Preparation and catalytic ability to reduce hydrogen peroxide of Ag nanoparticles highly dispersed via hyperbranched copolymer†
Lu
Yao
a,
Weiying
Yang
b,
Jie
Yang
b,
Linghao
He
a,
Jing
Sun
a,
Rui
Song
*a,
Zhi
Ma
c and
Wei
Huang
d
aKey Lab of Surface and Interface Sciences of Henan Provincial, Zhengzhou University of Light Industry, Zhengzhou, 450005, China. E-mail: rsong@gucas.ac.cn; Fax: +86-10-88256092; Tel: +86-10-88253067
bCollege of Chemistry and Chemical Engineering, Graduate University of Chinese Academy of Sciences, Beijing, 100049, China
cShanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai, 200032, China
dLaboratory of Advanced Polymer Materials, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100080, China
First published on 3rd December 2010
Abstract
Highly dispersed Ag nanoparticles, stabilized by hyperbranched copolymers (HPCs), were prepared by chemical reduction in toluene. These Ag NPs were used further for the fabrication of a hydrogen peroxide (H2O2) sensor, by which a good catalytic ability for the reduction of H2O2 was found.
Metal nanoparticles (NPs) have long been highly regarded owing to their important potential applications in catalysts, electronics, and chemical sensors, etc.1–4 Various techniques have been employed for the synthesis of metal NPs, for example, gas evaporation, arc plasma, sputtering, chemical reaction, electrochemical method and laser ablation.5–10 However, the big challenge met during the preparation of metal NPs is that, because of their high surface energy, a large number of dispersed metal NPs often readily congregate and separate from solutions, which results in an obvious decrease in their useful properties. So, the preparation of monodisperse metal NPs usually needs the addition of a stabilizer or dispersing agent, which may traditionally be organic molecules, linear polymers or surfactants.11 Recently, the application of hyperbranched polymers (HPs) has opened a new way of forming metal NPs with small sizes and narrow size distributions.12,24–26 As a novel kind of polymer, with highly branched three-dimensional globular architectures with many functional groups and inner cavities,12 HPs have been widely applied in different domains ranging from sensors13 to coatings14,15, and in other technologies such as blending modification,16–18 delivery devices19,20 and polymeric membranes.21–23 Due to their special structure, HPs are particularly suitable for use as a matrix which disperses and stabilizes the NPs to prevent agglomeration, especially for metal NPs.24–26 It has been demonstrated that a series of Ag NPs or Au NPs can be fabricated under the assistance of amine-terminated HPs.12,24 Highly stable, uniform and monodispersed Au NPs were successfully encapsulated within a hyperbranched poly(amine-ester).25 In addition, the monodisperse Ag NPs were prepared using hyperbranched polyurethane as the protective agent.26 In these works, the HPs possessed some strong electron-donating centers, such as amino or carboxylate groups, to facilitate the complex with the metal ions. Nevertheless, it is still a puzzle to obtain HPs with these functional groups in a scaled-up way. Furthermore, it is necessary to modify the terminal groups of the HPs by chemical methods, which usually involves a tedious, time-consuming and complicated process. Hence, HPs used as a stabilizer and dispersing agent are still limited. Herein, we present a hyperbranched polystyrene copolymer (without functional groups interacting with Ag NPs) assisted dispersion method for preparing Ag NPs in toluene by chemical reduction, and explore their potential as a H2O2 sensor for the catalytic ability for H2O2.
The synthesis approach of HPCs-assisted dispersed Ag NPs is schematically shown in Fig. 1. Firstly, a series of hyperbranched copolymers of St (styrene) and CMS (p-chloromethyl styrene) were prepared via ATR-SCVP (atom transfer radical self-condensing vinyl polymerization) using CuCl/2,2,-bipy as the catalysis system (see Fig. S1 in the ESI†). The detail about the synthesis steps of these HPCs can be found elsewhere,27 and some structure parameters are listed in Table S1 (in the ESI†). Then, Ag+ was quickly transferred from water to an organic medium, which involved mixing 50 ml of 0.6 mM aqueous AgNO3 solution with 50 ml of ethanol containing 1 ml of dodecylamine (DDA), and then extracting the Ag+ into 50 ml of toluene.28 At 60 °C, 3 mg HPCs (HPC-1 and HPC-2) was added to 20 ml the toluene solution of Ag+ under stirring. To this solution, 375 μl of 100 mM aqueous NaBH4 solution was rapidly added under vigorous stirring for several minutes, giving rise to a red-brown Ag hydrosol, namely HPCs-assisted dispersed Ag NPs (HPC-1/Ag NPs and HPC-2/Ag NPs) (Fig.1). As a reference, Ag NPs in the absence of HPCs were synthesized according to the above steps.
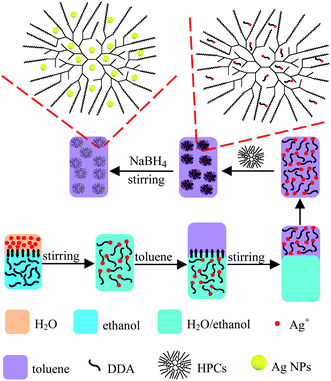 | ||
| Fig. 1 Schematic illustration of the preparation of HPCs-assisted dispersed Ag NPs. Ag+ was transferred from the aqueous solution to toluene using DDA as the cationic surfactant, and then reduced by NaBH4 after adding HPCs as the dispersing agent. | ||
The dispersion and stability of the obtained HPCs-assisted dispersed Ag NPs in toluene can be seen in the photographs in Fig. 2A. Here, in comparison with Ag NPs which precipitated almost completely after only 40 days, HPC-1/Ag NPs and HPC-2/Ag NPs did not show any trace of aggregation or precipitation for more than half a year. This observation showed that HPCs-assisted dispersed Ag NPs had a high solubility and dispersibility in toluene. Additionally, in this study, the mass ratio of Ag NPs to HPCs was 0.43 mg/mg, nearly three times higher than that previously reported, in which the loading ratio of Ag NPs to HPCs with tertiary amine groups was 0.15 mg/mg.9
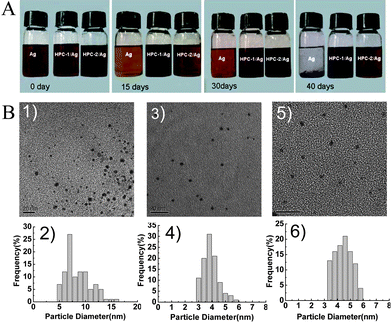 | ||
| Fig. 2 Optical photograph (A) and TEM micrograph and corresponding size distribution (B) of the Ag NPs and HPCs-assisted dispersed Ag NPs in toluene: 1) TEM micrograph and 2) size distribution of Ag NPs; 3) TEM micrograph and 4) size distribution of HPC-1/Ag NPs; 5) TEM micrograph and 6) size distribution of HPC-2/Ag NPs. | ||
The TEM (Tecnai G2 20, FEI, USA) micrographs of HPCs-assisted dispersed Ag NPs clearly demonstrated the high dispersity of spherical Ag NPs (Fig. 2B), and the resulting particle size distribution histogram were based on 100 randomly selected particles. It could be seen that compared to aggregated Ag NPs (Fig. 2B (1)), the HPC-1/Ag NPs (Fig. 2B (3)) and HPC-2/Ag NPs (Fig. 2B (5)) were isolated and dispersed homogeneously, and the particle size distribution of HPCs-assisted dispersed Ag NPs was narrow, as shown in the corresponding histograms (Fig. 2B (4) and 2B (6)). The mean diameter of Ag NPs stabilized by HPC-1 and HPC-2 was about 4.10 ± 0.60 nm (Fig. 2B (4)), and 4.63 ± 0.68 nm (Fig. 2B (6)), respectively, while the average diameter of Ag NPs was about 9.04 ± 2.45 nm (Fig. 2B (2)). Hence, it is obvious that the HPCs played an efficient role of a dispersing agent in preventing Ag NPs agglomeration.
UV-vis spectroscopy (Hitachi 7500, Japan) was employed for further characterization of free Ag NPs and Ag NPs dispersed by HPCs (Fig. 3A). The spectrum of Ag NPs in absence of HPCs in toluene showed an absorption band at 415 nm which is attributed to the SPR band of Ag NPs.28 It is interesting to note that the SPR bands of the Ag NPs in the presence of HPCs remained at approximately 415 nm, though the absorbance of the SPR bands was significantly enhanced, which implies a higher concentration of Ag NPs in the presence of HPCs.29 At the same time, the widening of the half-width of the SPR bands of Ag NPs dispersed by HPCs became evident, suggesting some interaction occurs between Ag NPs and HPCs.30 The interaction involved may not be the strong coordinating interactions like Ag NPs and amine groups,9 yet the essence of the interaction deserves further investigation.
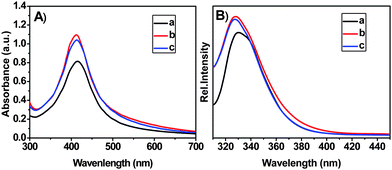 | ||
| Fig. 3 UV-vis spectra (A) and fluorescence spectra (excited at 292 nm) (B) for (a) Ag NPs, (b) HPC-1/Ag NPs, (c) HPC-2/Ag NPs. | ||
Eventually, after settling for half a year, the absorbance of the surface plasmon resonance (SPR) band of Ag NPs became very weak, and even almost disappeared, while those of HPC-1/Ag NPs and HPC-2/Ag NPs changed very little (see Fig. S1 in the ESI†), which was consistent with the result of the photograph.
In addition, a strong emission peak at ∼330 nm upon photoexcitation at 292 nm could be detected (Fig. 3B), which was assigned to transitions to an excited state from the d levels of the Ag NPs,30–32 the position of which remains approximately the same with the addition of HPCs. On the other hand, intensity of an emission peak depends upon the transition probability; i.e. highest intensity of a peak corresponds to maximum transition probability.31 The intensity of all peaks of Ag NPs after adding HPCs increased more sharply than those of Ag NPs, which is due to HPCs' quasispherical branched structure, with many inner cavities and almost no chain entanglements.24 Accordingly, the HPCs enable Ag NPs to disperse uniformly in toluene and thus greatly improve the transition probability of Ag NPs.
The ζ-potential is often used as an index of the magnitude of electrostatic interactions between colloidal particles, and it is thus considered a further measure of the colloidal stability of a suspension. Particles with ζ-potential less than −15 mV or more than 15 mV are expected to be stabilized by electrostatic repulsion interaction.33 The ζ-potential of sample dispersions were measured by using a Zeta Plus apparatus (Zeta Potential Analyzer, Brookhaven Instruments Corporation). For the as-prepared Ag NPs suspension, a ζ-potential of −12.6 ± 1.2 mV was obtained; while for the suspensions of HPC-1/Ag NPs and HPC-2/Ag NPs, the recorded ζ-potentials were 27.6 ± 1.6 mV and 34.3 ± 1.8 mV. These negative and highly positive values further confirm the stability observed over time for dispersions of Ag nanoparticles and HPCs-assisted dispersed Ag nanoparticles.
The reliable, precise and fast determination of H2O2 is of significant importance as it is a significant mediator in food, pharmaceutical, clinical, and environmental analyses, etc.34 Owing to its intrinsic sensitivity, high selectivity and simplicity,35,36 an electrochemistry technique (CHI660 electrochemical workstation, Chenhua, Shanghai) was used to detect H2O2 directly by the fabrication of HPCs-assisted dispersed Ag NPs deposited on to a glassy carbon (GC) electrode. For the electrocatalytic reduction of H2O2, a 5 μL aliquot of HPCs-assisted dispersed Ag NPs was placed on a polished GC electrode (5 mm in diameter) and then dried at room temperature. In the presence of 1.0 mM H2O2, a weak peak at −0.64 V was observed at the Ag NP electrode (Fig. 4a), which showed that the Ag NPs were very weakly electroactive in the potential range due to the serious agglomeration of Ag NPs. However, after the GC electrode was modified by HPCs-assisted dispersed Ag NPs, the peak potentials appeared at −0.6 V and shifted positively as compared with that of the Ag NP modified electrode (Fig. 4b and c). The responses of H2O2 to the bare GC, HPC-1 and HPC-2 electrodes were obviously weak and were disregarded (the inset of Fig. 4). These results suggested that the HPCs-assisted dispersed Ag NP modified electrode had a significant catalytic ability in H2O2 reduction, and that the catalytic current mainly came from the Ag NPs dispersed by HPCs in the electrocatalytic reduction of H2O2.37
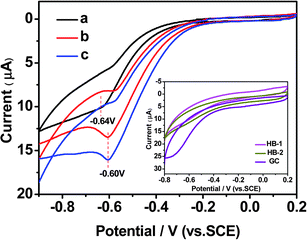 | ||
| Fig. 4 Cyclic voltammograms (CVs) of different electrodes in 0.1 M PBS of pH = 7.0 and 1.0 mM H2O2: (a) Ag NPs, (b) HPC-1/Ag NPs, (c) HPC-2/Ag NPs electrode. Scan rate: 0.05 mV s−1. (Inset: CVs of the electrodes modified with only HPCs and the bare GC electrode in 0.1 M PBS of pH = 7.0 and 1.0 mM H2O2). | ||
In summary, we have demonstrated a simple and facile scheme to prepare HPCs-assisted dispersed Ag NPs, which exhibits a stable suspension and thus enabled us to explore their application in electrochemical detection of H2O2. We believe that metal NPs such as gold NPs, platinum NPs and palladium NPs can be dispersed well by HPs like HPCs in a similar fashion, enabling the preparation of a series of novel HPs-assisted dispersed metal NPs with a wide range of important potential applications.
Acknowledgements
This work was supported by the President Fund of Graduate University of CAS (grant number 095101CY00), and grant KF2008-04 from the State Key Laboratory of Environmental Chemistry and Ecotoxicology, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences.Notes and references
- D. Tabuani, O. Monticelli, A. Chincarini, C. Bianchini, F. Vizza, S. Moneti and S. Russo, Macromolecules, 2003, 36, 4294 CrossRef CAS.
- L. I. Trakhtenberg, G. N. Gerasimov, L. N. Aleksandrova and V. K. Potapov, Radiat. Phys. Chem., 2002, 65, 479 CrossRef CAS.
- Y. Gao, P. Jiang, L. Song, J. X. Wang, L. F. Liu, D. F. Liu, Y. J. Xiang, Z. X. Zhang, X. W. Zhao, X. Y. Dou, S. D. Luo, W. Y. Zhou and S. S. Xie, J. Cryst. Growth, 2006, 289, 376 CrossRef CAS.
- S. Kidambi and M. L. Bruening, Chem. Mater., 2005, 17, 301 CrossRef CAS.
- G. T. Fei, R. Lu, Z. J. Zhang, G. S. Cheng, L. D. Zhang and P. Cui, Mater. Res. Bull., 1997, 32, 603 CrossRef CAS.
- K. Ohsaki and X. G. Li, Mater. Sci. Eng. A, 1999, 252, 141 CrossRef.
- C. Charton and M. Fahland, Surf. Coat. Technol., 2003, 174–175, 181 CrossRef CAS.
- N. Shirtcliffe, U. Nickel and S. Schneider, J. Colloid Interface Sci., 1999, 211, 122 CrossRef CAS.
- Z. Jian, Z. Xiang and W. Yongchang, Microelectron. Eng., 2005, 77, 58 CrossRef CAS.
- F. Mafune, Jun-ya Kohno, Y. Takeda, T. Kondow and H. Sawabe, J. Phys. Chem. B, 2000, 104, 9111 CrossRef CAS.
- J. S. Cho, A. Kimoto, M. Higuchi and K. Yamamoto, Macromol. Chem. Phys., 2005, 206, 635 CrossRef CAS.
- Y. W. Zhang, H. S. Peng, W. Huang, Y. F. Zhou and D. Y. Yan, J. Colloid Interface Sci., 2008, 325, 371 CrossRef CAS.
- K. Inoue, Polym. Sci., 2000, 25, 453 Search PubMed.
- A. Asif, C. Y. Huang and W. F. Shi, Colloid Polym. Sci., 2004, 283, 200 CrossRef CAS.
- J. Samuelsson, P. E. Sundell and M. Johansson, Prog. Org. Coat., 2004, 50, 193 CrossRef CAS.
- Y. Kaneko, Y. Imai, K. Shirai, T. Yamauchi and N. Tsubokawa, Colloids Surf., A, 2006, 289, 212 CrossRef CAS.
- J. Verrey, Y. Winkler and V. Michaud, Compos. Sci. Technol., 2005, 65, 1527 CrossRef CAS.
- S. M. Burkinshawa, P. E. Froehling and M. Mignanellia, Dyes Pigm., 2002, 53, 229 CrossRef.
- P. Kolhe, J. Khandare, O. Pillai, S. Kannan, M. L. Lai and R. Kannan, Pharm. Res., 2004, 21, 2185 CrossRef CAS.
- S. E. Stiriba, H. Kautz and H. Frey, J. Am. Chem. Soc., 2002, 124, 9698 CrossRef CAS.
- P. Gode, A. Hult and P. Jannasch, Solid State Ionics, 2006, 177, 787 CrossRef CAS.
- J. H. Fang, H. Kita and K. Okamoto, J. Membr. Sci., 2001, 182, 245 CrossRef CAS.
- J. H. Zou, Y. B. Zhao and W. F. Shi, J. Membr. Sci., 2004, 245, 35 CrossRef CAS.
- Y. W. Zhang, H. S. Peng, W. Huang, Y. F. Zhou, X. H. Zhang and D. Y. Yan, J. Phys. Chem. C, 2008, 112, 2330 CrossRef CAS.
- C. Y. Bao, M. Jin, R. Lu, T. R. Zhang and Y. Y. Zhao, Mater. Chem. Phys., 2003, 82, 812 CrossRef CAS.
- H. W. Lu, S. H. Liu, X. L. Wang, X. F. Qian, J. Yin and Z. K. Zhu, Mater. Chem. Phys., 2003, 81, 104 CrossRef CAS.
- C. H. Zhang, J. G. Li, J. Zhang, L. Y. Zhang and H. Y. Li, Polym. Adv. Technol., 2009, 21, 710 CrossRef.
- J. Yang and J. Y. Ying, Nat. Mater., 2009, 8, 683 CrossRef CAS.
- M. M. Alvarez, J. T. Khoury, T. G. Schaaff, M. N. Shafigullin, I. Vezmar and R. L. Whetten, J. Phys. Chem. B, 1997, 101, 3706 CrossRef CAS.
- J. Gao, J. Fu, C. Lin, J. Lin, Y. Han, X. Yu and C. Pan, Langmuir, 2004, 20, 9775 CrossRef CAS.
- O. P. Siwach and P. Sen, J. Lumin., 2009, 129, 6 CrossRef CAS.
- A. Mooradian, Phys. Rev. Lett., 1969, 22, 185 CrossRef CAS.
- P. C. Hiemez and R. Rajagopalan, Principles of Colloid and Surface Chemistry, Marcel Dekker, New York, 3rd edn, 1997 Search PubMed.
- O. Wolfbeis, A. Drkop, M. Wu and Z. Lin, Angew. Chem., Int. Ed., 2002, 41, 4495 CrossRef CAS.
- J. B. Jia, B. Q. Wang, A. G. Wu, G. J. Cheng, Z. Li and S. J. Dong, Anal. Chem., 2002, 74, 2217 CrossRef CAS.
- X. L. Luo, J. J. Xu, Q. Zhang, G. J. Yang and H. Y. Chen, Biosens. Bioelectron., 2005, 21, 190 CrossRef CAS.
- K. Cui, Y. H. Song, Y. Yao, Z. Z. Huang and L. Wang, Electrochem. Commun., 2008, 10, 663 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Structure and structure parameters of the HPCs, and UV-vis and XPS spectra of the NPs . See DOI: 10.1039/c0nr00567c |
| This journal is © The Royal Society of Chemistry 2011 |
