DOI:
10.1039/C0NR00684J
(Paper)
Nanoscale, 2011,
3, 741-745
Facile synthesis of magnetic metal (Mn, Fe, Co, and Ni) oxides nanocrystals via a cation-exchange reaction†
Received
16th September 2010
, Accepted 26th October 2010
First published on 26th November 2010
Abstract
Magnetic metal (Mn, Fe, Co, and Ni) oxides nanocrystals with small size and uniform size distribution are synthesized via a cation-exchange reaction. Two experimental stages are included in the synthesis of metal oxides nanocrystals. Firstly, Cu(OH)2 decomposes to CuO nanocrystals, induced by free metal cations. Compared to CuO nanocrystals produced without any free metal cation, the free metal cation has an important influence on the shape and size of CuO. Secondly, free metal cations exchange with the Cu2+ cation in the CuO nanocrystals to get Mn3O4, Fe2O3, CoO and NiO nanocrystals by cation-exchange reactions. The magnetic properties of these metal oxides nanocrystals have been investigated, all the nanocrystals are superparamagnetic at room temperature.
Introduction
When the size of a material becomes smaller, the thermodynamics and kinetic reaction properties of nanomaterials are different from those in bulk material. The size effect on the thermodynamics and kinetics of reactions is shown via decreasing the activation energy for the diffusion of atoms or ions, which makes the reaction easier in nanoscale materials.1 Temperature, pressure, and other experimental parameters of reaction, would be reduced by decreasing the size of materials. As an example, the ion-exchange reaction is easily achieved in nanomaterials and difficult for bulk materials. Recently, scientists have used ion-exchange reactions to synthesise semiconductor nanoparticles, nanospheres, core/shell nanostructure and heterostructures. By cation-exchange reactions, Ag2Se nanoparticles,2 PbSe nanoparticles,3 PdS, PdSe, PdTe, PtS, PtSe, PtTe nanoparticles or nanorods,4 Cu2S nanorods,5 PbS nanorods,6 PbTe/CdTe core/shell,7 Se/Ag2Se core/shell;8 carbon and SiC microscopic beads,9 cubic phase α-NaYF4 hollow nanoparticles,10 ZnS hollow nanoparticles,11 rare earth orthophosphate nanocrystals,12 titanate nanostructures13 and NaNbO3 nanorods14 are successfully achieved by anion-exchange reactions. Ion-exchange reactions are focused on semiconductor nanocrystals, few reports are related to synthesis of metal oxide nanocrystals. Only the synthesis of Co–Ni hydroxide nanosheets15 is used cation or anion-exchange reactions.
Herein, we synthesize metal oxides nanocrystals via a cation-exchange reaction. Magnetic metal oxide nanocrystals have received attention for their wide applications in bioanalysis, ferrofluids, magnetic resonance imaging, electronic, magnetic data storage, sensors, heavy metal ion removal and lithium ion batteries.16 Scientists have paid much attention to the synthesis of metal oxides with various shapes and sizes. MnO, Mn2O3, Mn3O4, and MnO2 nanocrystals with dot-shaped, rod-shaped and hierarchical structure have been synthesized.17 Iron oxide nanocrystals with various shapes and sizes have also been synthesized, such as nanoparticles, nanorods, nanoplates, nanorings etc.18 CoO nanocrystals19 and NiO nanocrystals20 with particle-shaped, rod-shaped and tube-shaped have also been achieved. Various synthetic routes have been developed to produce magnetic metal oxides nanocrystals with shape and size control, such as the decomposition of metal carboxylate or metal hydroxides, oxidation metal elements.
In this paper, we firstly synthesize magnetic metal oxides nanocrystals via a cation-exchange reaction. Metal chloride and Cu(OH)2 are the raw materials, OLA and ODE as the solution. Two experimental stages are included in the reaction. Firstly, Cu(OH)2 decompose to CuO nanocrystals, induced by the free metal cation. Secondly, magnetic metal cation diffuses into CuO nanocrystals and exchanges the Cu to form magnetic metal oxides by cation-exchange reaction.
Experimental
Materials
MnCl2 (99+ %), FeCl2 (99.9%), 1-octadecene (ODE, 90%) were purchased from Aldrich. CoCl2 (99.7%), NiCl2 (99%), and Cu(OH)2 (94%) were purchased from Alfa Aesar. Oleylamine (OLA, ≥70%) was purchased from Fluka. Toluene was purchased from the Beijing Chemical Company. All chemicals were used in the experiments without further purification.
Synthesis section
All experiments were carried out using standard airless techniques: a vacuum/dry nitrogen gas Schlenk line was used for synthesis and a nitrogen glove-box for storing and handling air- and moisture-sensitive chemicals.
Synthesis of magnetic metal oxides nanocrystals: typically, MnCl2 (0.48 mmol, 60.48 mg) (or FeCl2 (0.48 mmol), CoCl2 (0.48 mmol), NiCl2 (0.48 mmol)), Cu(OH)2 (0.32 mmol, 30.2 mg), OLA (2.35 mmol) and ODE (1.15 ml) were placed into a 50 ml three-neck flask in glove-box, then the flask was taken out and connected to Schlenk line. The flask was evacuated and flushed with nitrogen a number of times to remove oxygen. The mixture was heated to 160 °C, and kept for 20 min at this temperature. Then the red solution was taken out, and the solution color became blue black as soon as it was exposed to the atmosphere.
An equal volume of methanol was added into the solution and nanocrystals were collected by centrifuging. These nanocrystals were purified three times. Finally, the nanocrystals were dissolved in nonpolar solvents to form stable concentrated colloidal solutions.
Characterizations
Transmission electron microscopy (TEM) images were obtained with a Hitachi H-8100IV transmission electron microscope using an acceleration voltage of 200 kV. High resolution transmission electron microscopy (HRTEM) images were taken with a JEOL JEM-2100 at 200kV, the point-to-point resolution of this HRTEM was 0.19 nm. Powder X-ray diffraction (XRD) was obtained on a Bruker D8 diffractometer operating at 40 kV and 40 mA, using a Cu-Kα target. Data were collected from 15° to 80° with a sampling interval of 0.02° per step and a counting rate of 0.2 s per step. The valence state of iron was investigated by X-ray photoelectron spectroscopy (XPS: SSI, 2803-S). The magnetic properties of the nanocrystals were measured using a vibrating sample magnetometer (VSM) at room temperature.
Result and discussion
Powder X-ray diffraction (XRD) was used to confirm the crystal structure and chemical composition of the as-prepared sample. The XRD patterns of as-prepared magnetic metal oxides are given in Fig. 1. We characterized the sample produced with MnCl2, the XRD was consistent with the tetragonal Mn3O4, JCPDS No. 24-0734. The observed diffraction peaks are (112), (103), (211), (004), (220), (105), (321), (224) and (400) crystal planes for tetragonal Mn3O4. Similarly, the crystal structure and chemical composition of sample produced by FeCl2, CoCl2 and NiCl2 were all studied by XRD. XRD of the sample produced by FeCl2 was poor, which can confirm the chemical composition for Fe2O3 or Fe3O4. Figure S1 shows the XPS spectra of the samples, which can only give information on Fe(III).† XPS shows the sample is Fe2O3. Combining the XRD of samples, the chemical composition and crystal structure, the product is Fe2O3, JCPDS No. 39-1346. The diffraction peaks in the XRD patterns are in accordance with the (220), (311), (400), (511) and (440) crystal planes of cubic Fe2O3. The samples produced by CoCl2 and NiCl2 are CoO and NiO, respectively. The diffraction peaks in the XRD patterns of CoO are in response to the (101), (110), (112), (211) and (202) crystal planes of cubic CoO, JCPDS No. 65-5474. The (111), (200), (220) and (311) crystal planes of cubic NiO (JCPDS No. 65-2901) have obvious diffraction peaks in Fig. 1. In the XRD patterns, all of the diffraction peaks are in response to magnetic metal oxides, indicating the high purity of the samples. The low intensity and wide peaks in the XRD patterns of the samples show the lower crystallinity and smaller size of the nanocrystals. Compared to the XRD of Mn3O4 and CoO nanocrystals, the XRD patterns of Fe2O3 and NiO nanocrystals have lower intensity and wider peaks, indicating the much smaller size of Fe2O3 and NiO nanocrystals.
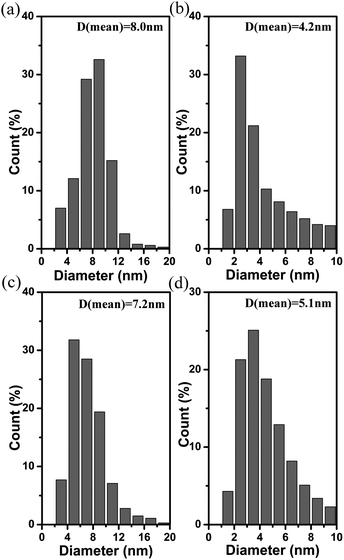
To ascertain the shape and size of these magnetic metal oxides nanocrystals, Fig. 2 gives the transmission electron microscopy (TEM) images of these samples. Mn3O4 nanocrystals are irregular nanoparticles; the size of nanoparticles is about 8.0 nm and size distribution is uniform. Fig. 2b gives the TEM images of Fe2O3 nanoparticles. The size of the Fe2O3 nanoparticles is smaller, they are about 4.0 nm. The histogram for size distribution of Fe2O3 nanoparticles is shown in Fig. 3b, the average size is 4.2 nm. The shape of the CoO nanocrystals are nanocubes with a size of about 7.2 nm, which also have uniform size distribution. The shape and size distribution of the NiO nanocrystals is shown in Fig. 2d and 3d. Monodisperse NiO nanoparticles have a size of about 5.1 nm. The Mn3O4 nanocrystals have the biggest size, and Fe2O3 nanoparticles are the smallest, which is consistent with the results of the XRD patterns. In order to confirm the crystal structure and shape of these magnetic metal oxides, we characterize these nanocrystals by high resolution transmission electron microscopy (HRTEM), as shown in Fig. 4. the Mn3O4 nanoparticles are irregular, single crystalline nanoparticles. The lattice of Mn3O4 nanoparticles can be clearly observed in Fig. 4a, indicating the high crystallinity of the Mn3O4 nanoparticles. The interplanar distance of 0.25 nm agrees with the (211) lattice fringe of tetragonal Mn3O4. The lattice of the Fe2O3 nanoparticles can also be observed and they are single crystalline in nature (Fig. 4b), the interplanar distance 0.30 nm is response to the (110) lattice fringe. The smaller size of the Fe2O3 nanoparticles is consistent with the weaker intensity and wider peaks in the XRD patterns. Fig. 4 also gives the HRTEM images of CoO nanoparticles and NiO nanoparticles. They are also single crystalline. The interplanar distance of 0.21 nm agrees with (110) lattice fringe of cubic CoO in Fig. 4c and the interplanar distance of 0.24 nm is consistent with the (111) lattice fringe of cubic NiO (Fig. 4d). The SAED images of these nanoparticles are inserted in Fig. 4, the given SAED images show the single crystal structure of the produced magnetic metal oxides nanocrystals. We investigated the influence of ligands, molar ratio of MCl2 (M = Mn, Fe, Co, and Ni)/Cu(OH)2, on the shape and size of the nanocrystals. We found the ligands had no effect on the shape and size of the nanocrystals, but the molar ratio had important influence on the nanocrystals. Figure S2 and S3 gave the TEM images of Mn3O4 and Fe2O3 nanocrystals with different molar ratios.†
With the aim of understanding the formation mechanism of the magnetic metal oxides nanocrystals, XRD and TEM were used to characterize the products taken at different reaction stages in the experiment. Figure S4, S5, S6 and S7 give the XRD patterns of products taken at different reaction stage in experiment.† The raw material Cu(OH)2 firstly decomposes to CuO nanocrystals, and then CuO nanocrystals transform to magnetic metal oxides. The free metal cation has an important influence on the decomposition of Cu(OH)2. When CuO nanocrystals are produced by decomposition of Cu(OH)2 without the free metal cation (the shape of the CuO nanocrystals is given in Figure S8†), the CuO nanocrystals are nanorods with size of 1–2 μm and the nanorods are constructed of smaller CuO nanoparticles. However, when free metal cation is present, the shape and size of the CuO nanocrystals produced changes greatly. Fig. 5 gives the TEM images of CuO nanocrystals which are produced with different free metal cation. The influence of anion or cation on the shape and crystal structure of nanocrystals has been reported before.21 Based on the CuO nanocrystals, the CuO nanocrystals gradually transform to magnetic metal oxides with existence of free metal cation. The cation-exchange reaction can be used to explain the transformation from CuO nanocrystals to magnetic metal oxides. The free cation inducement of decomposition and cation-exchange reaction are the two stages in the formation of magnetic metal oxides. The synthesis of magnetic metal oxides nanocrystals could be concluded as the following reactions from the experiments:
| | | 3Mn2+ + 4Cu(OH)2 → 3Mn2+ + 4CuO + 4H2O → Mn3O4 + 2Cu1+ + 2Cu2+ + 4H2O | (1) |
| | | 2Fe2+ + 3Cu(OH)2 → 2Fe2+ + 3CuO + 3H2O → Fe2O3 + 2Cu1+ + Cu2+ + 3H2O | (2) |
| | | Co2+ + Cu(OH)2 → Co2+ + CuO + H2O → CoO + Cu2+ + H2O | (3) |
| | | Ni2+ + Cu(OH)2 → Ni2+ + CuO + H2O → NiO + Cu2+ + H2O | (4) |
From the above reaction equations we can see that oxidation–reduction reactions were included in the synthesis of Mn3O4 and Fe2O3 nanoparticles, Cu+ was produced as a by-product. The existence of Cu+ in the produce of Mn3O4 and Fe2O3 nanoparticles could also be proved by the change of color when the reaction solution was exposed to air (Figure S9†). When the solution is exposed to air, the colorless Cu+ changes to blue Cu2+ immediately. The color of the reaction solution used to produce CoO and NiO nanoparticles did not changed when the solution was exposed to air. The experimental results prove that the proposed mechanism is reasonable in the experiment. Fig. 6 gives the schematic illustration of the formation process from Cu(OH)2 to magnetic metal oxides nanocrystals.
 |
| | Fig. 6 Schematic illustration of the evolution of Cu(OH)2 to metal oxide nanocrystals. | |
As typical magnetic metal oxides, magnetism is the most important property to these nanocrystals. Fig. 7 gives the magnetization hysteresis loop of Mn3O4, Fe2O3, CoO and NiO nanocrystals at room temperature, which is characterized by a SQUID magnetometer. If the size of these nanoparticles is small enough, they are single domain, and hysteresis would not be observed. The coercive fields for Mn3O4, Fe2O3, CoO and NiO nanocrystals is 7.7, 1.54, 6.18 and 12.8 Oe, respectively. Remnant magnetizations are 0.00032, 0.00039, 0.00018 and 0.00022 emu g−1 for Mn3O4, Fe2O3, CoO and NiO nanoparticles respectively. All the metal oxides nanocrystals are superparamagnetic at room temperature, which is different from the bulk materials. Bulk Mn3O4, Fe2O3, CoO and NiO are ferromagnetic, ferromagnetic, antiferromagnetic, and ferromagnetic at room temperature, respectively. Because of the size effect on the magnetic, these metal oxides are superparamagnetic at room temperature. Generally, when the size of magnetic nanocrystals decreases, they change from multidomain to single domain.22 Our experimental results are consistent with the previous reports on the magnetic of these magnetic metal oxides nanocrystals.17,23
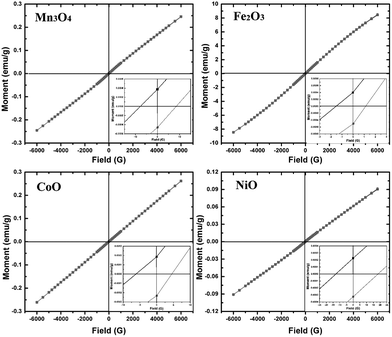 |
| | Fig. 7 Hysteresis loops of Mn3O4, Fe2O3, CoO and NiO nanocrystals at room temperature. | |
Conclusions
Mn3O4, Fe2O3, CoO and NiO nanocrystals with sizes of 8.0 nm, 4.0 nm, 7.2 nm and 5.1 nm are synthesized via cation-exchange reactions. The XRD patterns show that the composition changes in the experiment are from Cu(OH)2 to CuO, and then CuO transform to magnetic metal oxides. Two experimental stages are included in the experiment. Firstly, Cu(OH)2 decomposes to CuO nanocrystals. Compared with CuO nanocrystals produced without any free metal cation, the free metal cation has an important influence on the shape and size of CuO nanocrystals. Secondly, by cation-exchange reaction, free metal cation exchanges with the Cu2+ cation in the CuO nanocrystals to get Mn3O4, Fe2O3, CoO and NiO nanocrystals. All the nanocrystals are superparamagnetic, which is different from the magnetism of the bulk materials at room temperature.
Acknowledgements
Thank you for Dr Qiang Zhao for the characterizations and magnetic analysis of these metal oxides nanocrystals. This work was supported by NSFC (Nos. 21073071 and 10979001), the National Basic Research Program of China (No. 2007CB808000 and 2011CB808200).
Notes and references
- A. N. Goldstein, C. M. Echer and A. P. Alivisatos, Science, 1992, 256, 1425 CrossRef CAS
 ; G. Baldinozzi, D. Simeone, D. Gosset and M. Dutheil, Phys. Rev. Lett., 2003, 90, 216103 CrossRef CAS
; G. Baldinozzi, D. Simeone, D. Gosset and M. Dutheil, Phys. Rev. Lett., 2003, 90, 216103 CrossRef CAS  .
.
- D. H. Son, S. M. Hughes, Y. D. Yin and A. P. Alivisatos, Science, 2004, 306, 1009–1012 CrossRef CAS
 .
.
- M. V. Kovalenko, D. V. Talapin, M. A. Loi, F. Cordella, G. Hesser, M. I. Bodnarchuk and W. Heiss, Angew. Chem., Int. Ed., 2008, 47, 3029–3033 CrossRef CAS
 .
.
- S. E. Wark, C. H. Hsia and D. H. Son, J. Am. Chem. Soc., 2008, 130, 9550–9555 CrossRef CAS
 .
.
- B. Sadtler, D. O. Demchenko, H. M. Zheng, S. M. Hughes, M. G. Merkle, U. Dahmen, L. W. Wang and A. P. Alivisatos, J. Am. Chem. Soc., 2009, 131, 5285–5293 CrossRef CAS
 .
.
- J. M. Luther, H. M. Zheng, B. Sadtler and A. P. Alivisatos, J. Am. Chem. Soc., 2009, 131, 16851–16857 CrossRef CAS
 .
.
- K. Lambert, B. D. Geyter, I. Moreels and Z. Hens, Chem. Mater., 2009, 21, 778–780 CrossRef CAS
 .
.
- W. Zhu, W. Z. Wang and J. L. Shi, J. Phys. Chem. B, 2006, 110, 9785–9790 CrossRef CAS
 .
.
- L. Tosheva, J. Parmetier, S. Saadallah, C. Vix-Guterl, V. Valtchev and J. Patarin, J. Am. Chem. Soc., 2004, 126, 13624–13625 CrossRef CAS
 .
.
- F. Zhang, Y. F. Shi, X. H. Sun, D. Y. Zhao and G. D. Stuchy, Chem. Mater., 2009, 21, 5237–5243 CrossRef CAS
 .
.
- J. Park, H. M. Zheng, Y. Jun and A. P. Alivisatos, J. Am. Chem. Soc., 2009, 131, 13943–13945 CrossRef CAS
 .
.
- H. X. Mai, Y. W. Zhang, L. D. Sun and C. H. Yan, Chem. Mater., 2007, 19, 4514–4522 CrossRef CAS
 .
.
- D. V. Bavykin and F. C. Walsh, J. Phys. Chem. C, 2007, 111, 14644–14651 CrossRef CAS
 .
.
- C. Y. Xu, L. Zhen, R. S. Yang and Z. L. Wang, J. Am. Chem. Soc., 2007, 129, 15444–15445 CrossRef CAS
 .
.
- J. B. Liang, R. Z. Ma, N. Iyi, Y. Ebina, K. Takada and T. Sasaki, Chem. Mater., 2010, 22, 371–378 CrossRef CAS
 ; W. H. Chen, Y. F. Yang, H. X. Shao and J. Fan, J. Phys. Chem. C, 2008, 112, 17471–17477 CrossRef CAS
; W. H. Chen, Y. F. Yang, H. X. Shao and J. Fan, J. Phys. Chem. C, 2008, 112, 17471–17477 CrossRef CAS  .
.
- A. J. Zarur and J. Y. Ying, Nature, 2000, 403, 65–67 CrossRef CAS
 ; S. A. Majetich and Y. Jin, Science, 1999, 284, 470–473 CrossRef CAS
; S. A. Majetich and Y. Jin, Science, 1999, 284, 470–473 CrossRef CAS  ; A. K. Gupta, R. R. Naregalkar, V. D. Vaidya and M. Gupta, Nanomedicine, 2007, 2, 23 CrossRef CAS
; A. K. Gupta, R. R. Naregalkar, V. D. Vaidya and M. Gupta, Nanomedicine, 2007, 2, 23 CrossRef CAS  ; S. P. Gubin, Y. A. Koksharov, G. B. Khomutov and G. Y. Yurkov, Russ. Chem. Rev., 2005, 74, 489 Search PubMed
; S. P. Gubin, Y. A. Koksharov, G. B. Khomutov and G. Y. Yurkov, Russ. Chem. Rev., 2005, 74, 489 Search PubMed  ; M. A. Willard, L. K. Kurihara, E. E. Carpenter, S. Calvin and V. G. Jarris, Int. Mater. Rev., 2004, 49, 125 Search PubMed
; M. A. Willard, L. K. Kurihara, E. E. Carpenter, S. Calvin and V. G. Jarris, Int. Mater. Rev., 2004, 49, 125 Search PubMed  .
.
- D. Portehault, S. Cassaignon, N. Nassif, E. Baudrin and J. Jolivet, Angew. Chem., Int. Ed., 2008, 47, 6441–6444 CrossRef CAS
 ; M. Yin and S. O'Brien, J. Am. Chem. Soc., 2003, 125, 10180–10181 CrossRef CAS
; M. Yin and S. O'Brien, J. Am. Chem. Soc., 2003, 125, 10180–10181 CrossRef CAS  ; W. S. Seo, H. H. Jo, K. Lee, B. S. J. Kim, J. T. Oh and Park, Angew. Chem., Int. Ed., 2004, 43, 1115–1117 CrossRef CAS
; W. S. Seo, H. H. Jo, K. Lee, B. S. J. Kim, J. T. Oh and Park, Angew. Chem., Int. Ed., 2004, 43, 1115–1117 CrossRef CAS  .
.
- P. Guardia, N. Pérez, A. Labarta and X. Batlle, Langmuir, 2010, 26, 5843–5847 CrossRef CAS
 ; X. L. Yu, C. B. Cao and X. Q. An, Chem. Mater., 2008, 20, 1936–1940 CrossRef CAS
; X. L. Yu, C. B. Cao and X. Q. An, Chem. Mater., 2008, 20, 1936–1940 CrossRef CAS  ; L. Lacroix, S. Lachaize, A. Falqui, M. Respaud and B. Chaudret, J. Am. Chem. Soc., 2009, 131, 549–557 CrossRef CAS
; L. Lacroix, S. Lachaize, A. Falqui, M. Respaud and B. Chaudret, J. Am. Chem. Soc., 2009, 131, 549–557 CrossRef CAS  .
.
- W. S. Seo, J. H. Shim, S. J. Oh, E. K. Lee, N. H. Hur and J. T. Park, J. Am. Chem. Soc., 2005, 127, 6188–6189 CrossRef CAS
 ; L. H. Zhuo, J. C. Ge and B. Tang, Cryst. Growth Des., 2009, 9, 1–6 CrossRef CAS
; L. H. Zhuo, J. C. Ge and B. Tang, Cryst. Growth Des., 2009, 9, 1–6 CrossRef CAS  ; L. F. Chen, J. C. Hu, R. Richard, S. Prikhodko and S. Kodambaka, Nanoscale, 2010, 2, 1657–1660 RSC
; L. F. Chen, J. C. Hu, R. Richard, S. Prikhodko and S. Kodambaka, Nanoscale, 2010, 2, 1657–1660 RSC  .
.
- I. W. Lenggoro, Y. Itoh, N. Iida and K. Okuyama, Mater. Res. Bull., 2003, 38, 1819–1827 CrossRef
 ; J. W. Lang, L. B. Kong, W. J. Wu, Y. C. Luo and L. Kang, Chem. Commun., 2008,(35), 4213–4215 RSC
; J. W. Lang, L. B. Kong, W. J. Wu, Y. C. Luo and L. Kang, Chem. Commun., 2008,(35), 4213–4215 RSC  ; H. Pang, Q. Y. Lu, Y. C. Li and F. Gao, Nanoscale, 2010, 2, 920–922 RSC
; H. Pang, Q. Y. Lu, Y. C. Li and F. Gao, Nanoscale, 2010, 2, 920–922 RSC  ; C. Z. Yuan, X. G. Zhang, L. H. Su, B. Gao and L. F. Shen, J. Mater. Chem., 2009, 19, 5772–5777 RSC
; C. Z. Yuan, X. G. Zhang, L. H. Su, B. Gao and L. F. Shen, J. Mater. Chem., 2009, 19, 5772–5777 RSC  .
.
- Q. Wu, F. Zhang, P. Xiao, H. S. Tao, X. Z. Wang and Z. Hu, J. Phys. Chem. C, 2008, 112, 17076–17080 CrossRef CAS
 ; G. A. Tai, J. X. Zhou and W. L. Guo, Nanotechnology, 2010, 21, 175601 CrossRef
; G. A. Tai, J. X. Zhou and W. L. Guo, Nanotechnology, 2010, 21, 175601 CrossRef  ; A. Filanlembo, S. Giorgio, I. Lisiecki and M. P. Pileni, J. Phys. Chem. B, 2003, 107, 7492–7500 CrossRef
; A. Filanlembo, S. Giorgio, I. Lisiecki and M. P. Pileni, J. Phys. Chem. B, 2003, 107, 7492–7500 CrossRef  ; S. Jana, I. C. Baek, M. A. Lim and S. Seok, J. Colloid Interface Sci., 2008, 322, 473–477 CrossRef CAS
; S. Jana, I. C. Baek, M. A. Lim and S. Seok, J. Colloid Interface Sci., 2008, 322, 473–477 CrossRef CAS  ; J. J. Ning, Q. Q. Dai, T. Jiang, K. K. Men, D. H. Liu, N. R. Xiao, C. Y. Li, D. M. Li, B. B. Liu, B. Zou, G. T. Zou and W. W. Yu, Langmuir, 2009, 25, 1818–1821 CrossRef CAS
; J. J. Ning, Q. Q. Dai, T. Jiang, K. K. Men, D. H. Liu, N. R. Xiao, C. Y. Li, D. M. Li, B. B. Liu, B. Zou, G. T. Zou and W. W. Yu, Langmuir, 2009, 25, 1818–1821 CrossRef CAS  .
.
- N. Amin, S. Agags and E. Matjevic, Phys. Status Solidi A, 1985, 104, K65
 .
.
- S. Jana, S. Basu, S. Pande, S. K. Ghosh and T. Pal, J. Phys. Chem. C, 2007, 111, 16272–16277 CrossRef CAS
 ; P. Umek, A. Gloter, M. Pregelj, R. Mominko, A. Zimina, M. Brzhezinskaya, A. Potočnik, A. Levstik and D. Arčon, J. Phys. Chem. C, 2009, 113, 14798–14803 CrossRef CAS
; P. Umek, A. Gloter, M. Pregelj, R. Mominko, A. Zimina, M. Brzhezinskaya, A. Potočnik, A. Levstik and D. Arčon, J. Phys. Chem. C, 2009, 113, 14798–14803 CrossRef CAS  ; W. Jiang, Y. Wu, B. He, X. B. Zeng, K. L. Lai and Z. W. Gu, J. Colloid Interface Sci., 2010, 347, 1–7 CrossRef CAS
; W. Jiang, Y. Wu, B. He, X. B. Zeng, K. L. Lai and Z. W. Gu, J. Colloid Interface Sci., 2010, 347, 1–7 CrossRef CAS  .
.
|
| This journal is © The Royal Society of Chemistry 2011 |
Click here to see how this site uses Cookies. View our privacy policy here. 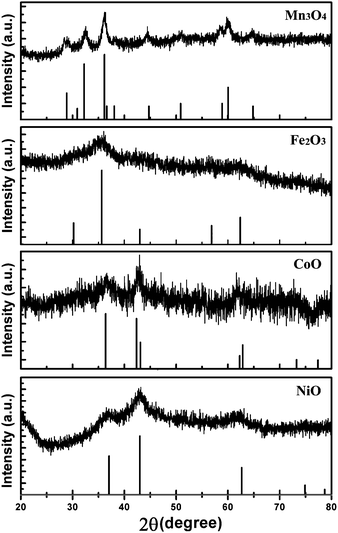





; G. Baldinozzi, D. Simeone, D. Gosset and M. Dutheil, Phys. Rev. Lett., 2003, 90, 216103 CrossRef CAS
.
.
.
.
.
.
.
.
.
.
.
.
.
.
; W. H. Chen, Y. F. Yang, H. X. Shao and J. Fan, J. Phys. Chem. C, 2008, 112, 17471–17477 CrossRef CAS
.
; S. A. Majetich and Y. Jin, Science, 1999, 284, 470–473 CrossRef CAS
; A. K. Gupta, R. R. Naregalkar, V. D. Vaidya and M. Gupta, Nanomedicine, 2007, 2, 23 CrossRef CAS
; S. P. Gubin, Y. A. Koksharov, G. B. Khomutov and G. Y. Yurkov, Russ. Chem. Rev., 2005, 74, 489 Search PubMed
; M. A. Willard, L. K. Kurihara, E. E. Carpenter, S. Calvin and V. G. Jarris, Int. Mater. Rev., 2004, 49, 125 Search PubMed
.
; M. Yin and S. O'Brien, J. Am. Chem. Soc., 2003, 125, 10180–10181 CrossRef CAS
; W. S. Seo, H. H. Jo, K. Lee, B. S. J. Kim, J. T. Oh and Park, Angew. Chem., Int. Ed., 2004, 43, 1115–1117 CrossRef CAS
.
; X. L. Yu, C. B. Cao and X. Q. An, Chem. Mater., 2008, 20, 1936–1940 CrossRef CAS
; L. Lacroix, S. Lachaize, A. Falqui, M. Respaud and B. Chaudret, J. Am. Chem. Soc., 2009, 131, 549–557 CrossRef CAS
.
; L. H. Zhuo, J. C. Ge and B. Tang, Cryst. Growth Des., 2009, 9, 1–6 CrossRef CAS
; L. F. Chen, J. C. Hu, R. Richard, S. Prikhodko and S. Kodambaka, Nanoscale, 2010, 2, 1657–1660 RSC
.
; J. W. Lang, L. B. Kong, W. J. Wu, Y. C. Luo and L. Kang, Chem. Commun., 2008,(35), 4213–4215 RSC
; H. Pang, Q. Y. Lu, Y. C. Li and F. Gao, Nanoscale, 2010, 2, 920–922 RSC
; C. Z. Yuan, X. G. Zhang, L. H. Su, B. Gao and L. F. Shen, J. Mater. Chem., 2009, 19, 5772–5777 RSC
.
; G. A. Tai, J. X. Zhou and W. L. Guo, Nanotechnology, 2010, 21, 175601 CrossRef
; A. Filanlembo, S. Giorgio, I. Lisiecki and M. P. Pileni, J. Phys. Chem. B, 2003, 107, 7492–7500 CrossRef
; S. Jana, I. C. Baek, M. A. Lim and S. Seok, J. Colloid Interface Sci., 2008, 322, 473–477 CrossRef CAS
; J. J. Ning, Q. Q. Dai, T. Jiang, K. K. Men, D. H. Liu, N. R. Xiao, C. Y. Li, D. M. Li, B. B. Liu, B. Zou, G. T. Zou and W. W. Yu, Langmuir, 2009, 25, 1818–1821 CrossRef CAS
.
.
; P. Umek, A. Gloter, M. Pregelj, R. Mominko, A. Zimina, M. Brzhezinskaya, A. Potočnik, A. Levstik and D. Arčon, J. Phys. Chem. C, 2009, 113, 14798–14803 CrossRef CAS
; W. Jiang, Y. Wu, B. He, X. B. Zeng, K. L. Lai and Z. W. Gu, J. Colloid Interface Sci., 2010, 347, 1–7 CrossRef CAS
.
