Seed-mediated growth of palladium nanocrystals: The effect of pseudo-halide thiocyanate ions†
Ling
Zhang
ab,
Wenxin
Niu
ab and
Guobao
Xu
*a
aState Key Laboratory of Electroanalytical Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, 130022, China. E-mail: guobaoxu@ciac.jl.cn; Fax: +86 431 85262747; Tel: +86 431 85262747
bGraduate School of the Chinese Academy of Sciences, Beijing, 100039, China
First published on 17th December 2010
Abstract
In synthesis in a solution phase, adsorbates such as halides can interact selectively with different metal crystal facets and affect the final morphology of nanocrystals. Pseudo-halide thiocyanate ions (SCN−) can also adsorb on the metal surface, but they have never been used for the synthesis of shape-controlled colloidal metal nanocrystals. In this study, we first investigated the effect of SCN− on the morphology of palladium nanocrystals through a seed-mediated growth method. The presence of 1 µM SCN− in the growth solutions could lead to the formation of palladium polyhedra: truncated rhombic dodecahedra enclosed by twelve {110}, eight {111} and six {100} facets. The products were nanocubes enclosed with six {100} facets if cetyltrimethylammonium bromide (CTAB) was the only capping agent. Meanwhile, the mechanism of the effect of SCN− on the morphology of Pd nanocrystals is discussed.
1. Introduction
Interest in palladium nanocrystals has been growing steadily due to their fascinating properties and practical applications. For example, cross-coupling reactions of aryl halides (Suzuki coupling reactions) and the coupling of aryl halides with alkenes (Heck reactions) are important organic reactions catalyzed by palladium.1 It also serves as a major catalyst in automobile industry for the reduction of vehicle exhausts.2 Moreover, Pd shows a very large capacity for hydrogen adsorption, and its structural change involved in the adsorption process offers a number of attractive features suitable for energy purpose.3 Meanwhile, surface-enhanced Raman scattering (SERS) and magnetic properties of Pd nanostructures have also been investigated.4–9 Because the morphology of metal nanoparticles affects the chemical and physical properties, many studies have been dedicated to the shape-controlled synthesis of palladium nanocrystals, especially the colloidal methods.10–15 A rich variety of shapes for Pd nanocrystals have been synthesized by Xia's group, including truncated octahedra, icosahedra, octahedra, decahedra, hexagonal and triangular thin plates, rectangular bars and cubes.16 Through using different reducing agents or adding other capping agents, the shape of Pd nanocrystals is successfully controlled only by manipulating the reduction kinetics in the poly(vinyl pyrrolidone) (PVP) solution. Meanwhile, Pd nanocubes or bars, nanorods and nanodendrities have also been obtained by other researchers.9,17–24 In the solution-phase synthesis of noble metals, capping agents such as cetyltrimethylammonium bromide (CTAB), cetylpyridinium chloride, sodium dodecyl sulfate, bis(2-ethylhexyl)sulfosuccinate or PVP are necessary for avoiding the aggregation of the noble metal nanoparticles.18–20,22,25–31 These capping agents are thought as growth-directing adsorbates on the metal surfaces, generating shape anisotropy during nanocrystal growth by stabilizing a particular facet through molecular interaction. Although the actual mechanism for this selective adsorption is still under consideration, some empirical rules have been concluded. For instance, CTAB appears to adsorb more strongly to the {100} than {111} facets of palladium and results in the formation of nanocubes or nanorods.9,20,25 PVP is believed to adsorb to the {111} and {100} facets of palladium preferentially, usually leading to the formation of cubes, octahedra, icosahedra, hexagonal and triangular nanoplates.16,32,33Selective adsorption onto different crystal planes is not limited to large surfactants and long-chain polymers, small molecules or inorganic ions could also affect the shape of metal nanocrystals through preferential binding.12,34Halide ions have been explored widely in the synthesis of colloidal metals due to their selective adsorption onto the metal surface. For example, in one-pot synthesis of palladium nanocubes capped by CTAB, the addition of chloride to the reaction solutions containing Na2PdCl4, ascorbic acid, and CTAB would lead to the formation of Pd nanodendrities. When chloride was substituted by bromide or iodide, the products were aggregated nanopheres with irregular shape due to the strong adsorption of bromide or iodide on palladium surface compared with chloride.35 But in some PVP systems, bromide played a critical role in the formation of Pd nanobars or nanorods enclosed by the {100} and {110} facets.16,19 Recently, our group have synthesized single-crystalline cubic, rhombic dodecahedral, and octahedral palladium nanocrystals by adding different amount of KI to the growth solutions.36 Apparently, the adsorption of halide ions on the metal surface plays an important role in the shape-controlled synthesis of colloidal metal nanocrystals.
Halides have provided an effective route for the shape-controlled synthesis of metal nanostructures. Meanwhile, pseudo-halide thiocyanate ions (SCN−) have been studied extensively by in situvibrational spectroscopies because of their strong adsorption on the metal surface and large infrared and Raman cross section of the C–N stretch.37–41 However, the effect of their adsorption on the growth kinetics of metal nanocrystals has not been studied so far. In this paper, we study the effect of SCN− on the shape of Pd nanocrystals by using a seed-mediated growth method. The experiments involved the preparation of 22 nm Pd nanocubic seeds and the subsequent addition of an appropriate quantity of the Pd seed solution to the aqueous growth solution, which contained desired quantities of CTAB, H2PdCl4, ascorbic acid and different amount of SCN−. The introduction of certain amount of KSCN will lead to the formation of polyhedral palladium single-crystalline nanocrystals, which were identified as truncated rhombic dodecahedra enclosed by twelve {110}, eight {111} and six {100} facets. Also, the mechanism of the effect of SCN− on the shape of the Pd nanocrystals was discussed.
2. Experimental section
Materials
Palladium(II) chloride (PdCl2) and potassium thiocyanate (KSCN) were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). CTAB was obtained from Fluka (Switzerland). L-Ascorbic acid and hydrochloric acid were obtained from the Beijing Chemical Reagent Company (Beijing, China). All the chemicals were of analytical grade and used without further purification. Doubly distilled water was used throughout the experiments. A 10 mM H2PdCl4 solution was prepared by dissolving 0.1773 g PdCl2 in 10 mL of 0.2 M HCl solution and further diluting to 100 mL with doubly distilled water.Synthesis of 22 nm Pd nanocubes as seeds
The method of producing Pd seeds is similar to our previous reports.20 Briefly, 0.5 mL aliquot of 10 mM H2PdCl4 solution was added to 9420 µL of 12.5 mM CTAB solution heated at 95 °C under stirring. After 5 minutes, 80 µL of freshly prepared 100 mM ascorbic acid solution was added, and the reaction was allowed to proceed for 20 min. The nanocube solution was stored at 30 °C for future use as seeds. Palladium seeds were collected by centrifugation (12000 rpm, 10 min) and washed by redispersing in doubly distilled water and centrifuging twice for scanning electron microscopy (SEM), transmission electron microscopy (TEM) and X-ray diffraction (XRD) characterization.Seed-mediated growth of polyhedral palladium nanocrystals
In a typical synthesis, 5 µL of 1 mM KSCN solution was added to 5 mL of 100 mM CTAB solution kept at a given temperature. 250 µL of 10 mM H2PdCl4 solution and 80 µL as-synthesized seed palladium nanocube solution were then added. Finally, 50 µL of freshly prepared 100 mM ascorbic acid solution was added and the solution was mixed thoroughly. The resulting solution was placed in a water bath at 40 °C, 60 °C and 80 °C, respectively. Reactions at the same temperature were set up in parallel. When the orange solutions became colorless, the reactions were complete. The reaction time were about 15 h, 2 h and 1 h for 40 °C, 60 °C and 80 °C, respectively. The precipitates were collected by centrifugation (6000 rpm, 5 min) and washed by redispersing in doubly distilled water and centrifuging twice for SEM, TEM and selected-area electron diffraction (SAED) characterization.Instrumentation
SEM images were taken using an FEI XL30 ESEM FEG scanning electron microscope operated at 25 kV. TEM, high-resolution transmission electron microscopy (HRTEM) and SAED studies were performed on a FEI Tecnai G2F20 microscope operated at 200 kV. X-ray diffraction (XRD) data were collected using Bruker D8 ADVANCE X-ray diffractometer (Cu-Kα radiation) operated at 40 kV and 40 mA.3. Results and discussion
The effect of SCN− on the morphology of palladium nanocrystals
Here, we used the seed-mediated method to investigate the effect of SCN− on the growth of Pd nanocrystals. First, the 22 nm Pd nanocubes capped by CTAB were synthesized and then used as seeds for the further growth of the Pd nanocrystals. The SEM, TEM images and XRD pattern of the Pd seeds were shown in Fig. S1 and S2 in the supporting information.†TEM image showed that these Pd seeds were almost ideal nanocubes, which was in accord with the previous reports.20 If the growth solution contained only CTAB, H2PdCl4 and the reducing agent ascorbic acid, these small Pd nanocubes would further grow into larger nanocubes which were about 90 nm in size as shown in Fig. 1A. When 5 µL of 1 mM KSCN solution was introduced to the growth solution (final concentration was 1 µM), the products were not larger nanocubes. As shown in the SEM image of Fig. 1B, the products were truncated rhombic dodecahedra, since the small triangles and quadrangles were observed on the surface of the polyhedra, and the faces with the largest areas approached a rhombus. The {111} and {100} truncations occurred on the corners of rhombic dodecahedra, thus the polyhedra should be enclosed by twelve {110}, eight {111} and six {100} facets. The geometrical models of the polyhedra labelled in Fig. 1B were shown in the bottom of Fig. 1. Fig. 1C showed the TEM image of the polyhedra, their sizes were about 100 nm according to the long axes. The projected shapes of these polyhedra approached round, hexagon and quadrangle shapes whose corners were slightly cut, depending on the orientations of the polyhedra. Fig. 1D showed the HRTEM image of a selected corner of an individual Pd polyhedron, and the lattice fringe spacings of the image were measured to be 0.225 nm, which is in agreement with the {111} lattice spacing of the fcc crystal of palladium, indicating that the polyhedral palladium nanocrystal is single-crystalline. | ||
| Fig. 1 (A, B) SEM images of the Pd nanocubes obtained in the absence of KSCN and polyhedra obtained in the presence of 1 µM KSCN at 60 °C, respectively. Scale bars: 200 nm. (C) TEM image of the Pd polyhedra produced in the same conditions as (B). (D) HRTEM image of a corner of an individual polyhedron. Bottom: geometrical models of three polyhedra labeled in the SEM image. The {110}, {111} and {100} facets are shown in purple, blue, and green, respectively. | ||
To further confirm the shape of the Pd polyhedra, the larger polyhedra were synthesizd by reducing the amount of Pd seeds. Fig. 2A and B showed the SEM images of the larger polyhedra produced when the volume of the added Pd seeds solution was decreased to 60 µL. The small triangles and quadrangles were obvious, and size of the polyhedra approached 110 nm according to the long axes. Most of the polyhedra lay flat on the substrate (the top and bottom {110} faces were parallel to the substrate), and the projected shapes approached hexagons, as shown by the TEM image of Fig. 2C. Fig. 2D showed the SAED pattern of a single flat-lying polyhedron, of which the TEM image was shown in the inset. The SAED pattern was obtained by directing the electron beam perpendicular to the upper face of the polyhedron and accorded with the flat-lying rhombic dodecahedral palladium nanocrystal along the [011] zone axis,36 the twelve faces with the largest area of an polyhedra should be {110} facets. Thus, we confirmed that these Pd polyhedra obtained here were truncated rhombic dodecahedra enclosed by twelve {110}, eight {111} and six {100} facets. The {111} truncations led to the formation of eight triangles of the polyhedron, and each triangle was surrounded by three {110} faces; The {100} truncations led to the formation of six quadrangles, and each quadrangle was surrounded by four {110} faces. It should be indicated that the shapes of these polyhedral nanocrystals were not as perfect as the geometrical models shown in Fig. 1. As shown in the SEM images in Fig. 2, the {110} faces were a little concave and the {111} faces with triangular shapes were stretched out, thus the projected shape of the flat-lying polyhedron appeared to be a hexagon with two concave edges. But the {110} faces seemed a little better for the polyhedra with smaller size, as shown in Fig. 1.
![(A, B) SEM images at different magnifications and (C) TEM image of the larger Pd polyhedra synthesized at 60 °C, respectively. (D) SAED pattern of a single flat-lying truncated rhombic dodecahedron recorded along the [011] ozone axis. Inset: the flat-lying truncated rhombic dodecahedron corresponding to SAED pattern, scale bar: 20 nm.](/image/article/2011/NR/c0nr00622j/c0nr00622j-f2.gif) | ||
| Fig. 2 (A, B) SEM images at different magnifications and (C) TEM image of the larger Pd polyhedra synthesized at 60 °C, respectively. (D) SAED pattern of a single flat-lying truncated rhombic dodecahedron recorded along the [011] ozone axis. Inset: the flat-lying truncated rhombic dodecahedron corresponding to SAED pattern, scale bar: 20 nm. | ||
The XRD patterns recorded on the cubic and polyhedral palladium nanocrystals were shown in Fig. 3. All of the peaks can be indexed to the fcc palladium metal (JPCDS card no. 05-0681). The XRD pattern of the palladium nanocubes showed an abnormally intense (200) peak, suggesting a relatively large proportion of palladium nanocubes were oriented with their {100} facets parallel to the substrate. The XRD pattern of the polyhedra showed the highest (111) peak and relatively higher (220) peak, which should be attributed to the randomly oriented palladium polyhedra.36
 | ||
| Fig. 3 XRD patterns of cubic (A) and polyhedral (B) palladium nanocrystals. | ||
The effect of SCN− on the growth of palladium nanocrystals was also investigated at the different temperatures. Fig. 4 showed the palladium nanocrystals obtained at 40 °C and 80 °C, respectively. Without SCN− in the growth solution, the main products were nanocubes, as shown in Fig. 4A and C. In the presence of 1 µM SCN−, the palladium nanocrystals obtained at 40 °C or 80 °C were still mainly truncated rhombic dodecahedra with twenty-six facets, as shown in Fig. 4B and D. At 80 °C, some palladium nanorods and nanocrystals with irregular shapes were formed due to the spontaneous nucleation at higher temperatures.
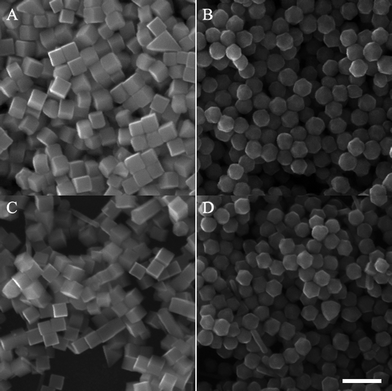 | ||
| Fig. 4 SEM images of the Pd nanocrystals obtained (A, C) in the absence of KSCN at 40 and 80 °C, respectively; (B, D) in the presence of 1 µM KSCN at 40 and 80 °C, respectively. Scale bars: 200 nm. | ||
The possible mechanism of the effect of SCN− on the growth of Pd nanocrystals
For palladium nanoparticles reported with a regular shape, such as a cube, cuboctahedron, or prism, their surfaces are usually bound by low-energy {100} and {111} facets. The {110} facet is rarely observed in palladium nanostructures due to its higher surface energy.9 In our synthetic conditions, the palladium seeds would further grow into larger nanocubes enclosed by six {100} facets when capped by CTAB. If CTAB was replaced with cetyltrimethylammonium chloride (CTAC), the final products were a mixture of polydisperse nanoparticles of irregular shapes, which suggested that Br− played an important role in the formation of nanocubes with well-defined {100} surfaces.20 Also, our group recently reported that the introduction of I− to the growth solution would lead to the formation of rhombic dodecahedral palladium nanocrystals.36 These results suggested that the adsorbates could alter the surface energies of palladium facets and affect the shape of the nanocrystals.The adsorption of SCN− on metal surfaces is of particular interest because this molecule presents ambidentate functionality with the possibility of either N or S coordination. And the N-bound, S-bound and bridging structures are usually present at the same time.42 In this study, the effect of adsorption of SCN− on the growth of the palladium nanocrystals was investigated. When SCN− was introduced into the growth solution, the rhombic dodecahedral palladium nanocrystals with {111} and {100} truncations were formed. The products were nanocubes enclosed with six {100} facets if CTAB was the only capping agent. Based on the observations, it was presumed that SCN− may adsorb to the {110} facets and protected them from disappearing during the growth of palladium nanocrystals.
When the final concentration of KSCN increased to 10 µM, the shapes were irregular and there were many indentations on the nanocrystals. It was possible that excess SCN− replaced CTAB adsorbed on the surface of Pd nanocrystals and weakened the structure-directing role of CTAB. But the small SCN− ions do not have remarkable stabilization to the Pd nanoparticles, so the final shapes of the nanocrystals were irregular. The SEM image and XRD pattern of these Pd nanocrystals was shown in Fig. S3 and S4.† The relative intensity of each peak was similar to the XRD pattern of Pd polyhedra of Fig. 3B.
4. Conclusions
With the seed-mediated growth method, we investigated the effect of SCN− on the growth of palladium nanocrystals. Through adding a certain amount of SCN− to the growth solution, polyhedral palladium single-crystalline nanocrystals which were identified as truncated rhombic dodecahedra enclosed by twelve {110}, eight {111} and six {100} facets were produced. It was presumed that SCN− may adsorb to the {110} facets and protect them, leading to the formation of the unstable {110} facets. In conclusion, the adsorbate SCN− ions could kinetically control the shape of noble metal nanocrystals like halides, which provides us a new way of synthesizing shape-controlled colloidal metal nanocrystals.Notes and references
- R. Narayanan and M. A. El-Sayed, Langmuir, 2005, 21, 2027 CrossRef CAS.
- Y. Nishihata, J. Mizuki, T. Kao, H. Tanaka, M. Enishi, M. Imura, T. Kamoto and N. Hamada, Nature, 2002, 418, 164 CrossRef CAS.
- F. Favier, E. C. Walter, M. P. Zach, T. Benter and R. M. Penner, Science, 2001, 293, 2227 CrossRef CAS.
- J. M. McLellan, Y. J. Xiong, M. Hu and Y. N. Xia, Chem. Phys. Lett., 2006, 417, 230 CrossRef CAS.
- Z. Q. Tian, Z. L. Yang, B. Ren, J. F. Li, Y. Zhang, X. F. Lin, J. W. Hu and D. Y. Wu, Faraday Discuss., 2006, 132, 159 RSC.
- M. E. Abdelsalam, S. Mahajan, P. N. Bartlett, J. J. Baumberg and A. E. Russell, J. Am. Chem. Soc., 2007, 129, 7399 CrossRef CAS.
- T. Taniyama, E. Ohta and T. Sato, Europhys. Lett., 1997, 38, 195 CrossRef CAS.
- B. D. Shanina, A. M. Danishevskii, A. I. Veinger, A. A. Sitnikova, R. N. Kyutt, A. V. Shchukarev and S. K. Gordeev, J. Exp. Theor. Phys., 2009, 109, 609 CrossRef CAS.
- C. W. Xiao, H. Ding, C. M. Shen, T. Z. Yang, G. Hui and H. J. Gao, J. Phys. Chem. C, 2009, 113, 13466 CrossRef CAS.
- C. Burda, X. B. Chen, R. Narayanan and M. A. El-Sayed, Chem. Rev., 2005, 105, 1025 CrossRef CAS.
- M. A. El-Sayed, Acc. Chem. Res., 2004, 37, 326 CrossRef CAS.
- S. E. Habas, H. Lee, V. Radmilovic, G. A. Somorjai and P. D. Yang, Nat. Mater., 2007, 6, 692 CrossRef CAS.
- T. K. Sau and C. J. Murphy, J. Am. Chem. Soc., 2004, 126, 8648 CrossRef CAS.
- T. K. Sau and A. L. Rogach, Adv. Mater., 2010, 22, 1781 CrossRef CAS.
- S. Cheong, J. D. Watt and R. D. Tilley, Nanoscale, 2010, 2, 2045 RSC.
- B. Lim, M. J. Jiang, J. Tao, P. H. C. Camargo, Y. M. Zhu and Y. N. Xia, Adv. Funct. Mater., 2009, 19, 189 CrossRef CAS.
- Y. C. Yu, Y. X. Zhao, T. Huang and H. F. Liu, Mater. Res. Bull., 2010, 45, 159 CrossRef CAS.
- G. Chang, M. Oyata and K. Hirao, Acta Mater., 2007, 55, 3453 CrossRef CAS.
- Y. J. Xiong, H. Cai, B. J. Wiley, J. Wang, M. J. Kim and Y. N. Xia, J. Am. Chem. Soc., 2007, 129, 3665 CrossRef CAS.
- W. X. Niu, Z. Y. Li, L. H. Shi, X. Q. Liu, H. J. Li, S. Han, J. A. Chen and G. B. Xu, Cryst. Growth Des., 2008, 8, 4440 CrossRef CAS.
- J. Liu, F. He, T. M. Gunn, D. Zhao and C. B. Roberts, Langmuir, 2009, 25, 7116 CrossRef CAS.
- Y.-H. Chen, H.-H. Hung and M. H. Huang, J. Am. Chem. Soc., 2009, 131, 9114 CrossRef CAS.
- N. Huang and N. Zheng, J. Am. Chem. Soc., 2009, 131, 4602 CrossRef CAS.
- J. Watt, N. Young, S. Haigh, A. Kirkland and R. D. Tilley, Adv. Mater., 2009, 21, 2288 CrossRef CAS.
- C. J. Johnson, E. Dujardin, S. A. Davis, C. J. Murphy and S. Mann, J. Mater. Chem., 2002, 12, 1765 RSC.
- J. E. Park, M. Atobe and T. Fuchigami, Ultrason. Sonochem., 2006, 13, 237 CrossRef CAS.
- B. Lim, H. Kobayashi, P. H. C. Camargo, L. F. Allard, J. Y. Liu and Y. N. Xia, Nano Res., 2010, 3, 180 CrossRef CAS.
- E. C. Cho, P. H. C. Camargo and Y. N. Xia, Adv. Mater., 2010, 22, 744 CrossRef CAS.
- M. P. Pileni, Nat. Mater., 2003, 2, 145 CrossRef CAS.
- W. X. Niu, S. L. Zheng, D. W. Wang, X. Q. Liu, H. J. Li, S. Han, J. A. Chen, Z. Y. Tang and G. B. Xu, J. Am. Chem. Soc., 2009, 131, 697 CrossRef CAS.
- X. Q. Huang, H. H. Zhang, C. Y. Guo, Z. Y. Zhou and N. F. Zheng, Angew. Chem., Int. Ed., 2009, 48, 4808 CrossRef CAS.
- A. R. Tao, P. Sinsermsuksakul and P. D. Yang, Angew. Chem. Int. Ed., 2006, 118, 4713 CrossRef.
- F. Kim, S. Connor, H. Song, T. Kuykendall and P. D. Yang, Angew. Chem. Int. Ed., 2004, 116, 3759 CrossRef.
- H.-L. Wu, C. H. Chen and M. H. Huang, Chem. Mater., 2009, 21, 110 CrossRef CAS.
- F. R. Fan, A. Attia, K. U. Sur, J. B. Chen, Z. X. Xie, J. F. Li, B. Ren and Z. Q. Tian, Cryst. Growth Des., 2009, 9, 2335 CrossRef CAS.
- W. X. Niu, L. Zhang and G. B. Xu, ACS Nano, 2010, 4, 1987 CrossRef CAS.
- K. Ashley, M. G. Samant, H. Seki and M. R. Philpott, J. Electroanal. Chem., 1989, 270, 349–364 CrossRef CAS.
- P. Cao, J. Yao, B. Ren, R. Gu and Z. Tian, J. Phys. Chem. B, 2002, 106, 7283 CrossRef CAS.
- B. Ren, Q. J. Huang, Y. Xie and Z. Q. Tian, Anal. Sci., 2000, 16, 225–234 CrossRef CAS.
- M. Bron and R. Holze, Electrochim. Acta, 1999, 45, 1121 CrossRef CAS.
- J. W. Hu, Y. Zhang, J. F. Li, Z. Liu, B. Ren, S. G. Sun, T. Lian and Z. Q. Tian, Chem. Phys. Lett., 2005, 408, 354 CrossRef CAS.
- X. Li and A. A. Gewirth, J. Am. Chem. Soc., 2003, 125, 11674 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional SEM, TEM and XRD data. See DOI: 10.1039/c0nr00622j |
| This journal is © The Royal Society of Chemistry 2011 |
