Made-to-order nanocarbons through deterministic plasma nanotechnology
Yuping
Ren
ab,
Shuyan
Xu
a,
Amanda Evelyn
Rider
cd and
Kostya (Ken)
Ostrikov
*dc
aPlasma Sources and Applications Centre, NIE and Institute of Advanced Studies, Nanyang Technological University, 1 Nanyang Walk, 637616, Singapore
bDepartment of Advanced Material Technology Development, Globalfoundries, Singapore 738406
cComplex Systems, School of Physics, The University of Sydney, Sydney, New South Wales 2006, Australia
dPlasma Nanoscience Center Australia (PNCA), CSIRO Materials Science and Engineering, P.O. Box 218, Lindfield, New South Wales 2070, Australia. E-mail: Kostya.Ostrikov@csiro.au
First published on 16th November 2010
Abstract
Through a combinatorial approach involving experimental measurement and plasma modelling, it is shown that a high degree of control over diamond-like nanocarbon film sp3/sp2 ratio (and hence film properties) may be exercised, starting at the level of electrons (through modification of the plasma electron energy distribution function). Hydrogenated amorphous carbon nanoparticle films with high percentages of diamond-like bonds are grown using a middle-frequency (2 MHz) inductively coupled Ar + CH4 plasma. The sp3 fractions measured by X-ray photoelectron spectroscopy (XPS) and Raman spectroscopy in the thin films are explained qualitatively using sp3/sp2 ratios 1) derived from calculated sp3 and sp2 hybridized precursor species densities in a global plasma discharge model and 2) measured experimentally. It is shown that at high discharge power and lower CH4 concentrations, the sp3/sp2 fraction is higher. Our results suggest that a combination of predictive modeling and experimental studies is instrumental to achieve deterministically grown made-to-order diamond-like nanocarbons suitable for a variety of applications spanning from nano-magnetic resonance imaging to spin-flip quantum information devices. This deterministic approach can be extended to graphene, carbon nanotips, nanodiamond and other nanocarbon materials for a variety of applications
1. Introduction
Nanocarbons1–4 in a range of structural configurations including graphene,5–8nanotubes,9–15nanowires,16, 17 nanocones,18 nanotips19–21nanofibres22 and a wealth of other variations have been the subject of intense research interest over the last few decades due to their unique mechanical, optical, electronic and other properties. Whilst some structures, e.g., nanotubes are ‘holey’ with factors like chirality affecting their behavior,23 nanocarbons that are fully filled such as nanotips, nanoparticlesetc. contain mixtures of sp2- and sp3-hybridized carbons, the relative amounts of which, to a great extent control their properties.It has been noted that sp3-hybridized carbons are largely responsible for the elastic and mechanical properties of nanocarbons such as diamond-like nanocarbon (DLNC), whereas the optical and electronic properties are controlled by the clustering of the sp2 phase.24,25 The ability to tailor the ratio of sp3 to sp2 bonds so the properties of the film are suited for a specific application is crucial. For example, some applications require water repellent coatings/lubricants, Paul et al.26 found that hydrophobicity in DLNC films increases with higher sp3/sp2 ratios. In DLNC films, high compressive stress limits applications to use of films of thickness 100 nm or less,27 however, Cheng et al. found that decreases in the sp3/sp2 ratio led to a reduction in internal stress.28 As the sp2 clustering in particular, is highly dependent on the specifics of the growth environment,25 choosing the most flexible and controllable fabrication technique is key to the efficient manufacturing of made-to-order diamond-like nanocarbon films for a broad range of applications spanning from nanoelectromechanical systems, to magnetic hard disc coatings to biomedical coatings for joint implants.29 Moreover, by achieving high percentages of sp3carbon in nanoparticles, one can produce nanodiamond clusters,30,31 which are particularly promising materials for nano-magnetic resonance imaging32 and spintronics.33 However, enabling sp3/sp2 control simultaneously with optimization of nanodiamond size and structure remains extremely challenging.
Many fabrication methods including ion beam deposition,34magnetron sputtering,26 filtered cathodic vacuum arc35 and chemical vapor deposition (CVD)36 have been used to make nanocarbon films. Inductively coupled plasma (ICP) CVD is a promising growth route as ICPs have a high plasma density, low ion bombardment energies (hence less damage to the forming film) and excellent uniformity.37–39 Reactive plasmas40,41 consist of a diverse range of species which undergo many chemical reactions in the plasma bulk.42,43 Some of the newly created radicals and ions, however, may detract from the desired film structure, composition and properties. Hence, intense modeling efforts are required to account for species production and loss,19,44 to enable us to optimize the production of plasma-generated species that will enhance rather than detract from the intended properties of ‘made-to-order’ diamond-like nanocarbon films.
However, despite numerous plasma modeling papers19,44 and experimental examinations of DLNC films24,26,34,45 the relationship between sp3/sp2 concentration in the gas phase (or plasma influx) and in the deposited nanoparticle film has not been sufficiently developed. Clearly elucidating this link, with particular attention to the effect of plasma parameters such as discharge power and hydrocarbon source gas flow rate and thus proposing a comprehensive combinatorial46 approach to DLNC nanoparticle film growth are the main aims of this paper. This approach incorporates theory, simulation and experiment and serves as an important road mark on the way to deterministic plasma fabrication of made-to-order diamond-like nanocarbon films and may ultimately be extended to produce highly tailored nanodiamonds and other nanocarbon films including graphene. For nanostructured films manufactured using plasma-based fabrication techniques, a high level of control is required even at the level of electrons (fostering such a capability was highlighted as a grand scientific challenge by the US Department of Energy47). In this work, we show that such a level of control may be exercised by modifying plasma parameters to fine tune the electron energy distribution function (EEDF) which influences the production of ions and neutral radicals in the plasma bulk.38 These plasma-generated species in turn, influence the film composition (i.e., sp3/sp2 ratio) and hence the film properties and potential applications.
The paper is structured as follows: the experimental set-up and methodology are presented in Section 2, with the results of a global plasma discharge model, plasma diagnostics and DLNC nanoparticle film deposition described in Section 3. A further discussion and comparison of the results is presented in Section 4, whereas Section 5 is a summary and outlook for future work.
2. Experiment and methodology
This section begins with a flow chart (Sec. 2.1) which provides an overview of the steps required to get from a Ar +CH4 plasma to a tailored DLNC nanoparticle film. Subsequent sections 2.2, 2.3 and 2.4 describe the physical experiment (including the deposition system and plasma diagnostics), the global discharge model and the characterization of the DLNC films, respectively, in greater detail.2.1. Process flowchart
A flow chart provided in Fig. 1 details the links between the experiment, theory and simulation tasks described in more detail in Sections 2.2, 2.3 and 2.4. The steps required to get from an Ar + CH4 ICP (described in Sec. 2.2) to a tailored diamond-like nanocarbon thin film are as follows. Firstly, the EEDF is calculated based on the measured plasma parameters. Electron temperature and number density (Te and ne, respectively) are derived from the EEDF in Step P1, which leads to the first decision - will ne and Te result in appropriate rates of electron impact reactions (Ke)? If not, the EEDF must be adjusted by varying the process parameters (e.g., Pin). If the resultant Ke is acceptable, it will be input into the global discharge model (described in Sec. 2.3) which will calculate the expected sp3/sp2-hybridized precursor species densities in the plasma discharge. The projected gas phase sp3/sp2 ratio from the global discharge model is calculated in Step P2.![. (Color online) Flow-chart for design of DLNC thin films. The red dashed line indicates one of the aims of this paper - to link the sp3/sp2 ratio in the gas phase to the sp3/sp2 ratio in the thin film (see in Sec. 3.4). Steps [P1] - [P4] are as follows: [P1] is the derivation of ne and Te from the EEDF, [P2] is calculation of the sp3/sp2 ratio based on the global discharge model, [P3] is the calculation of the sp3/sp2 ratio based on the plasma diagnostics measurements and [P4] is the measurement of the sp3/sp2 ratio in the film using Raman spectroscopy and/or XPS. See Secs. 2.1–2.4 for further details.](/image/article/2011/NR/c0nr00718h/c0nr00718h-f1.gif) | ||
| Fig. 1 . (Color online) Flow-chart for design of DLNC thin films. The red dashed line indicates one of the aims of this paper - to link the sp3/sp2 ratio in the gas phase to the sp3/sp2 ratio in the thin film (see in Sec. 3.4). Steps [P1] - [P4] are as follows: [P1] is the derivation of ne and Te from the EEDF, [P2] is calculation of the sp3/sp2 ratio based on the global discharge model, [P3] is the calculation of the sp3/sp2 ratio based on the plasma diagnostics measurements and [P4] is the measurement of the sp3/sp2 ratio in the film using Raman spectroscopy and/or XPS. See Secs. 2.1–2.4 for further details. | ||
Note, as indicated by the red dashed line in Fig. 1, one of the main aims of this paper is to elucidate the link between sp3/sp2 ratios in the plasma bulk and in the DLNC nanoparticle film. This will provide us with an indication of what the sp3/sp2 gas phase ratio should be in order to produce the desired sp3/sp2 ratio in the film (this will be addressed in Sec. 3.4). If the plasma sp3/sp2 is not appropriate, then return to the EEDF stage. If the ratio is acceptable, proceed to experimental measurements [Optical emission spectroscopy (OES) and Quadrupole mass spectroscopy (QMS)] of the species densities in the plasma discharge and in Step P3 calculate the actual gas phase sp3/sp2 ratio using the measured data. The ratio in Step P3 should be close to the ratio measured in Step P2, if not, return to the global discharge model and recalculate. If the ratio is acceptable, commence ICP-CVD deposition of the film and in Step P4 measure the film sp3/sp2 ratio using Raman spectroscopy and/or X-ray photoelectron spectroscopy (XPS). If the film sp3/sp2 ratio is not acceptable, return to the global discharge model stage. However, if the sp3/sp2 is as anticipated, then a diamond-like nanocarbon film with the desired properties should be obtained.
2.2. Experimental details
A middle frequency (2MHz) inductively coupled plasma source,38 depicted both schematically [Fig. 2 (a)] and pictorially [Fig. 2 (b)] was used to deposit DLNC nanoparticle films on Si (111) substrates with varying input power (Pin) and methane flow rate (JCH4). The DC bias voltage applied to the substrate holder was used to control the ion flux and energy, in this work it was maintained at −50 V. The temperature of the substrate holder is an important parameter for neutral particle flux and was kept at 300 °C. The deposition time was 40 min.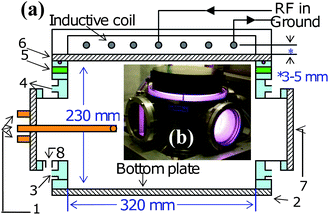 | ||
| Fig. 2 (Color online) (a) Schematic of the ICP experimental set-up, where 1: Diagnostic ports for Langmuir probe, OES and QMS measurements; 2: Vacuum chamber; 3: Cooling water in; 4: Cooling water out; 5: Gas inlet; 6: Quartz window; 7: Observation window; 8: To vacuum pump. (b) Photograph of the ICP source operating in H-mode Ar discharge. | ||
A single Langmuir probe was used to obtain the I–V curve, the second derivative of which is proportional to the EEDF, fe(V), which can be expressed as follows:
 | (1) |
where A is the surface collection area of the single radio frequency (RF)-compensated Langmuir probe (where the RF compensation was specifically designed for the 13.56 MHz operation frequency), e is the electron charge and me is the electron mass. The electron number density and temperature, ne and Te may be extracted from the EEDF (Step P1 from Fig. 1) as follows:
| ne(ε) = ∫∞0fe(ε)dε | (2) |
 | (3) |
The optical emission was measured by inserting a collimator into the holes at the sideport of the ICP chamber (recall Fig. 2 (a), port is designated as 1), the collected optical emission signal was transferred via optical fiber to the entrance slit of the SpectroPro-775 spectrometer (with a line resolution of 0.023 nm). Data acquisition was controlled by the SpectraSenser program. The Quadrupole Mass Analyzer system used to conduct QMS was “Microvision Plus” (manufactured by MKS Instrument Spectra Products), with data acquisition controlled by “Process Eye 2000” software.
2.3. Global discharge model
Electron impact ionization and dissociation, neutral–neutral reactions, neutral–ion reactions, proton impact ionization, and dissociative recombination reactions were included in a global, spatially-averaged model (190 reactions, 48 neutral and ionic species) which was used to predict the particle densities (fluxes of CH+4 and CH+3 obtained from the simulation are included in Table 1) for Ar + CH4 plasmas. Supported by previous experimental measurements,48,49 the plasma density was assumed to be uniform in the processing chamber. The rate constants for electron impact reactions were Ke = ∫f(ε)σ(ε)νe(ε)d(ε), where νe(ε) is the electron velocity, f(ε) is the EEDF and σ(ε) is the reaction cross section. As a weakly ionized Ar + CH4 (electropositive) plasma was considered, anions were not accounted for and it was assumed that upon ionization neutral particles would be singly ionized.| P in | n e | T e | CH+4 | CH+3 | sp3/sp2 (XPS) [%] |
| 400 | 1.29 | 3.78 | 7.63 | 6.61 | 7.1 |
| 800 | 2.53 | 3.64 | 7.44 | 6.48 | 12.0 |
| 1200 | 3.59 | 3.53 | 6.72 | 5.80 | 17.2 |
| 1600 | 4.41 | 3.75 | 6.43 | 5.50 | 19.0 |
| 2000 | 4.85 | 3.76 | 5.83 | 4.94 | 21.3 |
Some carbon containing species may be sp2- or sp3-hybridized in the gas-phase. As noted, the ratio of sp3- to sp2-hybridized carbons in nanomaterials can be used to tailor structure and properties to ensure that the resulting materials conform to the requirements of a given application. This paper seeks to establish a link between the EEDF, the hybridization in the gas-phase/plasma-bulk to hybridization in the DLNC film (as indicated by the red line in Fig. 1). Hence in our discussion of the plasma influx (both experimentally measured and simulated) we will focus on the following sets of species [CH+4, C2H+6, C2H5] and [CH3, CH+3, C2H+5, CH2, C2H3] as representative of plasma-generated species containing sp3 and sp2-hybridized carbons, respectively (this will be elaborated on in Sec. 4). Non-radical neutrals were not considered to directly insert into films as their sticking coefficients were assumed zero due to their saturated electronic structure.50
2.4. Characterisation of DLNC films
The sp3/sp2 ratio of DLNC thin films was estimated by Raman measurement and XPS (Step P4 in Fig. 1), compared to the plasma influx sp3/sp2 ratio and related to plasma parameters: input power, Pin and methane inlet, JCH4. X-ray diffraction was used to examine the effect of the plasma parameters on the crystalline structure of the samples.3. Results
3.1. Electron energy distribution
The effect of increasing Pin on the EEDF is an increase in the number of electrons, including those in the high-energy tail of the distribution - shown in the inset in Fig. 3. The electron number density, ne and temperature, Te have been derived from the EEDF and are listed in Table 1. The number and temperature of electrons (particularly those in the high-energy tail as the threshold energy for electron impact reactions, including ionization and dissociation, is typically around 10 eV as indicated by the dashed line in Fig. 3), influence the rate constants for electron impact reactions, Ke. Explicitly, Ke increases with Pin as f(ε) increases with ne and νe(ε) increases with Te. If Ke increases, then more electron impact reactions will occur which will affect the relative densities of hydrocarbon ions and neutral radical species in the plasma bulk, the sp3/sp2 ratio in the plasma-generated flux incident to the substrate and hence, ultimately, the sp3/sp2 ratio in the DLNC nanoparticle films. The EEDF was used for the calculation of Ke in the global discharge model described in Sec. 2.3.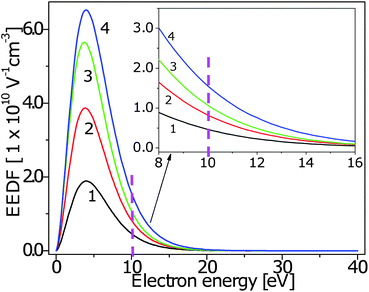 | ||
| Fig. 3 (Color online) The experimental EEDF of Ar + CH4 ICP at 1.5 Pa, JAr = 35 sccm and JCH4 = 8 sccm, inset shows a high-energy tail of the EEDF. Pin varies as follows 1: 400 W, 2: 800 W, 3: 1200 W, 4: 1600 W. EEDF calculated using I–V measurements obtained by a single RF-compensated Langmuir probe. Dashed line shows a typical energy threshold for electron impact reactions. | ||
3.2. Plasma generated species
Fig. 4 presents the results both of the global discharge model and data from the experimentally produced Ar + CH4 discharge, specifically, the effect of input power, Pin on the densities of ions and neutral radicals in the plasma bulk. The results of the Optical Emission Spectroscopy and Quadrupole Mass Spectrometry of a 2 MHz ICP plasma (JAr = 35 sccm, JCH4 = 8 sccm) discharge in a Ar + CH4 gas mixture at 1.5 Pa with different input powers (0 – 2000 W) are presented in Fig. 4 (a) and (b), respectively. For the majority of the 48 species included in the global model, the OES and QMS results are largely consistent with number density tendencies found in the modeling. Experimentally and computationally obtained neutral radical and ion fluxes are presented in Fig. 4 (c) and (d), respectively. | ||
| Fig. 4 (Color online). (a) OES relative intensity of species α to Ar (416 nm), Ia/IAr_416, with different Pin. The legend represents neutral radicals as follows: 1: CH(387), 2: H2(420), 3: CH(431), 4: Hγ (434), 5: H2(471), 6: Hβ (486), 7: C2(517), 8: Hα (656), 9: C(833). (b) The partial pressures of radicals where JAr = 35 sccm and JCH4 = 8 sccm at 1.5 Pa with varying Pin. The fluxes (cm−2s−1) of (c) radicals containing sp3 and sp2 carbons, (d) ions containing sp2 and sp3 carbons, where: 1: CH+4 (sp3), 2: CH+3 (sp2), 3: C2H+5 (sp2), 4: C2H+6 (sp3). | ||
The relative intensity of OES lines of species α to Ar (416 nm) lines, Iα/IAr_416, with different Pin up to 2000 W is plotted in Fig. 4 (a). The intensities of the optical emission lines with varying Pin serves as a qualitative representation of the neutral radicals densities and enables us to verify the validity of the global discharge model without interfering with the ongoing discharge. The OES results in Fig. 4 (a) show an increase of intensities of Hβ and Hα emissions and a decline of intensities of CH, H2, Hγ, C2 and C lines with increasing Pin. Whilst these species are not the ones that we have singled out for analysis in Fig. 4 (c) and (d), they may play an important role in determining the concentration of relevant sp3 and sp2 hybridized hydrocarbons through reactions in the plasma discharge. We emphasize that the OES results are in a good level of agreement with species densities predicted by the global discharge model (specific comparisons are omitted for space considerations).
The partial pressures of C, CH, H, CH2, C2H5 and CH3versusPin measured by QMS are plotted in Fig. 4 (b). We will single out CH2, C2H5 and CH3 for the purpose of this discussion. The density of a CH2 radical initially decreases with increased Pin until Pin = 1200 W, when it begins to rise with further increased Pin. As in Fig. 4 (c), the QMS results show that the concentration/flux of C2H5 is much lower than the other species of interest. The density of a methyl radical is shown by QMS to increase with Pin until 1200 W, whereupon it begins to decrease. For radicals, the flux plotted in Fig. 4 (c) is calculated by Ψj = 0.25njνth,iγj, where γj is the sticking coefficient, νth,j is the thermal speed and nj is the radical density. Fluxes of all species appear to decrease with greater Pin except for CH2 which increases. There is better agreement between the CH3 and CH2 radical fluxes and QMS results at higher powers. Fig. 4 (d) presents ion fluxes - both from simulation and experimentally obtained by measuring the current on the substrate and taking into account the relative intensity ratio of the ions. It should be noted that the ion fluxes are comparable, if not higher than the plotted neutral radicals in Fig. 4 (c). This, combined with the higher sticking probability of ions versus radicals points to the importance of the ion flux to the deposited film properties.
The simulated fluxes with JCH4 varying between 8 and 32 sccm, with Pin = 1600 W are plotted in Fig. 5. Unsurprisingly, with increased methane influx, all species increase with increasing JCH4, excepting CH2 which decreases. This decrease could be due to more chemical reactions involving CH4 favouring consumption of CH2 rather than production.
 | ||
| Fig. 5 (Color online). Ion/radical number densities (simulated) with varying methane flow rate, where Pin is 1600 W and 1: CH3(sp2), 2: CH2(sp2), 3: C2H3 (sp2), 4: CH+4 (sp3), 5: CH+3 (sp2), 6: C2H5 (sp3), 7: C2H+5 (sp2), 8: C2H+6 (sp3). | ||
3.3. DLNC films
Fig. 6 presents scanning electron microscope (SEM) images of DLNC films with varied discharge power and methane inlet. The SEM image at low power [Fig. 6 (a)] shows uniform coverage of nanoparticles with an approximate size of 40 nm. The cobble-like nanoparticles congregate into a film, with average nanoparticle size increasing to approximately 100 nm at 1600 W as shown in Fig. 6 (b). It can be clearly observed from SEM micrographs that lower Pin and higher JCH4 [Fig. 6 (a) and (c)] result in a more graphite-like surface morphology (indicating a higher percentage of sp2 bonding, see Fig. 7 for further justification). On the other hand, at lower JCH4 and higher Pin, Fig. 6 (b) shows the emergence of some faceted diamond-like crystalline structure (sp3 bonding). This is emphasized in Fig. 6 (e) which is a zoomed-in inset of Fig. 6 (b) and shows a clear, faceted structure, indicating the increased crystallinity (crystallinity of the samples will be discussed further using X-ray diffraction (XRD) results plotted in Fig. 7).![(Color online) SEM images of DLNC thin films on Si(111) deposited at: (a) Pin = 400 W, JCH4 = 8 sccm (b) Pin = 1600 W, JCH4 = 8 sccm (c) Pin = 1600 W with JCH4 = 32 sccm. (d) XPS where Pin = 400 W, JCH4 = 8 sccm, legend as follows: 1) Pristine peak, Deconvoluted peaks: 2) C-sp2, 3) C-sp3, 4) C–O, 5: C = 0, (e) Zoomed in inset of (b) showing crystalline facets [sp3]. (f) XPS where Pin = 1600 W, JCH4 = 32 sccm, legend as in (d).](/image/article/2011/NR/c0nr00718h/c0nr00718h-f6.gif) | ||
| Fig. 6 (Color online) SEM images of DLNC thin films on Si(111) deposited at: (a) Pin = 400 W, JCH4 = 8 sccm (b) Pin = 1600 W, JCH4 = 8 sccm (c) Pin = 1600 W with JCH4 = 32 sccm. (d) XPS where Pin = 400 W, JCH4 = 8 sccm, legend as follows: 1) Pristine peak, Deconvoluted peaks: 2) C-sp2, 3) C-sp3, 4) C–O, 5: C = 0, (e) Zoomed in inset of (b) showing crystalline facets [sp3]. (f) XPS where Pin = 1600 W, JCH4 = 32 sccm, legend as in (d). | ||
 | ||
| Fig. 7 (Color online) Raman spectra of DLNC thin films on Si(111) varying as a function of (a) Pin,(b) JCH4. D and G peak positions marked. Increase of ID/IG with Pin clearly visible. XRD patterns with varying (c) Pin and (d) JCH4. | ||
This illustrates that the sp3/sp2 ratio is higher for lower methane flow rates. The observation of more sp2 bonding for lower Pin [Fig. 6 (a)] and higher JCH4 [Fig. 6 (c)] is supported by X-ray photoelectron spectroscopy (XPS) measurements in Fig. 6 (d) and (f), respectively. The XPS spectra both show a strong contribution of the C-sp2 peak [series 2] to the experimental data, in marked contrast to a relatively weak contribution from the C-sp3 peak [series 3]. The sp3/sp2 ratio may be calculated by taking the ratio of the areas under the sp2 and sp3 peaks. For Fig. 6 (d) it is 7.1%, whereas for 1600 W [Fig. 6 (f)] it is 19.0% (see Table 1). Hence, we can conclude that high methane flow rates and low Pin result in a lower sp3/sp2 ratio in the DLNC films.
For varying power, the behavior of the sp3 fraction of the films may also be derived from the visible Raman spectra plotted in Fig. 7 (a), application of the 3 stage model of Ferrari and Robertson24 and X-ray diffraction results [Fig. 7 (b)]. The ratio of the D peak to the G peak (ID/IG) clearly increases with Pin. Hence we recognize that the films are in stage 2 (nanocrystalline[NC]-graphite to a-C), thus the sp3 fraction is between 10% ∼ 20% and qualitatively note that the sp3/sp2 will increase with Pin [in agreement with the conclusion drawn from the XPS in Fig. 6 (d) and (f)]. Crystallinity is also an important factor in tailoring film properties, the only direct information of crystalline graphite is the weak peak G(002) at 2θ = 26.2° shown for the 1600 W series, but not observable for the 2000 W series in Fig. 7 (b). Hence, it could be argued that XRD also shows a shift from NC-graphite to a-C with increasing Pin (supporting our classification of the films being in stage 2 of the Ferrari-Robertson model24). Therefore, the sp3 fraction of carbon thin films indeed strongly depends on the input power.
The corresponding Raman and XRD spectra for the varying JCH4 case are shown in Fig. 7 (c) and (d), respectively. The slight shift of the G peak in Fig. 7 (c) towards lower wavenumbers with increased JCH4 indicates that the composition of the film is changing from amorphous carbon to NC-graphite, placing it in stage 2 of the Ferrari-Robertson model,24 predicting a decline of sp3/sp2 with increased JCH4. The trend towards a NC-graphite structure with greater methane fraction is supported by the XRD spectra presented in Fig. 7 (d), specifically, the clear increase of the (101) and (202) phases for graphite at 2θ = 44.4° and 51.4°, respectively. The increase in intensity of the D and G peaks with increased JCH4 in the Raman spectra in Fig. 7 (c) leads us to conclude that the increase of the peaks at 2θ = 44.4° and 51.4° in the XRD spectra is due to graphite - the G(101) and G(202) phases, respectively. These results suggest that sp2 content increases with JCH4, whereas sp3 content decreases.
3.4. Comparison of sp3/sp2 for influx species and DLNC films
Recall from Fig. 1 that one of the primary aims of this paper was to establish a link between the sp3/sp2 ratio in the gas phase to the sp3/sp2 ratio in the DLNC film. Fig. 8 compares the normalized sp3/sp2 ratios in the XPS-measured films to the measured (Langmuir probe) and calculated (global discharge model) plasma species densities. The effect of discharge power, Pin is considered in Fig. 8 (a). The film composition is normalized with respect to the sp3/sp2 ratio (0.213) at 2000 W, whereas, the fluxes of the plasma species are normalized with respect to the sp3/sp2 ratio at 2000 W derived from the measured and simulated fluxes. For the purpose of this work, the following ion and neutral radical species were taken into account when calculating the sp3 and sp2 contributions - (sp3): CH+4, C2H+6, C2H5 and (sp2): CH3, CH+3,C2H+5,CH2,C2H3. CH4 flow rate.](/image/article/2011/NR/c0nr00718h/c0nr00718h-f8.gif) | ||
| Fig. 8 (Color online). The normalized sp3/sp2 ratio in the total fluxes from model analysis and the bond ratio in the DLNC thin film by XPS measurement with varying (a) input power [E refers to experimentally measured ion flux, T refers to ion fluxes obtained through the global discharge model](b) CH4 flow rate. | ||
It is shown that sp3/sp2 increases as Pin is increased from 400 to 2000 W, indicating that the relative amount of sp3 increases as power is increased. It is observed that the normalized sp3/sp2 ratio for the plasma species increases less rapidly than film composition at high sp3/sp2 and high Pin. Similarly, the effect of CH4 flow rate was considered both on the plasma species and the film composition in Fig. 8 (b). The sp3/sp2 ratio was normalized with respect to the maximum value obtained at 8 sccm (19%). It can be seen that sp3/sp2 decreases as JCH4 increases [supported by Fig. 6 (b) and (c)], with increased disparity between sp3/sp2 for the plasma species and film composition at higher methane inputs. Hence, the normalised sp3/sp2 ratio for the film composition decreases at more marked rate than the plasma species. Given the correspondence between the sp3/sp2 ratio behaviour of the plasma species and film composition, the species density variation with plasma parameters may be used to predict the sp3/sp2 ratios for DLNC films as indicated in Fig. 1.
4. Discussion
As mentioned in Sec. 3, whilst the global discharge model accounts for 48 species, we singled out the following eight ion and neutral radical species for analysis due to their relative abundance in the discharge: CH+4, CH3, C2H+6, C2H5, CH+3, C2H+5, CH2 and C2H3. Recall, non-radical neutrals were not considered to directly contribute to developing films as their sticking coefficients are close to zero due to their saturated electronic structure.50 We will now expand on our assignation of various species as sp2- or sp3-hybridized. hybridization is a fundamental issue for carbon-containing species, however, it is not always entirely straightforward. The methyl radical, CH3, for example, has been assigned as sp2-hybridized.51 It has a planar structure,52 with the unpaired electron residing in a vacant p orbital and the 3 sp2 orbitals doubly occupied.51 Qualitatively, the sp2 orbitals are more stable in this case as the electrons will be closer to the nucleus - i.e., the sp2 orbitals have more of an ‘s’ character than sp3.51 This assignment is supported in the literature by both theoretical (Molecular Orbital Theory53) and experimental data (Infrared spectroscopy,54 microwave studies,55etc.). It is, however, noted in Electron Spin Resonance studies56 that whilst the trigonal planar structure is more stable, there is only a small additional amount of energy needed to favour a shallow pyramidal (sp3) structure.57 Note, the methyl cation (CH+3) is also classified as sp2,58 whilst the methane cation (CH+4), by contrast, is clearly sp3 (pyramidal).59One indication of sp2 -hybridization is the presence of a π-bond. Whilst this is indeed the case for the vinyl radical (C2H3) which is documented as sp2 hybridized,57,60–62 the distinction is not always so clear cut. For example, the CH2 radical does not have a double bond, yet it is clearly sp2-hybridized (even though it has a ‘bent’ structure, it can be rotated so it is planar). The ethyl cation (C2H+5), in the gas phase, exhibits a bridge structure (with a π-bond) as this is a more stable configuration than the classical open isomer,57 thus we have classified it as sp2. It should be noted that this cation is hyperconjugated63 and hence exhibits a higher degree of stability than a non-hyperconjugated structure.
The ethyl radical and C2H+6 in particular, bear mentioning due to their asymmetrical nature - only one side may be definitively designated as sp3 hybridized, the other is unknown - it bears mentioning, however, that the ethyl radical has been widely studied and it has been noted that the radical centre should be non-planar in terms of stability arguments.64 The asymmetry of these two species is not accounted for in our model and both have been assigned as sp3.
A common belief is that the methyl radical is the most important species for DLNC deposition,65–67 this is the view put forward by Shiratani et al.67 who found that CH3 contributed a maximum of 60% to film growth and Teii et al.68 who noted that CH3 represented the major adspecies in diamond CVD. It has also been stated that the methyl radical is the most important species in promoting diamond-like (or sp3) bonds in deposited films,69,70 this is despite its sp2 hybridization. An alternative interpretation is that of Dagel et al., who numerically and experimentally considered hard carbon film growth in RF methane plasmas66 and concluded that whilst CH3 was important, it did not appreciably contribute to a-C:H film growth, rather it was of interest as it produced C2H6 through the following recombination reaction 2CH3→C2H6.66Ethane (a non-radical neutral, hence not directly incorporated in the film) could undergo dissociation reactions in the plasma, the products of which (i.e.C2H5, C2H+5etc.) contribute to film growth. Recall from Table 1 that the electron number density increases with Pin. This implies that more electron impact reactions are likely to take place, i.e., ionization such as e− + C2H6→C2H+6 + 2e−, e− + C2H6→C2H+5 + H + 2e− and dissociation such e− + C2H6→C2H5 + H + e−. So whilst C2H6 may not directly contribute to film growth, its ions and radical products created through increased electron impact reactions can incorporate in the film. In this way the density of CH3 (sp2) can be linked with the densities of C2H+6 (sp3), C2H+5 (sp2) and C2H5 (sp3) and hence the sp3/sp2 gas phase ratio may be compared to the sp3/sp2 ratio in the film.
As noted in Sec. 3, the fluxes of ion species were comparable with the fluxes of neutral radicals. This is because applied external voltage [-50 V] accelerated cations and remarkably increased the ion flux. The increased flux, in combination with their higher (than radical) sticking coefficients (which in most cases are close to unity71) points to importance of ion species in determination of the film composition/properties. This is in line with the investigation of Miyagawa et al.72 who considered the effect of the ion(CH+3)/neutral radical (CH3) arrival ratio on the sp3 fraction of DLNC films in a plasma-based ion implantation process using a Monte Carlo simulation. They found that higher sp3 fractions were obtained for higher ion/neutral ratios at lower ion energies (∼50–150 eV).
What happens on the surface, i.e. film formation, whilst influenced by the plasma chemistry is not solely determined by it. We also have to consider how radicals/ions incorporate into the growing film, the influence of substrate conditions, etc. An in-depth modelling of reactions (i.e., radical recombinations, adsorption, desorption, energy transfer in plasma-surface interactions, etc.) on the surface of forming DLNC films was beyond the scope of this study. However, using logic and similar available studies52,65,66,72 we can qualitatively describe what is likely to happen on the surface under relevant experimental conditions.
The plasma species upon arriving at the surface of the forming film will undergo surface reactions, i.e., adsorption, desorption etc.Hydrogen atoms from the gas phase remove surface H [H(gas) + H(surface) → H2(gas)] thus creating dangling bonds which can then either be passivated by atomic H or act as radical sites for the chemisorption of hydrocarbon radicals/ions.73 Similarly, Ar+ may also serve to activate the surface and create dangling bonds.44 Radicals may undergo recombination and be released (as non-radical neutrals) back into the plasma to undergo subsequent gas-phase reactions. Ions, as well as directly incorporating into the film, can lead to loss/damage due to etching or sputtering or can further facilitate the incorporation of radicals into the film through stitching.65 It is noted that the direct chemisorption of a methyl radical at a surface site is the rate-limiting step in CVD modelling of film growth using methane plasmas.73 Moreover, Hori and Goto note that due to its planar structure CH3 is only very weakly physisorbed on the surface - thus to enable the chemisorption of CH3 both H abstraction and ion bombardment are required. It is commonly believed that film growth proceeds mainly by direct incorporation of ions or through the transformation of a physisorbed layer of radicals into a chemisorbed state that the surface of the growing film rather than by direct incorporation of radicals into chemisorbed sites.71 This is in accordance with our earlier assertion about the relative importance of the ion flux over the radical flux for tailoring film properties.
Surface processes can influence the plasma influxes - i.e. through ‘backflux’71 resulting from the desorption of radicals from the surface, the subsequent radical recombinations leading to the expulsion of non-radical neutral products from the surface to the plasma where they can undergo further reactions. As we do not model surface fluxes, this backflux is not accounted for and could also contribute to the discrepancy between theoretical (simulated) and measured ion fluxes mentioned above and in Sec. 3. It should also be noted that we do not take into account displacement damage due to ion irradiation or the possibility of random network stabilization74 - both are surface effects which can contribute to modification of bonding (and by extension, mechanical and optoelectronic properties) in the film. Hence, the plasma species sp3/sp2 ratio alone does not provide an exact indication of the sp3/sp2 ratio in the film. It does, however, give a reasonable idea of the expected film sp3/sp2 ratio trend variation with plasma parameters such as Pin and JCH4.
We have also considered the effect of varying the substrate temperature and the applied bias on DLNC films. This study suggests that increasing bias will increase the ion flux incident to the substrate. We found that whilst the deposition rate was increased, higher applied bias had very little effect on the composition of the films. This can be seen to support our assertion in Sec. 3 that it is the ions that are the significant constituents of the films formed even at lower substrate bias. Higher substrate temperature was observed to increase the sp3/sp2 ratio of the films, this is possibly due to its role in reducing neutral density (recall Γj = 0.25njγjνth,j), whilst the ions are controlled by the bias voltage. We should note that whilst raising the temperature of the substrate would enable us to obtain higher fractions of sp3 in deposited films, it would negate the benefit of a low temperature plasma process and limit the range of applications - thus a better way to tailor the sp3 content is by manipulating plasma parameters (i.e., Pin and JCH4) rather than surface parameters such as the substrate temperature or the applied bias.
Investigations such as these fall at the intersection of plasma physics, materials science and surface science, given the massive number of reactions both in the gas phase (i.e., electron impact, etc. as accounted for in our global discharge model) and occurring on the surface (i.e., self-sputtering, ion-induced damage, sub-plantation, etc.) the plasma-based deposition of DLNC may be easily classified as a complex system. In such systems, a combination of experimental studies and advanced multi-stage modeling (there are about 9 orders of magnitude that need to be accounted for in order to model the whole deposition process from the ICP system down to diffusion of adatoms/adions about the substrate surface42) is required to come up with effective (both in terms of cost and time) fabrication recipes for tailor made carbon-based films and nanostructures. As we noted, whilst there exist papers which link plasma parameters to the sp3/sp2 ratio in DLNC films71,75 the link between the sp3/sp2 ratio in the plasma influx to sp3/sp2 in the deposited film remains largely unexplored. Clarifying this issue was one of the main aims of this paper. Moreover, this work may be made applicable to a range of carbon-based nanostructures, by combining it with relevant growth and surface modelling.19,21–23
Potential extensions of this work include noting that the percentage of the total number of electrons with energies above the dissociation threshold can be estimated using the areas (in the same energy range) under the EEDF plot; thus it is also possible to tailor the percentage of mid-energy electrons to produce excited species relevant for DLNC fabrication as excitation will require less energy. Note also that whilst in this work, OES and QMS were used to indicate the relative densities of reactive neutral species, such species can also be measured unambiguously using other techniques such as fiber optic catalytic probes;77,78 incorporating such a diagnostic technique may further improve the process framework presented in this paper.
5. Conclusions
We have demonstrated a deterministic approach for the fabrication of DLNC thin films, with a reasonable level of control starting from electrons in the plasma to sp3/sp2 ratios in the deposited film. We have also shown that the sp3/sp2 ratio is higher for increased input power and lower methane flow rate. By tuning the sp3/sp2 ratio one is able to tailor the elastic, mechanical and optoelectronic properties of diamond-like nanocarbon thin films. Given the close relationship between sp3/sp2 in the plasma influx and deposited film, we have shown that one may effectively tailor nanostructured film properties, to the level of bond lengths,76by careful adjustment of plasma parameters at the level of electrons by controlling the EEDF. This highlights the importance of controlling species production in the plasma bulk by manipulation of parameters such as discharge power and gas composition in order to effect a higher level of control over the characteristic properties, not only of diamond-like nanocarbon thin films but also a variety of other solid/filled carbon nanostructures including nanoparticles, nanotips and highly tailored nanodiamonds. The integral role of predictive modeling in modern, deterministic fabrication processes, in particular has been highlighted as crucial in order to fully realize the potential of plasma nanofabrication all the way to made-to-order carbon-based nanostructures/films for a range of advanced applications. This approach can also be used for the production of graphene, nanodiamond, nanotips and other nanocarbon materials.Acknowledgements
S.X. and Y.P.R acknowledge support from the National Research Foundation of Singapore, A.E.R. was supported by an APA and the CSIRO PSS, K.O. acknowledges the partial support from the CSIRO's Science Leadership Program and the Australian Research Council (ARC).References
- L. Y. Chiang, R. Anandakathir, T. S. Hauck, L. Lee, T. Canteenwala, P. A. Padmawar, K. Pritzker, F. F. Bruno and L. A. Samuelson, Nanoscale, 2010, 2, 535 RSC.
- R. K. F. Lee, B. J. Cox and J. M. Hill, Nanoscale, 2010, 2, 859 RSC.
- J. A. Rodriguez-Manzo, A. Tolvanen, A. V. Krasheninnikov, K. Nordlund, A. Demortiere and F. Banhart, Nanoscale, 2010, 2, 901 RSC.
- N. Wang and L. H. Guan, Nanoscale, 2010, 2, 893 RSC.
- J. Cai, P. Ruffieux, R. Jaafar, M. Bieri, T. Braun, S. Blankenburg, M. Muoth, A. P. Seitsonen, M. Saleh, X. Feng, K. Müllen and R. Fasel, Nature, 2010, 466, 470 CrossRef CAS.
- R. Mas-Balleste, C. Gomez-Navarro, J. Gomez-Herrero and F. Zamora, Nanoscale, 2010 10.1039/C0NR00323A.
- S. Malik, A. Vijayaraghavan, R. Erni, K. Ariga, I. Khalakhan and J. P. Hill, Nanoscale, 2010, 2, 2139 RSC.
- M. K. Singh, E. Titus, G. Goncalves, P. A. A. P. Marques, I. Bdikin, A. L. Kholkin and J. J. A. Gracio, Nanoscale, 2010, 2, 700 RSC.
- M. Keidar, I. Levchenko, T. Arbel, M. Alexander, A. M. Waas and K. Ostrikov, Appl. Phys. Lett., 2008, 92, 043129 CrossRef.
- Z. J. Han, B. K. Tay, M. Shakerzadeh and K. Ostrikov, Appl. Phys. Lett., 2009, 94, 223106 CrossRef.
- M. Keidar, J. Phys. D: Appl. Phys., 2007, 40, 2388 CrossRef.
- R. M. Sankaran and W. H. Chiang, Nat. Mater., 2009, 8, 882 CrossRef.
- O. Volotskova, I. Levchenko, A. Shashurin, Y. Raitses, K. Ostrikov and M. Keidar, Nanoscale, 2010, 2, 2281 RSC.
- F. Villalpando-Paez, H. Muramatsu, Y. A. Kim, H. Farhat, M. Endo, M. Terrones and M. S. Dresselhaus, Nanoscale, 2010, 2, 406 RSC.
- B. C. Thompson, J. Chen, S. E. Moulton and G. G. Wallace, Nanoscale, 2010, 2, 499 RSC.
- I. Levchenko, K. Ostrikov, D. Mariotti and V. Švrček, Carbon, 2009, 47, 2379 CrossRef CAS.
- I. Levchenko, K. Ostrikov and D. Mariotti, Carbon, 2009, 47, 344 CrossRef CAS.
- Z. L. Tsakadze, I. Levchenko, K. Ostrikov and S. Xu, Carbon, 2007, 45, 2022 CrossRef.
- I. B. Denysenko, S. Xu, P. P. Rutkevych, J. D. Long, N. A. Azarenkov and K. Ostrikov, J. Appl. Phys., 2004, 95, 2713 CrossRef CAS.
- I. B. Denysenko and K. Ostrikov, Appl. Phys. Lett., 2007, 90, 251501 CrossRef.
- I. Levchenko, K. Ostrikov, M. Keidar and S. Xu, J. Appl. Phys., 2005, 98, 064304 CrossRef.
- I. Denysenko and K. Ostrikov, J. Phys. D: Appl. Phys., 2009, 42, 015208 CrossRef.
- E. Tam and K. Ostrikov, Nanotechnology, 2009, 20, 375603 CrossRef.
- A. C. Ferrari and J. Robertson, Philos. Trans. R. Soc. London, Ser. A, 2004, 362, 2477 CrossRef CAS.
- J. Robertson, Surf. Coat. Technol., 1992, 50, 185 CrossRef CAS.
- R. Paul, S. N. Das, R. N. Gayen, R. K. Roy, R. Bhar and A. K. Pal, J. Phys. D: Appl. Phys., 2008, 41, 055309 CrossRef.
- D. H. Lee, S. Fayeulle, K. C. Walter and M. Nastasi, Nucl. Instrum. Methods Phys. Res., Sect. B, 1999, 148, 216 CrossRef CAS.
- Y. Cheng, Y. Wu, J. Chen, D. Xu, X. Qiao and C. Xie, Surf. Coat. Technol., 1999, 111, 141 CrossRef CAS.
- C. Casiraghi, J. Robertson and A. C. Ferrari, Mater. Today, 2007, 10(1–2), 44 CrossRef CAS.
- J. E. Butler and A. V. Sumant, Chem. Vap. Deposition, 2008, 14, 145 CrossRef CAS.
- A. S. Barnard, Cryst. Growth Des., 2009, 9, 4860 CrossRef CAS.
- L. M. Manus, D. J. Mastarone, E. A. Waters, X. Q. Zhang, E. A. Schultz-Sikma, K. W. MacRenaris, D. Ho and T. J. Meade, Nano Lett., 2010, 10, 484 CrossRef CAS.
- C. Bradac, T. Gaebel, M. Naidoo, M. J. Sellars, J. Twamley, L. I. Brown, A. S. Barnard, T. Plakhotnik, A. V. Zvyagin and J. R. Rabeau, Nat. Nanotechnol., 2010, 5, 345 CrossRef CAS.
- A. Grigonis, Ž. Rutkūnienė, V. Kopustinskas, G. J. Babonas and A. Rėza, Vacuum, 2005, 78, 593 CrossRef CAS.
- N. Yasui, H. Inaba and N. Ohtake, Appl. Phys. Express, 2008, 1, 035002 Search PubMed.
- Q. J. Cheng, S. Xu, J. D. Long, Z. H. Ni, A. E. Rider and K. Ostrikov, J. Phys. D: Appl. Phys., 2008, 41, 055406 CrossRef.
- S. Xu, K. Ostrikov, J. D. Long and S. Y. Huang, Vacuum, 2006, 80, 621 CrossRef CAS.
- Y. P. Ren, J. D. Long and S. Xu, Phys. Plasmas, 2007, 14, 073301 CrossRef.
- Q. J. Cheng, E. Tam, S. Xu and K. Ostrikov, Nanoscale, 2010, 2, 594 RSC.
- U. Cvelbar, B. Markoli, I. Poberaj, A. Zalar, L. Kosec and S. Spaić, Appl. Surf. Sci., 2006, 253, 1861 CrossRef CAS.
- K. Ostrikov, I. Levchenko, U. Cvelbar, M. Sunkara and M. Mozetic, Nanoscale, 2010, 2, 2012 RSC.
- K. Ostrikov, Rev. Mod. Phys., 2005, 77, 489 CrossRef CAS.
- K. Ostrikov and A. B. Murphy, J. Phys. D: Appl. Phys., 2007, 40, 2223 CrossRef CAS.
- K. Ostrikov, H. J. Yoon, A. E. Rider and S. V. Vladimirov, Plasma Processes Polym., 2007, 4, 27 Search PubMed.
- R. Kleber, M. Weiler, A. Krüger, S. Sattel, G. Kunz, K. Jung and H. Ehrhardt, Diamond Relat. Mater., 1993, 2, 246 CrossRef CAS.
- A. D. Arulsamy, A. E. Rider, Q. J. Cheng, S. Xu and K. Ostrikov, J. Appl. Phys., 2009, 105, 094314 CrossRef.
- “Directing Matter and Energy: Five Challenges for Science and the Imagination”, BESAC Subcommittee on Grand Challenges for Basic Energy Sciences, http://www.sc.doe.gov/bes/reports/list.html Report prepared at Lawrence Berkeley National Laboratory, Editor: Arthur L. Robinson (Advanced Light Source Division), Writer: Lynn Yarris (Communications Department).
- S. Xu, K. N. Ostrikov, Y. Li, E. L. Tsakadze and I. R. Jones, Phys. Plasmas, 2001, 8, 2549 CrossRef CAS.
- K. N. Ostrikov, S. Xu and A. B. M. S. Azam, J. Vac. Sci. Technol., A, 2002, 20, 251 CrossRef CAS.
- J. Perrin, M. Shiratani, P. Kae-Nune, H. Videlot, J. Jolly and J. Guillon, J. Vac. Sci. Technol., A, 1998, 16, 278 CrossRef.
- M. K. Fox and J. K. Whitesell, Organic Chemistry, Jones and Bartlett Publishers, Inc., USA, 3rd edn, 2004 Search PubMed.
- M. Hori and T. Goto, Appl. Surf. Sci., 2007, 253, 6657 CrossRef CAS.
- F. M. Bickelhaupt, T. Ziegler and P. von Ragué Schleyer, Organometallics, 1996, 15, 1477 CrossRef CAS.
- L. Andrews and G. C. Pimentel, J. Chem. Phys., 1967, 47, 3637 CAS.
- M. C. Lawrence and G. N. Robertson, J. Phys. Chem., 1983, 87, 3375 CrossRef.
- M. Karplus and G. K. Fraenkel, J. Chem. Phys., 1961, 35, 1312 CrossRef CAS.
- F. A. Carey and R. J. Sundberg, Advanced Organic Chemistry Part A: Structure and Mechanisms, Springer, New York, 5th edn, 2007 Search PubMed.
- D. A. Dixon and A. J. Arduengo III, J. Phys. Chem., 1991, 95, 4180 CrossRef CAS.
- F. Penotti, J. Gerratt, D. L. Cooper and M. Raimondi, THEOCHEM, 1988, 169, 421 CrossRef.
- T. Fujii, H. Tokiwa and H. Ichikawa, THEOCHEM, 1995, 339, 209 CrossRef CAS.
- L. A. Curtiss and J. A. Pople, J. Chem. Phys., 1988, 88, 7405 CrossRef.
- K. A. Peterson and T. H. Dunning Jr, J. Chem. Phys., 1997, 106, 4119 CrossRef CAS.
- S. Ajith Perera, R. J. Bartlett, P. von, R. Schleyer and J. Am, J. Am. Chem. Soc., 1995, 117, 8476 CrossRef.
- J. Pacansky and M. Dupuis, J. Chem. Phys., 1978, 68, 4276 CrossRef CAS.
- N. V. Mantzaris, E. Gogolides, A. G. Boudouvis, A. Rhallabi and G. Turban, J. Appl. Phys., 1996, 79, 3718 CrossRef.
- D. J. Dagel, C. M. Mallouris and J. R. Doyle, J. Appl. Phys., 1996, 79, 8735 CrossRef CAS.
- M. Shiratani, J. Jolly, H. Videlot and J. Perrin, Jpn. J. Appl. Phys., 1997, 36(7B), 4752 CrossRef CAS.
- K. Teii, M. Hori and T. Goto, J. Appl. Phys., 2002, 92, 4103 CrossRef CAS.
- R. Chakrabarti, K. Chakrabarti, A. B. Maity, S. Chaudhuri and A. K. Pal, Diamond Relat. Mater., 1997, 6, 991 CrossRef CAS.
- F. G. Celii, P. E. Pehrsson, H. T. Wang and J. E. Butler, Appl. Phys. Lett., 1988, 52, 2043 CrossRef CAS.
- W. Möller, Appl. Phys. A: Mater. Sci. Process., 1993, 56, 527 CrossRef.
- Y. Miyagawa, Nakadate, M. Ikeyama, S. Nakao and S. Miyagawa, Diamond Relat. Mater., 2003, 12, 927 CrossRef CAS.
- J. C. Richley, J. N. Harvey and M. N. R. Ashfold, J. Phys. Chem. A, 2009, 113, 11416 CrossRef CAS.
- J. C. Angus and F. Jansen, J. Vac. Sci. Technol., A, 1986, 6, 1778.
- W. Möller, Appl. Phys. Lett., 1991, 59, 2391 CrossRef.
- C. Q. Sun, Prog. Mater. Sci., 2009, 54, 179 CrossRef CAS.
- U. Cvelbar, M. Mozetic and A. Ricard, IEEE Trans. Plasma Sci., 2005, 33, 834 CrossRef CAS.
- D. Babic, I. Poberaj and M. Mozetic, Rev. Sci. Instrum., 2001, 72, 4110 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
