Growth stimulation of gram (Cicer arietinum) plant by water soluble carbon nanotubes
Shweta
Tripathi
,
Sumit Kumar
Sonkar
and
Sabyasachi
Sarkar
*
Department of Chemistry, Indian Institute of Technology, Kanpur, Kanpur, 208016, India. E-mail: abya@iitk.ac.in
First published on 21st January 2011
Abstract
Water soluble carbon nanotubes (wsCNTs) show enhancement of the growth rate of common gram (Cicer arietinum) plants. Treating plants with up to 6.0 μg mL−1 of wsCNT shows an increased growth rate in every part of the plant including the roots, shoots and also in branching. The noticeable difference between the wsCNT treated and controlled gram is the water uptake; in the former it is dramatically enhanced, suggesting better water absorption and retention related to enhanced growth. This work shows that unlike CNTs, wsCNTs are non-toxic to plant cells that conserve water transport in plants.
1. Introduction
Amongst all nanomaterials, carbon-based materials (especially carbon nanotubes (CNTs)) have attracted much interest since their discovery1 due to their unique mechanical, electrical, thermal and chemical properties.2–5 Furthermore, their potential use in hydrogen storage,6drug delivery,7 and tumor imaging8 are some important fields of interest being vigorously pursued. From the wet side of its application, most of the work is focused on its interaction with different animal or mammalian cells9–13 and its influence on plant cells is scarcely explored. Plants as important ecological receptors have not received enough attention with regard to the use of carbon-based nanomaterials.Recently it was shown that the single-walled carbon nanotubes (SWNTs) and multiwalled carbon nanotubes (MWNTs) may penetrate the cell walls and cell membranes of tobacco cells14 and tomato plants15 respectively. There are reports on opening up the new nano-technological prospects of these CNTs in agriculture with or without the conventional fertilizers.16 But the uphill problem in such use is the lack of solubility of these CNTs in water. To understand the influence of CNTs in plant physiology and plant development at the organism level, considering the water solubility of CNTs is a must so that its biocompatible role may first be addressed before any agricultural application is investigated. Therefore the most important task is to derivatize CNTs, by attaching hydrophilic groups which will help to make it water soluble. We have made a simple approach to achieve this and this water soluble carbon nanotube (wsCNT)17 is being used as a probe in different biological fields. As shown in the previous reports of water insoluble SWNTs and MWNTs, due to their tubular structure and extremely high aspect-ratio, these can readily penetrate through various biological barriers in mammals,18 plants,14,15 and microorganisms.19 However, for such an effect there are many possibilities of damaging the biological barriers (membranes). In this respect a completely water soluble form of CNT can completely avoid such a mishap with the introduction of its beneficial effect.
Herein, we report the effect of such wsCNTs on the growth of gram (Cicer arietinum) seed, also known as bengal gram, under different concentrations. We choose a dicotyledonous plant of gram (C. arietinum) seed as it has a very short life cycle, and its vascular bundles are arranged in rings (as opposed to a monocotyledonous plant in which the vascular bundles are scattered). The molecular transport across cellular membranes is essential to sustain life. In this process, water and minerals are essential components for growth and their transport to different cells and organs is critically important for the survival of a living entity. In vascular plants, the root helps the plant to transport water and solutes20 and so is involved in the pumping of water due to the water potential caused by transpiration across the plasma membrane into the cytoplasm of cells.21 Molecular dynamic simulations of the osmotically driven transport of water molecules through hexagonally packed carbon nanotube membranes was simulated with respect to the ability of CNT semi-permeable membranes to separate components of pure water and salt solution.22 Molecular dynamics simulations of flows of water inside CNTs were also studied23 to show the pulse like transmission of water through a nanotube. However, experimental findings on such phenomena are lacking. Based on this, we present here our experimental result carried out with wsCNTs instead of CNTs, which is more relevant.
2. Experimental
Materials
All reagents were of analytical grade, purchased from Sigma Aldrich, and used without further purification.Synthesis of water soluble multiwalled carbon nanotubes
A wsCNT was synthesized by the reported method17 with some modification. CNT-containing carbon soot was collected over a glass surface from the top of the flame burning from an oil (mustard) lamp. The soot was collected and washed with toluene, alcohol and acetone and finally with water to remove all unburnt oil and any hydrocarbon produced. This washed soot was placed under concentrated nitric acid overnight in cold conditions. The acid was then diluted with water and decanted off carefully, leaving the black slurry. Several washings of the acid-treated soot by water removed the trace amount of nitrate as tested using Griess reagent24 and finally the black slurry was dried over a water bath to yield 35% wsCNT from the raw soot.Extent of the derivatization (the density of the carboxylic acid groups on surface of CNT)
An acid–base titrimetric25,26 method to assay the number of carboxylic acid groups present in the CNT showed the presence of 20–22% by weight and such heavy carboxylation rendered this wsCNT able to dissolve readily in water without sonication and the wsCNT remained in solution for months without precipitation (see Fig. 1c). Such heavily derivatized wsCNT possesses porous outer surface to allow the permeability of fluid materials readily. A stock solution of wsCNT (150 mg L−1) was used in all the experiments.Seeds
Seeds of common gram C. arietinum was placed in a dry place under ambient conditions before use. One-day-old sprouted gram seeds were used for monitoring the growth under the treatment with different concentrations of wsCNT.Germination
Seeds were immersed in a 10% sodium hypochlorite solution for 10 min to ensure surface sterility, then they were soaked in water for one day for germination.Preparation of CdS for fluorescence
For fluorescence microscopic studies, roots of well grown plants under wsCNT were placed in a vial in water containing 300 μL CdSO4 per mL of water for an hour. After one hour of such exposure, the roots were washed with running water to free them from external CdSO4. The washed seed roots were then dipped into water pre-saturated with H2S gas. After 10 min, these plant roots were removed from this and washed with running water until free from H2S. Slides were then prepared from the transverse section (T.S) and lateral section (L.S) of the root. Fluorescence microscopic images of CdS incorporated into wsCNT showed very distinct tubular structures due to the fluorescence of CdS aligned with the wsCNT. Images of fluorescence microscopy as shown in Fig. 8e and f clearly showed the channels fluoresce due to the presence of CdS which is formed by the passage of Cd2+ ion through the defect sides (porous) of the wsCNT which subsequently precipitates the fluorescing material CdS when exposed with hydrogen sulfide. As a check we used our underivatized CNT (with appreciable surface defect but not soluble in water due to the absence of hydrophilic carboxylated groups) and these were stirred under dilute CdSO4 aqueous solution for a couple of hours. The insoluble CNTs were centrifuged and washed several times to free them from any contamination of external CdSO4. Brief exposure of this material to water saturated with H2S gas and finally washing the material with plenty of water to remove traces of H2S produced clean CNTs impregnated with CdS. This was subjected to fluorescence microscopy (Fig. 8g). The figure shows a fluorescing line (marked by arrows) due to the trapping of CdS by CNT. As the starting material was not oxidised with nitric acid the CNT was contaminated with amporphous carbon. Such amorphous carbon can also trap CdS, contributing fluorescing spots. The fluorescence is understandable as the CNTs used here are defective in nature with enough porosity to allow the passage of the ions. When blank roots (without wsCNT exposure) were treated similarly with CdSO4 and H2S, such a treated root showed a blurred hue of weak fluorescence suggesting that whatever the concentration of Cd2+ ion inside the root by that time, it was readily removed on washing without its retention, which resulted in virtually no deposition of CdS fluorophore on reaction with H2S. This also confirms the enhanced micro ion uptake through the root under the wsCNT channel.Sample preparation for SEM and TEM analyses of root with wsCNT
Gram roots were cut into small pieces with a razor blade and these roots were pinned onto Silgard-coated plastic Petri dishes and placed these submerged with a fixing solution containing 2.5% glutaraldehyde in 0.1M cacodylate buffer, pH 7.4 for 6 h at room temperature. Thereafter, these roots were washed with cacodylate buffer with 4% sucrose at pH 7.4. Then, these roots were immersed in 1% osmium tetraoxide (aqueous) pH 7.4 for 2.5 h at room temperature. Again these roots were washed three times (each 15 min duration) in 0.1 M cacodylate buffer, pH 7.4. These were then treated by step wise dehydration in ascending ethanol series; 2 × 15 min in 30% ethanol, 2 × 15 min in 50% ethanol, 2 × 15 min in 70% ethanol, 2 × 15 min in 90% ethanol, 2 × 15 min in 100% ethanol. This automated dehydration process takes approximately 40–50 min to complete. After the fixation was complete, roots were dewaxed in 100% xylene for 1 min followed by incubating the dewaxed roots in 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 xylene
50 xylene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) paraffin (30 min.) and finally transferred to 100% paraffin wax for 10 h. After 10 h thin sections were cut from the embedded samples using an microtome (Leica microtome RM225), and sections were again washed with xylene followed by ethanol for the removal of excess wax and then mounted on copper grids for TEM analysis and on brass stub with sequential gold coating for SEM analysis.
paraffin (30 min.) and finally transferred to 100% paraffin wax for 10 h. After 10 h thin sections were cut from the embedded samples using an microtome (Leica microtome RM225), and sections were again washed with xylene followed by ethanol for the removal of excess wax and then mounted on copper grids for TEM analysis and on brass stub with sequential gold coating for SEM analysis.
Characterization
The Quanta 200 Hv Envoirnmental Scanning Electron Microscope (ESEM) (FEI Company, USA) equipped with an energy dispersive X-ray (EDX) was used for the visualization of the size and morphology of wsCNT in high vacuum mode.For high-magnification visualization of the size and morphology of wsCNT inside the xylem vessels of roots were made by the SUPRA 40VP Field Emission Scanning Electron Microscope (Carl Zeiss NTS GmbH, Oberkochen (Germany)) equipped with an energy dispersive X-ray (EDX), in high vaccum mode operated at 10 kV.
Samples were prepared by taking small amount of wsCNT which was ultrasonicated in about 3 mL of HPLC grade ethanol and allowed to stand. A drop from the upper part of the solution was then placed onto brass stubs that was dried and finally under vacuum made sequential gold sputtering.
The morphology and electron diffraction (ED) of the wsCNT were observed by using a Tecnai 20 G2 200 kV, STWIN model, transmission electron microscope (TEM). TEM and high resolution TEM (HRTEM) analyses was done with an acceleration voltage of 200 kV. Samples were prepared by placing a drop of the sonicated solution on the surface of carbon coated copper grid.
Raman spectra were recorded using a Raman spectrometer, WITEC MODEL, under 514 nm laser excitation
Fluorescence images were taken by a LEICA TCS SP2 fluorescence microscope at 488 and 561 nm band pass filters.
3. Results and discussion
Characterization of wsCNT
FT-IR and Raman spectroscopy (Fig. 1) were used to characterize the wsCNT.17 The FT-IR spectrum (Fig. 1a), clearly shows the carboxylic acid groups present in the wsCNT (very broad around 3300 cm−1 for ν(O–H) and a peak at around 1720 cm−1ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)). The representative Raman spectrum (Fig. 1b) of the sample showed the typical features of multi-walled CNT. The spectrum demonstrates that the peak frequencies of the graphite (G) mode are at 1575
O)). The representative Raman spectrum (Fig. 1b) of the sample showed the typical features of multi-walled CNT. The spectrum demonstrates that the peak frequencies of the graphite (G) mode are at 1575![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cm−1 as E2g mode vibrations of the graphitic plane and contains disorder (D) modes that are at 1345 cm−1 attributed to the presence of defects that corresponds to the highly derivatized and 2D band or the second-order harmonic of the D band at 2680 cm−1. The free solubility of this wsCNT in water is shown in some photographs as in Fig. 1c.
cm−1 as E2g mode vibrations of the graphitic plane and contains disorder (D) modes that are at 1345 cm−1 attributed to the presence of defects that corresponds to the highly derivatized and 2D band or the second-order harmonic of the D band at 2680 cm−1. The free solubility of this wsCNT in water is shown in some photographs as in Fig. 1c.
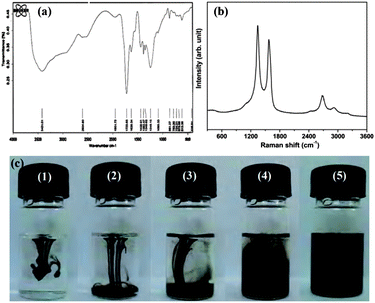 | ||
| Fig. 1 (a) FT-IR; (b) corresponding Raman spectra showing the characteristic D and G bands of CNT; (c) photographs showing the solubility of wsCNT with time interval; (c-1) just dropped the sample; (c-2) after 20 s; (c-3) after 40 s; (c-4) 1.5 min; (c-5) 5 min without any sonication. It remains in solution for several months without any precipitation. | ||
The wsCNT used in this experiment was characterized by conventional techniques like the scanning electron micrographs (Fig. 2a) with its energy dispersive X-ray (EDX) analysis (Fig. 2b) that showed the presence of carbon and oxygen due to heavy carboxylation. In Fig. 2c, the TEM images showed that these wsCNTs possess a 10–30 nm outer diameter and 4–6 nm inner diameter along with its HRTEM image (Fig. 2d). In addition these wsCNTs showed a crystalline structure consisting of parallel crystal planes with a lattice spacing of 0.34 nm (Fig. 2d and e) (inset shows the diffraction pattern). It is to be noted that the synthesized CNT used here has more surface defects than conventionally synthesised by standard CVD or electric arc or laser ablation technique. Such defects render these CNTs ready for carboxylation incorporating high density electrophilic groups which help enhance its solubility in water. Further its HRTEM image (Fig. 2d) clearly shows the damage on its surface and such damages are helpful to transport water or ions readily. Regarding the length of these wsCNTs, the lower limit of the length could be around 400 nm (Fig. 2e). However, the incorporation of wsCNTs inside the root is of assorted size as may be seen by the SEM image (Fig. 6b).
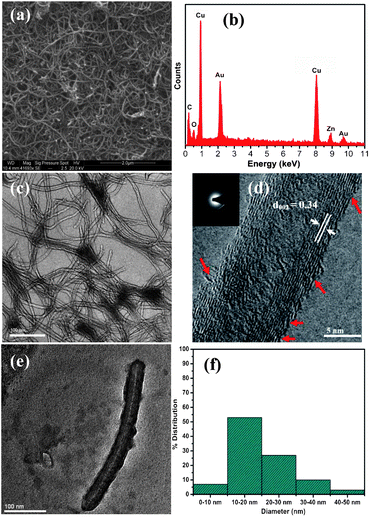 | ||
| Fig. 2 (a) SEM; (b) corresponding energy dispersive X-ray (EDX) analysis showing the presence of carbon and oxygen only; (c) TEM; (d) HRTEM image showing interlayer spacing in the wsCNT of 0.34 nm, corresponding to the (002) plane of graphite carbon and broken edged (porous) marked with red arrows, (inset, corresponding diffraction); (e) TEM image of a small wsCNT of length ∼ 400 nm; (f) a diameter distribution histogram of wsCNT. | ||
Growth of gram plants with wsCNT
To compare the growth in all the different parts of the plants like shoot length, branching, number of roots and length, water uptake etc. with or without wsCNT (shown in Fig. 3), three different sets of 10 vials were used.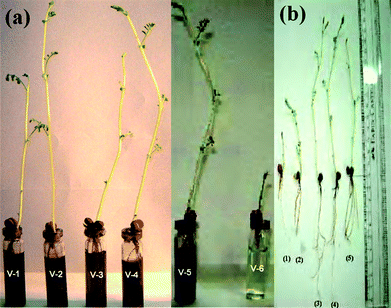 | ||
| Fig. 3 Phenotypes of gram plants after 10 days, (a) gram plants grown (v-1 to v-5) with wsCNT, in comparison with blank (v-6) without wsCNT (b) Phenotypes of gram plants after 10 days marked with (b-1, b-2) grown without wsCNT and (b-3, b-4) under 200 μL and (b-5) under 100 μL of wsCNT respectively. | ||
In the first set, seeds were grown without wsCNT, i.e. only grown in double distilled water (5 mL of water); in the second set 100 μL wsCNT from the stock solution was used and double distilled water added to make it 5 mL; in the third set 200μL of wsCNT of the stock solution was made upto 5 mL using double distilled water. For comparison the root length, shoot length, number of roots and water uptake by gram plants were monitored for 10 days (Fig. 3a and b). Results are shown with ± SE (standard error) of measurements based on 10 plants (Fig. 4).
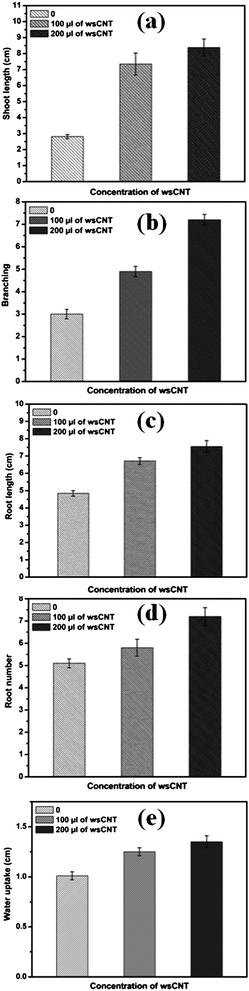 | ||
| Fig. 4 The effect of wsCNT on the growth and development of gram plants, shown as an average ± SE of measurements of 10 plants under different conditions. Up to 10 days’ growth of gram plants was observed and used for all these measurements: (a) growing shoot length of gram plants with and without wsCNT; (b) branching; (c) root length of gram plants growing with and without wsCNT; (d) root numbers of growing gram plants with and without wsCNT (100 μL and 200 μL of stock solution); (e) water uptake. | ||
Comparing the outer diameter of wsCNT (10–30 nm, shown in Fig. 2) with xylem (a few microns), it can be presumed that the wsCNT capillaries may get introduced inside the lumen of tracheal elements. Due to the size difference between these two, wsCNT should be incorporated within the xylem according to the concept of the formation of a “large capillary”. This is formed inside the tracheal elements of the xylem by head to tail arrangement of CNTs and, in addition, wsCNTs construct an aligned network and the narrow space in between these wsCNTs can act as the formation of several new capillaries which increase the water uptake mechanism of the plant in addition to their natural flow. Packing in bundles could also be a possibility. Thus it provides channels and capillaries through which water is conducted (Fig. 5). This can be clearly seen in the SEM image (Fig. 6b) which shows that wsCNTs of different lengths can be embedded inside. Further, because of the softness due to porosity, such wsCNTs are accommodated even through a zig-zag path. This is encouraging as it may adopt the alignment in the path of the channels readily. This is corroborated by TEM images of the roots. A comparison between wsCNT treated and blank roots (Fig. 7a and b) shows clearly the aligned incorporation of wsCNT.
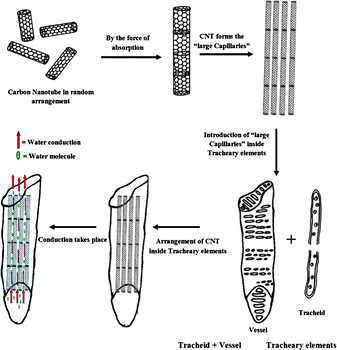 | ||
| Fig. 5 Schematic diagrams of alignment of wsCNT inside roots. | ||
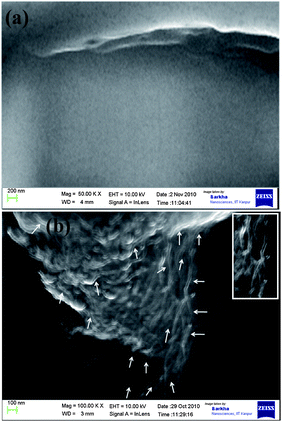 | ||
| Fig. 6 SEM images of L.S of root (top) without and (bottom) with wsCNT, showing the aligned wsCNT (white arrows) inside the root; inset shows zoomed image of root showing wsCNT. | ||
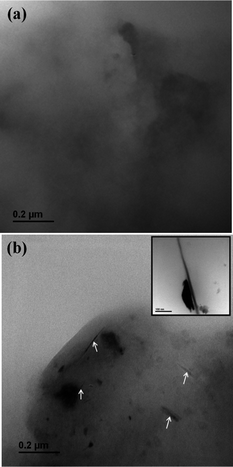 | ||
| Fig. 7 TEM images of L.S of root (a) without and (b) with wsCNT showing its alignment (marked with white arrow); inset shows an aligned wsCNT. | ||
For the induced growth mechanism, it might be possible that wsCNT, after attaching to the root surface or an inner portion of root (such as vascular bundles, cortical region etc.), aligned itself to enhance the capillary action of water absorption as shown by simulation work.26,27 Our present work suggested that water not only occupies these channels but also speeds up the molecular transport inside the plant via xylem where wsCNT acts as tubular membrane that offers both high selectivity and high flux. This results in very rapid flow, and the transport is expected to be as predicted by molecular dynamics, which correlates this enhancement of the flux to the atomic smoothness of the nanotube surface and to molecular ordering phenomena that may occur on a range of confined length scales.28
The presence of intracellular wsCNT inside the plants has been verified via examination of T.S and L.S of plants treated with and without wsCNT through optical microscopy. An effect was observed that wsCNTs, which were initially in the form of cluster aggregates like spaghetti, become aligned in the vascular bundle due to an endo-osmotic root pressure caused by xylem.29 These vascular bundles run all through the plant and consist of xylem and phloem. The presence of wsCNT in the plant root (especially in the xylem) can be detected by optical microscopy (Fig. 8b and d), and fluorescence microscopy (Fig. 8e and f) using the excitation wavelength of 371 nm. Images of fluorescence microscopy (Fig. 8e and f) clearly show the channels fluorescing throughout the passage of CdS as stated above.
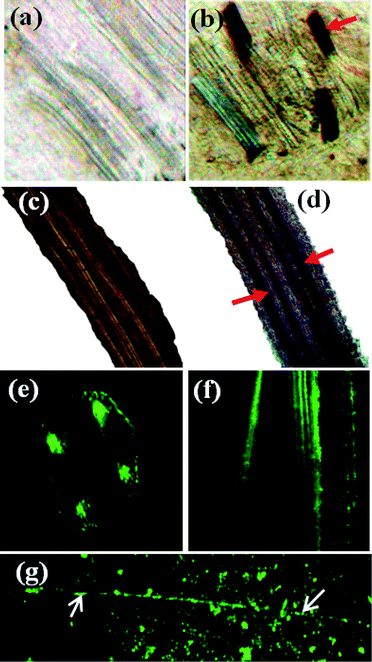 | ||
| Fig. 8 Optical images: (a,c) T.S and L.S of blank root; (b,d) T.S and L.S with wsCNT (red arrows mark wsCNT). Fluorescence images: (e,f) T.S and L.S of CdS treated root; (g) CdS incorporated untreated CNT (see text). | ||
In summary, our work describes a simple and rapid method for the application of wsCNT in the field of agriculture and to investigate the interactions of wsCNT with plant cells. We provide evidence by optical, fluorescence, SEM and TEM microscopy that these wsCNT are taken up by plants. Finally, we demonstrate that the continuous use of wsCNT is non-toxic and that the growth rate of the plants studied here increased several times in the presence of wsCNT. A key implication of this work is that the wsCNT could possibly be utilized for optimum water transport with larger water uptake to prevent water loss in the field of agricultural sciences. There are many unresolved things regarding the mechanism for the alignment of these wsCNTs across the xylem and the enhanced flow of fluid and ions either through these wsCNTs, or through the porous exterior walls of the aligned wsCNTs, or both, need further investigation. Further work will focus on understanding the influence of such interaction under the different stages like flowering, fruit ripeningetc. The present finding clarified the non-toxic behaviour of wsCNTs.
The use of wsCNTs in plant growth can play an important role in the arid areas of agriculture where the supply of water is crucial and requires maximum conservation and fruitful usage.
Acknowledgements
S.S. thanks the DST, New Delhi for granting the project under the Nano Science and Technology Initiative Scheme. S.T. thanks UP State Council of Science and Technology, Lucknow for a JRF and S.K.S. thanks IITK for a Senior Teaching Assistantship.Notes and references
- S. Iijima, Nature, 1991, 354, 56 CrossRef CAS.
- R. H. Baughman, A. A. Zakhidov and W. A. De Heer, Science, 2002, 297, 787 CrossRef CAS.
- M. S. Dresselhaus, G. Dresselhaus and P. C. Eklund, Science of Fullerenes and Carbon Nanotubes; Academic Press: San Diego, CA, 1996 Search PubMed.
- S. Helveg, C. Lopez-Cartes, J. Sehested, P. L. Hansen, B. S. Clausen, J. R. Rostrup-Nielsen, F. Abild-Pedersen and J. K. Norskov, Nature, 2004, 427, 426 CrossRef CAS.
- M. Endo, ChemTech, 1988, 18, 568 CAS.
- A. C. Dillon, K. M. Jones, T. A. Bekkedahl, C. H. Kiang, D. S. Bethune and M. J. Heben, Nature, 1997, 386, 377 CrossRef CAS.
- A. Bianco, K. Kostarelos and M. Prato, Curr. Opin. Chem. Biol., 2005, 9, 674 CrossRef CAS.
- C. Zavaleta, A. de la Zerda, Z. Liu, S. Keren, Z. Cheng, M. Schipper, X. Chen, H. Dai and S. S. Gambhir, Nano Lett., 2008, 8, 2800 CrossRef CAS.
- D. Pantarotto, J. Briand, M. Prato and A. Bianco, Chem. Commun., 2004, 16 RSC.
- W. Yang, P. Thordarson, J. Gooding, S. Ringer and F. Braet, Nanotechnology, 2007, 18, 412001 CrossRef.
- S. Fiorito, M. Monthioux, R. Psaila, P. Pierimarchi, M. Zonfrillo, E. D. Emilia, S. Grimaldi, A. Lisi, F. Beguinc, R. Almairac, L. Noe and A. Serafino, Carbon, 2009, 47, 2789 CAS.
- J. Wang, R. H. Sun, N. Zhang, H. Nie, H. J-Liu, J. N. Wang, H. Wang and Y. Liu, Carbon, 2009, 47, 1752 CrossRef CAS.
- C. Klumpp, K. Kostarelos, M. Prato and A. Bianco, Biochim. Biophys. Acta, Biomembr., 2006, 1758, 402.
- Q. L. Liu, B. Chen, Q. L. Wang, X. L. Shi, Z. Y. Xiao, J. X. Lin and X. H. Fang, Nano Lett., 2009, 9, 1007 CrossRef CAS.
- M. Khodakovskaya, E. Dervishi, M. Mahmood, Y. Xu, Z. Li, F. Watanabe and A. S. Biris, ACS Nano, 2009, 3, 3221 CrossRef CAS.
- M. C. DeRosa, C. Monreal, M. Schnitzer, R. Walsh and Y. Sultan, Nat. Nanotechnol., 2010, 5, 91 CrossRef CAS.
- P. Dubey, D. Muthukumaran, S. Dash, R. Mukhopadhyay and S. Sarkar, Pramana, 2005, 65, 681 CrossRef CAS.
- G. B. Qu, Y. H. Bai, Y. Zhang, Q. Jia, W. D. Zhang and B. Yan, Carbon, 2009, 47, 2060 CrossRef CAS.
- S. Kang, M. Herzberg, D. F. Rodrigues and M. Elimelech, Langmuir, 2008, 24, 6409 CrossRef CAS.
- F. N. Dalton, P. A. C. Raats and W. R. Gardner, Agron. J., 1965, 67, 334.
- A. de. Angeli, S. Thomine, J. Frachisse, G. Ephritikhine, F. Gambale and H. Barbier-Brygoo, FEBS Lett., 2007, 581, 2367 CrossRef CAS.
- A. Kalra, S. Garde and G. Hummer, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 10175 CrossRef.
- G. Hummer, J. C. Rasaiah and J. P. Noworyta, Nature, 2001, 414, 188 CrossRef CAS.
- S. Roy and S. Sarkar, J. Chem. Soc., Chem. Commun., 1994, 275 RSC.
- H. Hu, P. B. Bhowmik, B. Zhao, M. A. Hamon, M. E. Itkis and R. C. Haddon, Chem. Phys. Lett., 2001, 345, 25 CrossRef CAS.
- W. Huang, S. Fernando, L. F. Allard and Y. P. Sun, Nano Lett., 2003, 3, 565 CrossRef CAS.
- A. Kalra, S. Garde and G. Hummer, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 10175 CrossRef.
- A. S. Crafts and T. C. Broyer, Am. J. Bot., 1938, 25, 529 CrossRef CAS.
- A. S. Crafts, Plant Physiol., 1938, 13, 791 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
