Efficient synthesis of tailored magnetic carbon nanotubesvia a noncovalent chemical route†
Xianglong
Li
*a,
Joe D.
Thompson
b,
Yingying
Zhang
a,
Christina I.
Brady
c,
Guifu
Zou
a,
Nathan H.
Mack
c,
Darrick
Williams
a,
Juan G.
Duque
c,
Quanxi
Jia
a and
Stephen K.
Doorn
*a
aCenter for Integrated Nanotechnologies, Materials Physics and Applications Division, Los Alamos National Laboratory, Los Alamos, New Mexico 87545, USA. E-mail: xianglongli@lanl.gov; skdoorn@lanl.gov
bCondensed Matter and Magnet Science, Materials Physics and Applications Division, Los Alamos National Laboratory, Los Alamos, New Mexico 87545, USA
cPhysical Chemistry and Applied Spectroscopy, Chemistry Division, Los Alamos National Laboratory, Los Alamos, New Mexico 87545, USA
First published on 29th November 2010
Abstract
We report here an efficient noncovalent chemical route to dense and uniform assembly of magnetic nanoparticles onto multi-walled carbon nanotubes within a single-layer configuration. While preserving the electrical conduction behavior of the nanotube network itself, the resulting carbon nanotube derivatives exhibit a distinct superparamagnetism, and can be magnetically manipulated via a quick and reversible mode.
Introduction
Carbon nanotubes (CNTs)1 and inorganic nanoparticles (NPs)2,3 attract considerable scientific and technological interest due to their unique structure-dependent physical and chemical properties. The surface functionalization of CNTs with NPs has been proven to be a powerful tool for rationally exploiting innovative materials with desirable functionalities and applications. For example, nanohybrids of CNTs and noble metal NPs have been successfully utilized in catalysis, gas sensors, and fuel cells,4–6 and CNTs uniformly assembled with appropriate metal NPs have been shown to be attractive platforms for constructing new functional core-shell nanostructures.7,8 Likewise, CNTs integrated with different semiconductor NPs or quantum dots have found applications in optoelectronic devices and sensors9–12 based on interactions between components of the hybrid ensembles, as well as drug delivery13 on the basis of their respective merits. Furthermore, CNTs bearing magnetic NPs14–18 have demonstrated potential in many fields including biomedical imaging, biomanipulation, supercapacitor, and environmental treatments. A continuing challenge, however, is the ability to generate such hybrid assemblies with uniform NP coatings that avoid undesirable self-agglomeration (left panel, Scheme 1a).19 This is particularly important when the interaction and interface between the hybrid components is being exploited. Nonuniform hybridization or simple mechanical mixing (right panel, Scheme 1a), in fact, results in degradation of the desired performance.20 | ||
| Scheme 1 (a) Schematic illustration of uniform (left panel) and nonuniform (right panel) assembly of NPs onto CNTs. (b) Illustration of the procedure for preparing the magnetic nanotube derivatives (MNP-MWNT) that are uniformly coated with the MNPs. | ||
In order to efficiently assemble CNT-based nanohybrids with different NP types, it is necessary to activate the normally chemically inert graphitic surface of the nanotubes and introduce sufficient active binding sites (or surface anchoring groups) for attaching either NP precursors or as-synthesized NPs. Besides direct doping8,21–23 of the CNT walls with other elements (e.g., nitrogen) during the nanotube growth process, to-date, a variety of scalable post-growth strategies13,19,24–27 have been extensively investigated for introducing active binding points onto the CNTs, including aggressive oxidative treatment of CNTs with strong acids, polymer wrapping of CNTs, and noncovalent modification of CNTs with pyrene derivatives by π stacking. Despite the recent progress made on hybrid nanostructures, few scalable approaches have been developed for uniformly assembling magnetic NPs onto the CNTs without disrupting the pristine CNT structures. Here, we present a simple and efficient noncovalent chemical approach (Scheme 1b) in which maghemite (γ-Fe2O3) NPs (MNPs) are uniformly assembled onto oleylamine-functionalized multi-walled carbon nanotubes (f-MWNTs) in a single-layer modality, while preserving the electrical conduction behavior of the nanotube network itself. The resulting superparamagnetic carbon nanotube derivatives (MNP-MWNT) can be easily dispersed in common organic solvents, and can be quickly and reversibly manipulated by an external magnet.
Experimental
Pristine maghemite nanoparticles with a mean diameter of 5.7 nm (Fig. S1, ESI†) were synthesized following a previously-reported method.28 The MWNT arrays were grown by a chemical vapor deposition (CVD) process.29 A high density of active binding sites for anchoring the MNPs are introduced by noncovalently functionalizing the MWNTs with oleylamine molecules, thus resulting in subsequent efficient attachment of the MNPs. Briefly, 2 mg MWNTs were mildly sonicated in 10 mL toluene solution containing 0.1% (v/v) oleylamine (Acros) for 2 h. Subsequently, the oleylamine-functionalized MWNTs (f-MWNTs) were isolated from the solution by centrifugation and washed with ethanol. As-prepared f-MWNTs were weighed and again dispersed in toluene to make a suspension with desirable concentrations (typically, 0.01∼0.1 mg/ml). A hexane solution (50 μL) of maghemite nanoparticles (0.17 mg/ml) was then added to the above suspension. The mixture was mildly sonicated in an ultrasonic bath at room temperature for 5 min. The resulting hybrids (MNP-MWNT) were magneto-separated by holding the above mixture close to a small external magnet, and further washed with toluene for two times. The hybrids thus prepared can be easily and stably dispersed in common organic solvents.Samples were characterized by a JEOL 3000 F transmission electron microscope operating at 300 kV and a FEI Inspect F scanning electron microscope. UV–vis absorption spectra were recorded in a Varian Cary 6000i instrument. Raman spectra were recorded using laser excitation at 785 nm with a liquid nitrogen cooled CCD array using a single grating monochromator. XRD data were acquired using a Rigaku Ultima III θ/2θ powder diffractometer with Cu Kα radiation (1.5418 Å). The magnetic properties were studied by SQUID magnetometer at temperatures ranging from 5 K to room temperature. Electrical measurements were conducted using the four-probe method at temperatures from 85.9 K to 293 K.
Results and discussion
Fig. 1 shows representative scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images of the MWNT starting material and the MNP-MWNT hybrids (with 8 wt % MNP content in this work). As indicated in Fig. 1a and 1e, the MWNTs grown viaCVD are vertically aligned on the substrate, and exhibit smooth and clean surfaces. In contrast, the hybrid material is more highly textured (Fig. 1b-d), which suggests that most of the nanotubes have been completely coated with the introduced MNPs. This is verified in TEM images (Fig. 1f and 1g, see also Fig. S2, ESI†), in which the introduced nanoparticles are seen to be uniformly immobilized onto the nanotube surfaces as a single layer. Furthermore, no aggregation between the MNPs is observed. It is also evident that introduction of MNPs at a level of 8 wt % MNPs enables nearly complete coverage of the nanotube surfaces. Lower MNP content (e.g., 2 wt %) results in partial and inconsecutive coating of the nanotubes with the MNPs (Fig. S3, ESI†). | ||
| Fig. 1 (a) Typical SEM image of the MWNTs; the inset shows a lower magnification image of a MWNT array. (b–d) SEM images of the MNP-MWNT hybrids. (e) Typical TEM image of the MWNTs; the inset shows a high resolution TEM image. (f,g) TEM images of the MNP-MWNT hybrids. (h) High resolution TEM image of the MNP-MWNT hybrids. | ||
A deeper insight into the structure of the hybrids is provided by high-resolution TEM images. As shown in both the inset of Fig. 1e and in Fig. 1h, the (002) fringes of the nanotube with ca. 0.34 nm interplanar spacing are well resolved; the observed interplanar distances of ca. 0.25 nm in the case of the hybrids correspond to the (311) lattice planes of the maghemite structure.28 Furthermore, close examination suggests all the assembled MNPs are randomly oriented on the nanotubes, and there is not an apparent crystallographic relationship between the planes of the nanotubes and the assembled nanoparticles. Compared to the above results, a simple physical mixture of the nanoparticles and the nanotubes obtained by employing the pristine MWNTs as a support (Fig. S4, ESI†) show poor distribution over the MWNT surface, with significant aggregation of the MNPs. This indicates that the noncovalently-introduced amine functionalities play an important role in successful assembly of the MNPs onto the nanotubes. This can be attributed to specific conjugation between the MNPs and the amine groups of the f-MWNTs as reported earlier.30
Fig. 2 shows UV-vis absorption spectra of the MWNTs, the MNPs, and the hybrids. While the MWNTs present a typical featureless spectrum, the absorption spectrum of the hybrids reveals a broad peak at ca. 475 nm, corresponding to the characteristic peak of the MNPs.31 The result implies that the MNPs are sucessfully assembled onto the nanotubesvia the here-presented noncovalent functionalization route. Raman spectra (Fig. S5, ESI†) show negligible change in the relative intensity ratio of the D band to the G band of the nanotubes, which is an expected result considering the fact that the noncovalent assembly route is employed in this work. Furthermore, the face-centered cubic structure of the assembled maghemite nanoparticles is confirmed by X-ray diffraction (XRD) analyses (Fig. S6, ESI†).
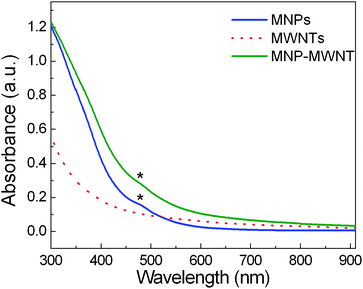 | ||
| Fig. 2 UV-vis absorption spectra of the MNPs, the MWNTs, and the MNP-MWNT hybrids. The characteristic peaks of the maghemite nanoparticles in both the MNP sample and the hybrids were marked with asterisks. | ||
The magnetic properties of the hybrids and their constituents were investigated using a superconducting quantum interference device (SQUID) magnetometer. Fig. 3a and 3b show magnetization hysteresis curves of the MNPs (as a control) and the MNP-MWNT hybrids acquired at room temperature (300 K), respectively. While the MWNTs are observed to be nonmagnetic (Fig. S7, ESI†), both the MNPs and the MNP-MWNT hybrids behave like superparamagnets exhibiting saturation magnetization Ms (45.7 emu/g and 6.3 emu/g, respectively), negligible coercitivity Hc (15 Oe for both) and remnant magnetization Mr (0.2 and 0.1 emu/g, respectively). Such magnetic properties are consistent with the fact that the MNPs in both cases have diameters (5.7 nm) far less than the critical size (ca. 20 nm) at which the magnetic properties of maghemite are expected to change from superparamagnetic to ferrimagnetic. Although the Ms for the hybrids is comparable to that for earlier-reported maghemite-impregnated magnetic carbon nanotubes (with about 13 wt % γ-Fe2O3),32 it should be noted that the Ms for the hybrids is associated with the low MNP content (8 wt %) in our case. If we only take the quantity of the MNPs in the hybrids, a much higher Ms value (78.8 emu/g), about 70% increase compared to that of the MNPs, can be estimated. The increase in Ms is similar to other reports in the literature for magnetic nanoparticles filled33 inside or decorated26 onto the nanotubes.
 | ||
| Fig. 3 (a,b) Hysteresis curves measured at 300 K for the MNPs and the MNP-MWNT hybrids, respectively. (c,d) Temperature dependence of the ZFC and FC magnetization measured at 100 Oe for the MNPs and the MNP-MWNT hybrids, respectively. (e) Photographs of the MWNTs and the MNP-MWNT hybrid materials in toluene demonstrating the response of the hybrids to an external magnetic field. | ||
We have also measured zero-field-cooled (ZFC) and field-cooled (FC) magnetization data for the hybrids and the MNPs. As shown in Fig. 3d for the hybrids, the ZFC curve exhibits an evident rounded maximum at Tmax (20 K), whereas the FC branch continues to increase with decreasing temperature. Such temperature dependence of magnetization further validates that the hybrids are superparamagnetic. In comparison, the pristine MNPs (Fig. 3c) bear a Tmax value of ca. 30 K. The Tmax represents the average superparamagnetic transition temperature of the MNPs with different diameters.21 Generally the larger the average diameter, the higher is the Tmax. For the hybrids, a smaller Tmax compared to that of the pristine MNPs, as well as an observation of a small gap between ZFC and FC curves at above Tmax, are suggested to result from the unique 1D assembly status of the MNPs attached onto the nanotubes. While the 8 wt % MNPs has densely covered most of the surface of the nanotubes, as exhibited in the above TEM images, the introduction of too many MNPs (e.g., 15 wt %) results in formation of nanoparticle agglomerates. These can include aggregation of secondary nanoparticles on the already assembled MNPs (Fig. S8, ESI†). Aggregation limits further demonstration of the above distinct magnetic properties (especially, the increase in Ms) of the single-layer MNPs within a one-dimensional (1D) assembly status.
It seems unlikely that the Ms enhancement is due to interaction between the MNPs and the nanotubes, since the nanotubes are nonmagnetic. In magnetic nanoparticle assemblies, generally, the dominant magnetic interactions are dipole–dipole interparticle interactions and exchange interactions through the surfaces of particles. Srikanth et al.33 demonstrated that highly-packed Fe3O4 nanoparticles inside carbon nanotubes possess a stronger dipolar interparticle interaction and hence enhance magnetic properties. In that case, it is also shown that a stronger dipolar interparticle interaction leads to higher Tmax and Hc. In contrast, the change in the dipolar interparticle interaction is not responsible for the magnetic enhancement in our system due to the single-layer characteristic of the assembled MNPs as well as the lower Tmax of the hybrids than that of the MNPs. Here, we assume that the exchange interactions between the one-dimensionally assembled MNPs may be correlated to the increase in Ms for the hybrids, although the detailed origin for those phenomena must be investigated further.
The magnetic response of the MNP-MWNT hybrid material to an external magnet is demonstrated in Fig. 3e. While the MWNTs cannot be dispersed, even under stronger sonication, the hybrids are easily dispersed in common organic solvents (e.g., toluene) by simple shaking. As shown in the right panel of Fig. 3e, the initially-dispersed MNP-MWNT hybrid material is efficiently separated from the solution by holding the solution close to a permanent magnet. In contrast, both the MWNTs (Fig. 3e) and the mixture exhibited in Fig. S4 (ESI†) are not influenced by the applied magnetic field. Depending on the hybrid concentration, the black solution becomes clear within one minute after applying the magnet (see the movie in the ESI†). This quick dispersion-separation process of the hybrids is reversible, allowing potential extension to applications including time-sensitive magneto-driven separation/collection, environmental analysis/treatment, and magnetorheological fluid devices and sensors.
We have also explored the temperature-dependent resistance of these hybrid materials on a macroscopic scale. As exhibited in Fig. 4a, the sheet resistance of the drop-cast hybrid film (inset of Fig. 4a) decreases with temperatures from 85.9 K to 293 K. The negative temperature dependence of sheet resistance shows a nonmetallic behavior of the hybrid network. In general, two main mechanisms have been suggested to explain the conduction behavior of disordered CNT films: a three-dimensional variable range hopping mechanism in Mott's hopping model and a tunneling conduction mechanism.34,35 These two mechanisms can be respectively expressed as Rs∝exp(A/T1/4) and Rs∝exp(B/T1/2), where Rs is the sheet resistance, A and B are constants, and T is the absolute temperature. As shown in Fig. 4b, lnRsversus T−1/4 based on the first equation shows a relatively higher degree of linearity than that of lnRsversus T−1/2 based on the second equation. This suggests that the conduction in our hybrid system is mainly controlled by a three dimensional hopping mechanism. The electrical conduction behavior is consistent with those observed in the pristine nanotube films and/or yarns,34,36 which implies that the assembly of the MNPs onto the nanotubes does not block the electron hopping from one localized site to another, or possibly from one nanotube to another. The preservation of electrical conduction behavior of the nanotube network is important especially for hybrid film-based electronic and magnetoelectronic applications.
 | ||
| Fig. 4 (a) Temperature-dependent sheet resistance of the MNP-MWNT hybrid film. Inset: Typical SEM image of a drop-cast hybrid film. (b) Scaling of the sheet resistance with the three-dimensional variable range hopping mechanism on a plot of lnRs versus T−1/4, and with the tunneling conduction mechanism on a plot of lnRsversus T−1/2. | ||
Conclusions
In conclusion, we have developed a simple noncovalent chemical route for synthesizing magnetic carbon nanotube derivatives while still preserving the original electrical conduction behavior of the nanotube network. This method is not only a convenient route for uniformly assembling magnetic nanoparticles onto carbon nanotubes within the single-layer configuration, but also as a versatile way for constructing other functional hybrids.9 As a result of the dense and uniform assembly of the MNPs on the nanotubes, the MNP-MWNT hybrids exhibit a distinct superparamagnetism with superparamagnetic transition temperature of 20 K, and can be magnetically manipulated in a quick and reversible mode. Upon further optimization of their sturcture and the interface between their components, these magnetic carbon nanotube derivatives are remarkable materials and should be suitable for a wide range of applications.Acknowledgements
This work was supported by LANL-LDRD funding. This work was performed, in part, at the Center for Integrated Nanotechnologies, a U.S. Department of Energy, Office of Basic Energy Sciences user facility. Los Alamos National Laboratory, an affirmative action equal opportunity employer, is operated by Los Alamos National Security, LLC, for the National Nuclear Security Administration of the U.S. Department of Energy under contract DE-AC52-06NA25396.References
- Ph. Avouris, Z. Chen and V. Perebeinos, Nat. Nanotechnol., 2007, 2, 605–615 CrossRef CAS.
- Z. Nie, A. Petukhova and E. Kumacheva, Nat. Nanotechnol., 2010, 5, 15–25 CrossRef CAS.
- P. V. Kamat, J. Phys. Chem. C, 2008, 112, 18737–18753 CAS.
- (a) S. Guo, S. Dong and E. Wang, Adv. Mater., 2010, 22, 1269–1272 CrossRef CAS; (b) P. Singh, J. Kumar, F. M. Toma, J. Raya, M. Prato, B. Fabre, S. Verma and A. Bianco, J. Am. Chem. Soc., 2009, 131, 13555–13562 CrossRef CAS.
- D. R. Kauffman and A. Star, Angew. Chem., Int. Ed., 2008, 47, 6550–6570 CrossRef CAS.
- G. G. Wildgoose, C. E. Banks and R. G. Compton, Small, 2006, 2, 182–193 CrossRef CAS.
- M. Grzelczak, M. A. Correa-Duarte, V. Salgueiriño-Maceira, B. Rodríguez-González, J. Rivas and L. M. Liz-Marzán, Angew. Chem., Int. Ed., 2007, 46, 7026–7030 CrossRef CAS.
- X. Li, Y. Liu, L. Fu, L. Cao, D. Wei, Y. Wang and G. Yu, J. Phys. Chem. C, 2007, 111, 7661–7665 CrossRef CAS.
- X. Li, Y. Jia and A. Cao, ACS Nano, 2010, 4, 506–512 CrossRef CAS.
- S. Liu, J. Li, Q. Shen, Y. Cao, X. Guo, G. Zhang, C. Feng, J. Zhang, Z. Liu, M. L. Steigerwald, D. Xu and C. Nuckolls, Angew. Chem., Int. Ed., 2009, 48, 4759–4762 CrossRef CAS.
- L. Hu, Y. Zhao, K. Ryu, C. Zhou, J. F. Stoddart and G. Grüner, Adv. Mater., 2008, 20, 939–946 CrossRef CAS.
- D. M. Guldi, G. M. A. Rahman, V. Sgobba, N. A. Kotov, D. Bonifazi and M. Prato, J. Am. Chem. Soc., 2006, 128, 2315–2323 CrossRef CAS.
- K. Kostarelos, A. Bianco and M. Prato, Nat. Nanotechnol., 2009, 4, 627–633 CrossRef CAS.
- J. H. Choi, F. T. Nguyen, P. W. Barone, D. A. Heller, A. E. Moll, D. Patel, S. A. Boppart and M. S. Strano, Nano Lett., 2007, 7, 861–867 CrossRef.
- C. Gao, W. Li, H. Morimoto, Y. Nagaoka and T. Maekawa, J. Phys. Chem. B, 2006, 110, 7213–7220 CrossRef CAS.
- D. Yang, F. Yang, J. Hu, J. Long, C. Wang, D. Fu and Q. Ni, Chem. Commun., 2009, 4447–4449 RSC.
- X. Zhao, C. Johnston and P. S. Grant, J. Mater. Chem., 2009, 19, 8755–8760 RSC.
- C. L. Chen, X. K. Wang and M. Nagatsu, Environ. Sci. Technol., 2009, 43, 2362–2367 CrossRef CAS.
- D. Eder, Chem. Rev., 2010, 110, 1348–1385 CrossRef CAS.
- N. Yang, J. Zhai, D. Wang, Y. Chen and L. Jiang, ACS Nano, 2010, 4, 887–894 CrossRef CAS.
- Y. Huang, J. Lin, Y. Bando, C. Tang, C. Zhi, Y. Shi, E. Takayama-Muromachi and D. Golberg, J. Mater. Chem., 2010, 20, 1007–1011 RSC.
- J. W. Lee, R. Viswan, Y. J. Choi, Y. Lee, S. Y. Kim, J. Cho, Y. Jo and J. K. Kang, Adv. Funct. Mater., 2009, 19, 2213–2218 CrossRef CAS.
- A. Zamudio, A. L. Elías, J. A. Rodríguez-Manzo, F. López-Urías, G. Rodríguez-Gattorno, F. Lupo, M. Rühle, D. J. Smith, H. Terrones, D. Díaz and M. Terrones, Small, 2006, 2, 346–350 CrossRef CAS.
- V. Georgakilas, D. Gournis, V. Tzitzios, L. Pasquato, D. M. Guldi and M. Prato, J. Mater. Chem., 2007, 17, 2679–2694 RSC.
- Y. Deng, C. Deng, D. Yang, C. Wang, S. Fu and X. Zhang, Chem. Commun., 2005, 5548–5550 RSC.
- M. A. Correa-Duarte, M. Grzelczak, V. Salgueiriño-Maceira, M. Giersig, L. M. Liz-Marzán, M. Farle, K. Sierazdki and R. Diaz, J. Phys. Chem. B, 2005, 109, 19060–19063 CrossRef CAS.
- V. Georgakilas, V. Tzitzios, D. Gournis and D. Petridis, Chem. Mater., 2005, 17, 1613–1617 CrossRef.
- T. Hyeon, S. S. Lee, J. Park, Y. Chung and H. B. Na, J. Am. Chem. Soc., 2001, 123, 12798–12801 CrossRef CAS.
- Y. Zhang, G. Zou, S. K. Doorn, H. Htoon, L. Stan, M. E. Hawley, C. J. Sheehan, Y. Zhu and Q. X. Jia, ACS Nano, 2009, 3, 2157–2162 CrossRef CAS.
- A. Glaria, M. L. Kahn, A. Falqui, P. Lecante, V. Colliėre, M. Respaud and B. Chaudret, ChemPhysChem, 2008, 9, 2035–2041 CrossRef CAS.
- P. K. Jain, Y. Xiao, R. Walsworth and A. E. Cohen, Nano Lett., 2009, 9, 1644–1650 CrossRef CAS.
- J. Jang and H. Yoon, Adv. Mater., 2003, 15, 2088–2091 CrossRef CAS.
- S. Pal, S. Chandra, M.-H. Phan, P. Mukherjee and H. Srikanth, Nanotechnology, 2009, 20, 485604 CrossRef.
- Q. Li, Y. Li, X. Zhang, S. B. Chikkannanavar, Y. Zhao, A. M. Dangelewicz, L. Zheng, S. K. Doorn, Q. X. Jia, D. E. Peterson, P. N. Arendt and Y. Zhu, Adv. Mater., 2007, 19, 3358–3363 CrossRef CAS.
- S. Banerjee and D. Chakravorty, J. Appl. Phys., 1998, 84, 1149–1151 CrossRef CAS.
- Z. Li, H. R. Kandel, E. Dervishi, V. Saini, Y. Xu, A. R. Biris, D. Lupu, G. J. Salamo and A. S. Biris, Langmuir, 2008, 24, 2655–2662 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: Fig. S1–S8 and the movie for magnetic manipulation of the MNP-MWNT hybrids. See DOI: 10.1039/c0nr00771d/ |
| This journal is © The Royal Society of Chemistry 2011 |
