeated centrifugation, analytical ultracentrifugation, re-dispersion and ultrafiltration have been proposed.6,7 However, such methodologies lack quantitative capabilities, their separation resolution is poor and available membrane porous sizes are limited. Size exclusion chromatography (SEC) coupled with UV/Vis detection3 and capillary electrophoresis with laser-induced fluorescence detection (CE-LIF) have been used also for bioconjugates purification and characterization.8–10 However, CE-LIF requires previous steps to purify bioconjugated QDs from the reagents in excess, e.g.ultrafiltration or SEC. In such cases the separation resolution showed a strong dependence on the pI and Mw of the biomolecule attached to the QD. In addition, due to the sample loading limitation associated to CE, this technique cannot be efficiently used for collecting purified fractions.
In this communication, a novel concept based on the combination of SEC separation coupled online with elemental (inductively coupled plasma-mass spectroscopy, ICP-MS) and molecular (fluorescence) detection is proposed as a diagnostic tool. In this way, a deeper understanding of the QDs status after their solubilization in aqueous media (e.g. with an amphiphilic polymer coating) and the effectiveness of QD bioconjugation to a typical functional biomolecule (monoclonal mouse anti-progesterone antibody, selected as a “model” antibody) were proved using this general platform. Of course, both steps are crucial for future development of QD based bioassays. The procedure, to carry out QDs bioconjugation viaethyl-3-(dimethylaminopropyl) carbodiimide (EDC) chemistry, is available in the ESI†. Of course, the purification and characterization steps are crucial to assure that any free antibodies (and even QDs) are absent in the purified bioconjugate reagent and in order to carry out a proper optimization of the derivatization reaction. Following previous work carried out in our lab,3SEC coupled with UV/Vis detection was first evaluated as purification/separation technique. Unfortunately, the observed QD-antibody (bioconjugated QDs) could not be distinguished from the individual QDs with such detection (see Fig. 1).
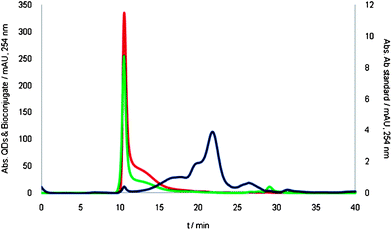 | ||
| Fig. 1 SEC-UV/Vis chromatograms using NH4HCO3 100 mM pH 7.4 as the mobile phase. Chromatograms corresponding to the anti-progesterone antibody (2.1 µM), the standard of QDs (3 µM), and the mixture of species after bioconjugation are shown in blue, red and green, respectively. | ||
A more selective detector should be desirable. In this vein, the molecular native fluorescence emission from QD cores and from the polymer coating measured at 600 and 332 nm, respectively (see Fig. S1†) can be combined to the quantitative and high specific multi-elemental information (e.g.114Cd) from the nanocrystals, viaICP-MS.5 To check such integrated approach, water soluble CdSe and CdSe/ZnS QDs were obtained and studied first. Although bioconjugation of the nanoparticles is carried out at pH = 7.4, QDs are much more stable in basic media. Thus, two different pHs of the mobile phase were studied, 7.4 and 10.5, while maintaining the same ionic strength. Along the chromatographic separation, ion signals from 114Cd (QDs core) and 32S (QDs shell and proteins) and fluorescence emission both at 600 and 332 nm were collected. Additionally, flow injection analysis (FIA) was used to evaluate column recoveries.
No difference was found at pH 7.4 between the chromatographic profiles obtained for both core and core–shell types of QDs under scrutiny (see Fig. S2†). However, column recoveries obtained by ICP-MS were low (ranging from 46 to 54% and from 73 to 78% for CdSe and CdSe/ZnS QDs, respectively). Bringing the pH to 10.5 led to higher recoveries in both cases (92–97% and 99–108%), while chromatographic profiles differed significantly, as can be clearly seen in Fig. 2. Interestingly, information given by fluorescence at both wavelengths (600 and 332 nm) allows an assessment of the quality of the QDs eluting from the SEC column (since a likely degradation on their surface would result in a lower emission quantum yield). In the case of complete loss of fluorescence, QDs could only be detected using the 114Cd signal (obtained by ICP-MS). In addition, integration of such ICP-MS elemental profile would provide the precious quantitative column recovery information. Following such reasoning, the fluorescence peaks observed at 10 min in Fig. 2a seem to indicate that such CdSe QDs were of high quality. Unfortunately, such good quality QDs amounted to just 3–5% of the total QDs injected (as demonstrated by the huge peak eluting at 26 min showing only 114Cd signal without any fluorescence associated). It is worth stressing that both peaks partially match the second polymer broad peak centred at 15 min (332 nm) and the tail of the CdSe QD peak from 10 to 15 min (114Cd) might be a clear indicator of degradation of QDs after loosing of polymer chains from the surface (which could result on final aggregation). Following such reasoning, only the fluorescence peaks observed at 10 min (Fig. 2a) seem to indicate high quality CdSe QDs.
 | ||
| Fig. 2 SEC chromatogram obtained for CdSe (a) and CdSe/ZnS (b) QDs obtained at pH 10.5. The 114Cd profile and the fluorescence with emission of 600 nm (core) and 332 (polymer coating) are shown in blue, red and green, respectively. | ||
In this way, the degradation of the polymer coating could take place completely, thus releasing uncoated CdSe nanoparticles of much smaller hydrodynamic volume (therefore eluting, along with eventual released elements, much later in the SEC column, i.e. 26 min). To corroborate this hypothesis three elution fractions were collected at different time periods (8–11, 11–16, 24–27 min) of the SEC chromatogram. These fractions were pre-concentrated and analyzed by TEM after drying. The TEM results are shown at the bottom images of Fig. 2a and demonstrate the presence of nanoparticles at the three elution times. As expected, QDs of the last fraction tend to aggregate, which eventually migrate to the edge of the copper grid (hydrophobic part) used to deposit the sample. This eventual degradation of the QDs is also confirmed by the complete absence of fluorescence at 26 min.
In a similar set of experiments, Fig. 2b shows the results obtained for the shell-protected CdSe/ZnS QDs. In this case the largest proportion of 114Cd eluted from the column at 10 min (following the same behaviour observed at pH 7.4, see Fig. S2†). Again, the eluted species at 10 min corresponds to high quality QDs as indicated by the intense fluorescence peak observed (600 nm) and the TEM image. It is worth stressing that, in this case, high quality QDs amounted to 55–58% of the total QDs injected. Loss of the polymer coating still occurs here, but to fewer extent (shoulder at 13 min on the 114Cd signal) resulting again in a loss of fluorescence and a slight decrease of the hydrodynamic volume, as demonstrated by the longer retention in the SEC column. However, in agreement with the TEM image obtained for the 26 min fraction in Fig. 2a, Cd detected seems to be still as nanoparticles, but already very hydrophobic. Such partially degraded QDs amounted to 42–46%. Therefore it seems that polymer coating is much better attached to shell protected CdSe/ZnS QDs resulting in more stable nanoparticles in water media (i.e. more appropriate for further bioconjugation to antibodies).
In our bioconjugation studies anti-progesterone antibodies were characterised first by the here proposed instrumental approach. Obtained chromatograms are shown in Fig. 3 (fluorescence and ICPMS detection). As can be seen, the vast majority of the antibody eluted at a single peak at 20 min (note that the free antibody can be detected by fluorescent measurements using the same conditions employed for the fluorescence detection of the polymer coating of QDs, see Fig. S1†).
 | ||
| Fig. 3 Ab anti-progesterone characterization chromatograms by SEC-fluorescence, using 332 nm as the emission signal (green line) and by SEC-ICP-MS, monitoring the 32S isotope (orange line). | ||
Under the same chromatographic conditions the bioconjugation reaction mixture was injected and results measuring fluorescence at 600 nm and 332 nm are shown in Fig. 4a. Again, as in Fig. 1, the excess of free antibodies (20 min vertical line) could be separated from the bioconjugates (10 min), but the QD-bioconjugated antibody could not be distinguished from the individual QDs as the peak profile is identical to that obtained for the individual QDs (see Fig. 2b). However, having a closer look at the fluorescence signals it is possible to observe a differential behaviour occurring when the QDs are bioconjugated just by looking at the peak area ratio of the fluorescent signals 332 (polymer)/600 (QDs core). In fact, such ratio increased up to 15 times when bioconjugate exits. This increase is due to the high emission intensity observed at 332 nm, likely caused by the incorporation of Ab (maximum emission wavelength 342 nm as shown in Fig. 3). In fact, no signal corresponding to the free antibody at 20 min (see Fig. 3a) was observed. Interestingly, the decrease suffer at the 600 nm fluorescence emission, that other authors have rationalised as a result of an inner filter effect when the biomolecule is bound to the QD,10 also contributes to the peak area ratio change computed. To the best of our knowledge this is the first time that a fluorescence signal ratio is used as diagnostic tool for bioconjugation success assessment. Unfortunately, the inner filter effect observed hinders its final use in a deep quantitative evaluation (e.g. establishment for bioconjugates stoichiometry). In this situation the complementary ICP-MS information (given in Fig. 4b and c) proved to be critical. In fact, the 32S and 114Cd profiles obtained provided the final demonstration of the effectiveness of the bioconjugation process: the poorly resolved peak at an elution time of 10 min showed a dramatic increase of its S/Cd ratio in the supposed bioconjugated species (even an inversion was noticed in the ratio mentioned in Fig. 4b and c), indicating the much higher amount of S present here, most likely due to the contribution from the high sulfur content of the anti-progesterone antibody containing cysteines and methionines bioconjugated to the CdSe/ZnS QDs. Also, no 32S peak was observed at the retention time of the free antibody (20 min, Fig. 3c). This increase in the S/Cd ratio in the bioconjugates (0.118 ± 0.002) is very significant compared to the value for CdSe/ZnS QDs (0.070 ± 0.001). Note that these data could be considered as a first attempt to obtain an approximate bioconjugate stoichiometry (biomolecule/QD), information highly demanded today in order to use such bioconjugated QD complexes as reagents for reliable quantitative immunoassays.
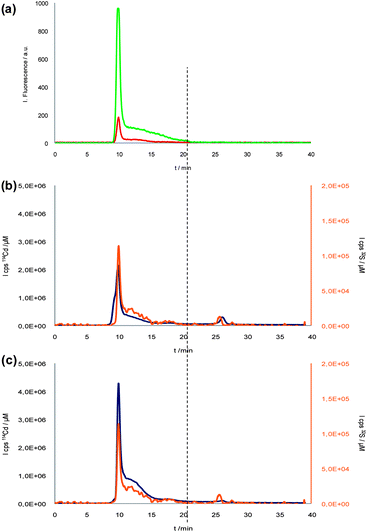 | ||
| Fig. 4 SEC-Fluorescence (a) and SEC-ICP-MS (b) chromatograms of Ab-QDs bioconjugation reaction mixture. CdSe/ZnS SEC-ICP-MS chromatogram (c). Red and green lines represent 600 nm core fluorescent emission from bioconjugated QDs and 332 nm polymer and Ab fluorescent emission, respectively. Blue and orange lines correspond to 114Cd and 32S from QDs and bioconjugate, respectively. Vertical line would show the elution time of the antibody. | ||
As demonstrated by the complementary detection techniques, a satisfactory isolation of high-quality QDs labelled antibodies from free antibodies can be achieved by the SE-chromatography. However, it would be desirable to demonstrate that the bioconjugated antibody maintains its functionality after their conjugation to the quantum dots and its subsequent purification. For such purpose, the first fraction (from 8 to 11 minutes) eluted from the SEC was collected. Further, a conventional ELISA-type spectrophotometric immunoassay was carried out in order to compare the activity of labelled (fraction collected from the SEC) with standard non-labelled antibodies. The conventional ELISA spectrophotometric assay was performed using an anti-mouse IgG peroxidase as a secondary antibody and 3,3′,5,5′ tetramethyl benzidine and H2O2 for colour development. Results from those studies clearly showed that the isolated anti-progesterone antibody labelled with QDs kept its functionality, specifically recognizing the presence of the antigen in the ELISA plate. Moreover, no significant differences were observed in the colour development obtained in the ELISA wells incubated with the standard antibodies and the antibodies bioconjugated to the QDs.
In summary, both elemental and molecular detection are required to interpret correctly the SEC chromatograms obtained and, therefore, to appropriately characterize water-soluble QDs, their bioconjugation reaction to proteins and their final correct purification.
Elemental information provides stoichiometry (elemental ratios) and recovery, but fluorescence is still required to assure that QDs are not degraded (i.e. they show an intense emission at 600 nm (core), accompanied by some at 332 nm (polymer coating)).
In fact, the information given by the integrated techniques reveals the great importance of the ZnS shell in the stable attachment of a polymer coating over the QD surface. Effectiveness of the bioconjugation of the water-soluble QDs to a given antibody can be assessed by the combination of the information given by the fluorescence and by elemental mass spectrometry techniques. For appropriate characterization of tailored bio-conjugated QDs, evaluation of the bio-conjugate stoichiometry is needed and information from the ICP-MS could again play a pivotal role in those studies. The approach and results provided in this work open the door to get such goals in a much more efficient way than non-conclusive approaches currently applied (such as slight blue shifts at the maximum emission wavelength).4
Acknowledgements
The authors are grateful for financial support of the Spanish Ministry of Education and Science, MEC (CTQ2006-02309), and FEDER program co-financing funds. L. T. A. and A. R. M. B. acknowledge the FPU program scholarship of the Spanish Education Ministry.Notes and references
- X. Huang, J. Weng, F. Sang, X. Song, C. Cao and J. Ren, J. Chromatogr., A, 2006, 1113, 251 CrossRef CAS.
- Q. Huo, Colloids Surf., B, 2007, 59, 1 CrossRef CAS.
- M. T. Fernández-Argüelles, J. M. Costa-Fernández, R. Pereiro and A. Sanz-Medel, Analyst, 2008, 133, 444 RSC.
- M. Dybiec, G. Chornokur, S. Ostapenko, A. Wolcott, Z. Zhang, A. Zajac, C. Phelan, T. Sellers and D. Gerion, Appl. Phys. Lett., 2007, 90, 263112 CrossRef; G. Chornokur, S. Ostapenko, E. Oleynik, C. Phelan, N. Korsunska, T. Kryshtab, J. Zhang, A. Wolcott and T. Sellers, Superlattices Microstruct., 2009, 45, 240 CrossRef CAS.
- A. R. Montoro Bustos, J. Ruiz Encinar, M. T. Fernández-Argüelles, J. M. Costa-Fernández, R. Pereiro and A. Sanz-Medel, Chem. Commun., 2009, 3107 RSC.
- B. Zhang, X. Liang, L. Hao, J. Cheng, X. Gong, X. Liu, G. Ma and J. Chang, J. Photochem. Photobiol., B, 2009, 94, 45 CrossRef CAS.
- E. E. Lees, M. J. Gunzburg, T.-L. Nguyen, G. J. Howlett, J. Rothacker, E. C. Nice, A. H. A. Clayton and P. Mulvaney, Nano Lett., 2008, 8, 2883 CrossRef CAS.
- G. Vicente and L. A. Colón, Anal. Chem., 2008, 80, 1988 CrossRef CAS.
- M. Pereira and E. P. C. Lai, J. Nanobiotechnol., 2008, 6, 10 CrossRef.
- H. Feng, W. S. Law, L. J. Yu and S. F. Y. Li, J. Chromatogr., A, 2007, 1156, 75 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Description of experimental section and Fig. S1 and S2. See DOI: 10.1039/c0nr00822b |
| ‡ Both authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2011 |
