Biocompatible dispersions of carbon nanotubes: a potential tool for intracellular transport of anticancer drugs†
Antonello
Di Crescenzo
a,
Diana
Velluto
b,
Jeffrey A.
Hubbell
b and
Antonella
Fontana
*a
aDipartimento di Scienze del Farmaco, Università “G. d'Annunzio”, Via dei Vestini, I-66013, Chieti, Italy. E-mail: fontana@unich.it; Fax: +39 0871 3554791; Tel: +39 0871 3554790
bInstitute for Bioengineering and Institute for Chemical Sciences and Engineering, École Polytechnique Fédérale de Lausanne (EPFL), Station 15, CH-1015, Lausanne, Switzerland
First published on 23rd December 2010
Abstract
The use of the biocompatible amphiphilic diblock copolymer poly(ethylene glycol-b-propylene sulfide) (PEG44PPS20) allows a tuned loading of doxorubicin onto the surface of non-functionalized multi-walled carbon nanotubes and an efficient cell internalization. The obtained multi-walled carbon nanotube-based systems show enhanced cytotoxic activity with respect to non-vehicled doxorubicin.
The possibility of developing carbon nanotubes (CNTs) for biomedical applications became1–3 a reality after the main drawback of CNTs, i.e. their insolubility in aqueous media,4 was overcome. Different strategies have been proposed to make CNTs soluble and compatible with physiological conditions, both covalent5 and non-covalent approaches.6 Compared to covalent functionalization of the CNT sidewall, the supramolecular approach has the advantage of establishing strong interactions with the nanotubes without altering their electronic nature and peculiar features (i.e. optical properties useful for various biological imaging and sensing applications).
It has been previously demonstrated7 that the amphiphilic block copolymers poly(ethylene glycol-b-propylene sulfide) [PEG–PPS] favour a better disentanglement and dispersion of single-walled carbon nanotubes (SWNTs) in aqueous solution than can be obtained with the widely investigated Pluronics. The good suspendability of SWNTs in PEG–PPS was attributed to the steric stabilization brought about by the PEG chains that flank the adsorbing hydrophobic PPS block.7,8 Furthermore, cytotoxicity studies highlighted the biocompatibility of PEG–PPS coated SWNT suspensions up to a nanotube concentration of 50 µg mL−1, a concentration comparable to that of hydrophilically functionalized CNTs.9 The presence of PEG chains into the block copolymer endows CNTs with a hydrophilic coating, which also reduces protein adsorption and phagocytosis, thus lengthening their blood circulation time.10 CNTs have been recently proposed as bioactive delivery vehicles11 for the anticancer agent doxorubicin;12–14 nevertheless, factors such as the amount of loaded drug, the location and timing of the release, “the mechanism by which the complexes between CNT and doxorubicin are delivered into cells”,12 need to be further investigated. In this regard, the present experiments were carried out to assess the ability of multi-walled carbon nanotubes (MWNTs), dispersed with PEG44PPS20, to assist and direct the entry of the desired amount of doxorubicin in cancer cells and to enhance its cytotoxic activity.
The diblock PEG44PPS20 (Fig. 1), synthesized following previously reported literature protocols,15 has been proved7 to ensure the highest dispersion of CNTs among the other investigated di- or triblock analogues. Thus, our first goal was to understand the role of PEG44PPS20 in CNT penetration inside the cell. It is well known16–18 that hydrophilically functionalized CNTs may passively cross cell membranes, via a so-called “nanoneedle mechanism”, without causing cell damage or death. Hela cells, already employed for previous cytotoxicity studies,7 have been used to find out whether PEG44PPS20-coated MWNTs behave like covalently functionalized CNTs. We have used MWNTs rather than SWNTs, as the former can be more easily detected via microscopic techniques, especially when images are taken inside the cell where cytoskeleton structures could hamper nanotube visualization. We chose to use fluorescence microscopy techniques as “fluorescence can be detected with high sensitivity and signal specificity and is well suited for observing spatial properties of molecules within, for example, cells”.19 Different types of fluorescent-labelled CNTs have been used up to now to allow their visualization by fluorescence microscopy. However, the chemical processing of the CNT surface required to achieve fluorescent labelling alters their electronic properties and may interfere with the capability of CNTs to interact with the living biological matter. Therefore, we tracked the intracellular trafficking of CNTs in vitro by confocal microscopy by simply labelling their copolymeric coating. Thus, the block copolymer was labelled by covalently binding fluorescein isothiocyanate (FITC) to the end of the PPS chains of the dispersant PEG–PPS copolymer.20
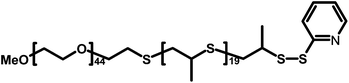 | ||
| Fig. 1 Chemical structure of PEG44PPS20. | ||
It has been recently demonstrated21 that the hydrophobic fluorescein group can bind to the sidewalls of CNTs (likely via π–π stacking) thus increasing the affinity of the block copolymer for the CNT surface. However, dispersing measurements, performed by following a previously published protocol,7 showed a dispersant efficiency of PEG44PPS20 13% higher than that of its fluorescent analogue (PEG–PPS–FITC).
Stable aqueous suspensions of fluorescent-labelled MWNTs were obtained by sonication of 1 mg of pristine MWNTs in 2 mL of 0.25 mg mL−1 PEG–PPS–FITC aqueous solution followed by centrifugation to remove non-dispersed MWNTs and other insoluble impurities (see detailed procedure in the ESI†). In order to eliminate unbound block copolymers, both in the form of monomers or micelles, size exclusion chromatography through Sepharose 6B columns was performed. Dynamic light scattering experiments gave rough evidence of the complete removal of unbound copolymer (see Fig. S1 of ESI†). Previously published studies,7 reporting on the stability of PEG44PPS20 coated carbon nanotubes under sink conditions, assured us that gel filtration and the subsequent dilution of the CNT dispersion did not induce the detachment of the copolymer from the CNT surface. Indeed, it has already been highlighted that other non-covalently bound solubilizing agents, such as properly functionalized phospholipids,22,23 or single stranded DNA,23 remain adsorbed to the SWNT surface upon dialysis and/or dilution.
Hela cells were incubated with 200 µL of PEG–PPS–FITC coated MWNTs dispersion eluted from gel filtration and 2 mL of cell culture Dulbecco's modified Eagle's medium (DMEM) for 48 hours (final concentration of MWNTs ≈ 25 µg mL−1).
The cells were washed several times with buffer and then observed by using a spinning disk confocal fluorescence microscope (Perkin Elmer). Fig. 2 highlights the presence within the cell of fluorescent structures due to single or small bundles (red arrows) of MWNTs covered by the fluorescent copolymer. Nevertheless, a few endosomal structures could also be visualized. These endosomes could either support the previously reported14,18,24 internalization of CNTs through endocytosis or be the result of endocytic uptake of the few polymeric micelles that still persist in the solution.
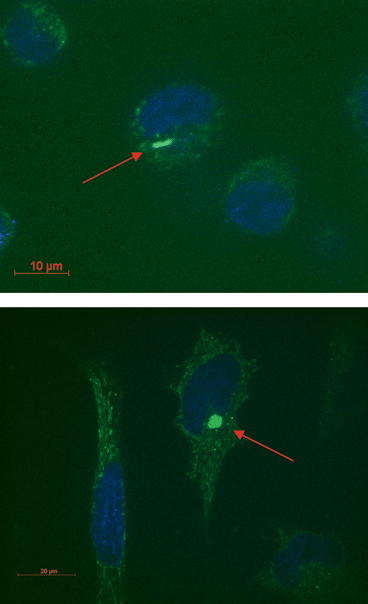 | ||
| Fig. 2 Confocal microscope images of Hela cells. Nuclei were stained with the fluorescent stain DAPI. MWNTs coated with the fluorescent copolymer can be visualized in the proximity of the nuclei (red arrows). | ||
Interestingly, the mentioned visualized fluorescent structures appear to reside mostly close to the nuclei with the consequent advantages for a prospective targeting of the CNT carrier. Actually the subcellular location of CNTs is still controversial. Some studies demonstrated that CNTs can enter the cells but not the nucleus,25,26 possibly translocating the transported drug24 or oligonucleotide23 into the nucleus, while other reports visualized SWNTs into the cell nucleus.16,27 We investigated the degree of cellular uptake of fluorescent-labelled MWNTs by using flow cytometry (see Fig. 3). After 36 hours of incubation with gel filtered PEG–PPS–FITC coated MWNTs solution, Hela cells were washed multiple times with buffer and analyzed. Fig. 3 shows that about 80% of cells internalized the non-covalently fluorescent-derivatized MWNTs. By using the size and the forward scattering, we gated the viable cells: Fig. 3d shows that the viability of treated cells is comparable to that of untreated cells used as the control (Fig. 3c), thus highlighting the non-toxicity of these MWNT dispersions under the investigated concentrations. Because one of the main causes of toxicity of CNTs is7,28,29 the insolubility of the nanotube material, the observed high viability gives further evidence of the strong adsorption of the block copolymer to the nanotubes that disfavours the reaggregation and precipitation of MWNTs.
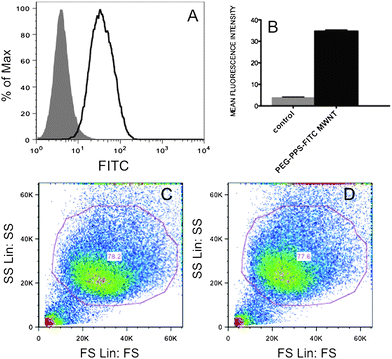 | ||
| Fig. 3 (A) Flow cytometric analysis of untreated Hela cells (gray solid histogram) and of Hela cells treated with PEG–PPS–FITC coated MWNT (black line histogram). (B) Mean fluorescence intensity of PEG–PPS–FITC coated MWNT treated cells calculated from flow cytometry histograms (n = 4). (C) Size scattering versus forward scattering of the main cell population of untreated and (D) treated sample respectively (n = 4). | ||
Once demonstrated that copolymer-coated MWNTs enter the cells without inducing toxicity, our aim was to evaluate whether these MWNTs could be efficiently loaded with doxorubicin (DOX) by supramolecular π–π stacking. Indeed, carbon nanotubes offer an undoubtedly large surface area for π–π interactions with the doxorubicin aromatic rings and CNTs coated with phospholipid–PEG13 or Pluronics12 have already been proposed as DOX carriers. Nevertheless, the advantages of using PEG–PPS copolymer with respect to the above mentioned solubilizing agents are at least threefold due to: (1) the better dispersant7 activity of the present block copolymer towards CNTs with respect to Pluronics and phospholipid–PEGs; (2) the superior stability and morphology control of supramolecular aggregates from PEG–PPS7,20 compared to supramolecular aggregates from Pluronic block copolymers or phospholipids, particularly relevant when aggregates have to withstand strong dilutions in the body fluids; (3) the low critical micellar concentration (CMC) of PEG44PPS20 block copolymer7,30 which allows block copolymer micelles to work in synergy with carbon nanotubes to favour the effective intracellular doxorubicin release. As a matter of fact, in the case of DOX loading, no gel filtration has been performed on the MWNT/DOX complex dispersions and several copolymer micelles could be present in the solution and work in synergy with MWNTs for DOX delivery. It has been previously demonstrated31 that copolymeric micelle (size ≈ 20 nm)30 can be large enough to avoid renal clearance yet sufficiently small to avoid uptake by the reticuloendothelial system.
To assess the loading capacity of the present nanotube carrier, we mixed the aqueous dispersion of PEG44PPS20 coated MWNTs, at various concentrations, with a solution of doxorubicin hydrochloride and characterized spectrofluorimetrically the resulting mixture. The DOX loading onto the nanotubes was performed by stirring the samples overnight at neutral pH in order to ensure a high drug loading and, at the same time, to avoid DOX decomposition.32DOX is a fluorescent molecule and shows a characteristic emission spectrum in the wavelength interval 500–750 nm (excitation wavelength 488 nm). A substantial decrease of fluorescence emission of DOX can be monitored upon increasing the amount of MWNTs relative to DOX (see Fig. 4). This decrease is likely due to the static quenching of DOX molecules whose chromophore groups interact via π–π stacking with carbon nanotube backbone.
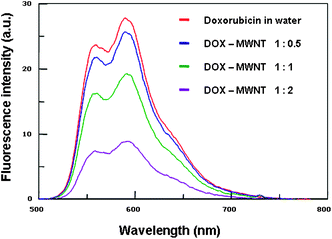 | ||
| Fig. 4 Fluorescence intensity of PEG44PPS20 coated MWNTs/DOX complexes at different weight ratios. The final concentration of DOX was 10 µg mL−1. The MWNT final concentration was gradually increased (5, 10, 20 µg mL−1). | ||
The obtained quenching of DOX upon increasing MWNTs concentration is indicative of increasing adsorption of DOX onto CNT surface. This evidence may be exploited for controlling DOX loading. As it has been demonstrated that the block copolymer does not detach from the carbon nanotubes under the adopted conditions, it is likely that the amount of loaded DOX depends on the surface area left free from the block copolymer adsorption. For this reason we performed several measurements following the above mentioned protocol but keeping the initial concentration of MWNTs and DOX constant (25 µg mL−1 each) and varying the concentration of the block copolymer in the range that assured the highest dissolution of MWNTs. It is worth mentioning that the investigated range of block copolymer concentration has been chosen in order to obtain stable MWNT/DOX solutions. The presence of copolymer in slight excess, with respect to the CMC value, allows non-adsorbed DOX to be solubilized inside the micelles and the prepared PEG44PPS20/MWNT/DOX complexes to remain in solution. PBS buffer was used throughout the experiment to control the pH of the solutions. In order to estimate the loading of DOX onto MWNT surface, the samples were filtered with Millex®GS 0.22 µm filters and the concentration of non-adsorbed DOX was quantified by using a Cary 100 (Varian) UV-Visible spectrophotometer. Fig. 5 shows that, under the investigated conditions, at constant amount of solubilized MWNT, the concentration of adsorbed DOX is inversely proportional to the concentration of block copolymer, thus allowing us to effectively tune the concentration of the drug at will. These data are confirmed by fluorescence measurements (see Fig. S2 in the ESI†).
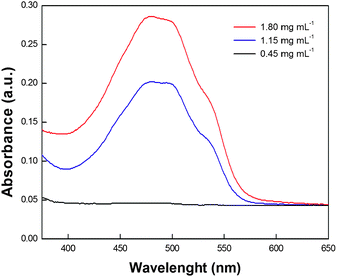 | ||
| Fig. 5 UV-vis spectra of filtered samples prepared by using different amounts of block copolymer: 0.45 mg mL−1 (black line), 1.15 mg mL−1 (blue line) and 1.80 mg mL−1 (red line) in PBS buffered solutions. The sample containing the lowest concentration of PEG44PPS20 (0.45 mg mL−1) does not evidence any DOX absorbance in agreement with a high adsorption of DOX onto MWNT sidewalls. | ||
Finally, we investigated the cytotoxic properties of the novel delivery system PEG44PPS20 coated CNT/DOX and compared it with the cytotoxic activity of a simple PEG44PPS20 coated MWNT dispersion and that of DOX alone. In vitro cytotoxicity studies were performed on the MDA-MB-435 human breast cancer cell line. The previously used7 Alamar Blue assay (see detailed procedure in the ESI†) was chosen to evaluate cytotoxicity, as this assay is considered the most reliable assay for CNTs. Fig. 6 shows that the tested aqueous copolymeric dispersion of MWNTs is non-toxic. Moreover, it highlights a statistically significant enhancement of cytotoxicity (∼20%) toward tumor cells in the presence of PEG44PPS20 coated MWNT/DOX as compared to DOX alone. Fig. 6 points out that the polymer favours the cytotoxic activity of DOX as well, because block copolymer micelles can behave as additional DOX carriers; nevertheless, the delivering capability of MWNTs appears to be much more significant (>10%) than that of the copolymer micelles.
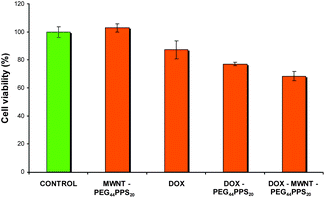 | ||
Fig. 6
Cell viability of MDA-MB-435 cells after 24 h incubation. Four experiments were performed in each case. Final concentrations: DOX 600 nM, MWNT 0.65 µg mL−1, PEG44PPS20 0.065 µg mL−1. DOX: MWNT weight ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. One-way analysis of variance (ANOVA), followed by Bonferroni's multiple comparison test, showed that the datum obtained for PEG44PPS20 coated MWNT/DOX is statistically different with respect to the ones obtained for the free DOX (p ≤ 0.05) or the PEG44PPS20/DOX (p ≤ 0.1). 2. One-way analysis of variance (ANOVA), followed by Bonferroni's multiple comparison test, showed that the datum obtained for PEG44PPS20 coated MWNT/DOX is statistically different with respect to the ones obtained for the free DOX (p ≤ 0.05) or the PEG44PPS20/DOX (p ≤ 0.1). | ||
In conclusion, the investigated PEG44PPS20 coated MWNTs appear to be good candidates for the delivery of anticancer drugs such as doxorubicin. We have demonstrated that PEG44PPS20 is a perfect biocompatible block copolymer capable of efficiently and stably dispersing CNTs and of finely tuning the DOX loading onto the nanotube surface. Confocal microscopy and flow cytometric analysis highlighted the capability of such complexes to penetrate the cell membrane and to reach the perinucleus region of the cell, although a mechanistic study needs to be carried out in order to investigate the real mechanism whereby these systems cross biological barriers. Finally, we have shown that the PEG44PPS20 coated MWNT/DOX complex exhibits enhanced cytotoxic activity compared to both doxorubicin alone and doxorubicin-loaded copolymer micelles.
Acknowledgements
We thank Prof. Maurizio Prato for supplying the nanotubes and for useful discussion. This work has been supported by MIUR (PRIN 2008, prot. 20085M27SS).References
- M. Prato, K. Kostarelos and A. Bianco, Acc. Chem. Res., 2008, 41, 60 CrossRef CAS.
- Z. Liu, S. Tabakman, K. Welsher and H. Dai, Nano Res., 2009, 2, 85 CrossRef CAS.
- Z. Liu, K. Chen, C. Davis, S. Sherlock, Q. Cao, X. Chen and H. Dai, Cancer Res., 2008, 68, 6652 CrossRef CAS; C. Tripisciano, K. Kraemer, A. Taylor and E. Borowiak-Palen, Chem. Phys. Lett., 2009, 478, 200 CrossRef CAS; J. Chen, S. Chen, X. Zhao, L. V. Kuznetsova, S. S. Wong and I. Ojima, J. Am. Chem. Soc., 2008, 130, 16778 CrossRef CAS; Y. Krupskaya, C. Mahn, A. Parameswaran, A. Taylor, K. Krämer, S. Hampel, A. Leonhardt, M. Ritschel, B. Büchner and R. Klingeler, J. Magn. Magn. Mater., 2009, 321, 4067 CrossRef CAS.
- M. F. Islam, E. Rojas, D. M. Bergey, A. T. Johnson and A. G. Yodh, Nano Lett., 2003, 3, 269 CrossRef CAS.
- J. Chen, M. A. Hamon, H. Hu, Y. Chen, A. M. Rao, P. C. Eklund and R. C. Haddon, Science, 1998, 282, 95 CrossRef CAS; V. Georgakilas, K. Kordatos, M. Prato, D. M. Guldi, M. Holzinger and A. Hirsch, J. Am. Chem. Soc., 2002, 124, 760 CrossRef CAS; D. Tasis, N. Tagmatarchis, A. Bianco and M. Prato, Chem. Rev., 2006, 106, 1105 CrossRef CAS.
- M. J. O'Connell, S. M. Bachilo, C. B. Huffman, V. C. Moore, M. S. Strano, E. H. Haroz, K. L. Rialon, P. J. Boul, W. H. Noon, C. Kittrell, J. P. Ma, R. H. Hauge, R. B. Weissman and R. E. Smalley, Science, 2002, 297, 593 CrossRef CAS; V. A. Sinani, M. K. Gheith, A. A. Yaroslavov, A. Rakhnyanskaya, K. Sun, A. A. Mamedov, J. P. Wicksted and N. A. Kotov, J. Am. Chem. Soc., 2005, 127, 3463 CrossRef CAS; A. Di Crescenzo, D. Demurtas, A. Renzetti, G. Siani, P. De Maria, M. Meneghetti, M. Prato and A. Fontana, Soft Matter, 2009, 5, 62 RSC.
- E. M. Di Meo, A. Di Crescenzo, D. Velluto, C. P. O'Neil, D. Demurtas, J. A. Hubbell and A. Fontana, Macromolecules, 2010, 43, 3429 CrossRef CAS.
- R. Shvartzman-Cohen, Y. Levi-Kalisman, E. Nativ-Roth and R. Yerushalmi-Rozen, Langmuir, 2004, 20, 6085 CrossRef CAS.
- A. Bianco, K. Kostarelos and M. Prato, Curr. Opin. Chem. Biol., 2005, 9, 674 CrossRef CAS; K. Kostarelos, Nat. Biotechnol., 2008, 26, 774 CrossRef CAS.
- M. S. Ryan, G. Mantovani, X. Wang, D. M. Haddleton and D. J. Brayden, Expert Opin. Drug Delivery, 2008, 5, 371 Search PubMed; Z. Liu, C. Davis, W. Cai, L. He, X. Chen and H. Dai, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 1410 CrossRef CAS.
- N. K. Mehra, A. K. Jain, N. Lodhi, R. Raj, V. Dubey, D. Mishra, M. Nahar and N. K. Jain, Crit. Rev. Ther. Drug Carrier Syst., 2008, 25, 169 Search PubMed.
- H. Ali-Boucetta, K. T. Al-Jamal, D. McCarthy, M. Prato, A. Bianco and K. Kostarelos, Chem. Commun., 2008, 459 RSC.
- Z. Liu, X. Sun, N. Nakayama-Ratchford and H. Dai, ACS Nano, 2007, 1, 50 CrossRef CAS.
- X. Zhang, L. Meng, Q. Lu, Z. Fei and P. J. Dyson, Biomaterials, 2009, 30, 6041 CrossRef CAS.
- A. Napoli, N. Tirelli, G. Kilcher and J. A. Hubbell, Macromolecules, 2001, 34, 8913 CrossRef CAS.
- K. Kostarelos, L. Lacerda, G. Pastorin, W. Wu, S. Wieckwski, J. Luangsivilay, S. Godefroy, D. Pantarotto, J. P. Briand, S. Muller, M. Prato and A. Bianco, Nat. Nanotechnol., 2007, 2, 108 CrossRef.
- D. Pantarotto, J. P. Briand, M. Prato and A. Bianco, Chem. Commun., 2004, 16 RSC.
- C. Ménard-Moyon, E. Venturelli, C. Fabbro, C. Samorì, T. Da Ros, K. Kostarelos, M. Prato and A. Bianco, Expert Opin. Drug Discovery, 2010, 5, 691 Search PubMed.
- M. Gumbletona and D. J. Stephens, Adv. Drug Delivery Rev., 2005, 57, 5 CrossRef CAS.
- S. Cerritelli, C. P. O'Neil, D. Velluto, A. Fontana, M. Adrian, J. Dubochet and J. A. Hubbell, Langmuir, 2009, 25, 11328 CrossRef CAS.
- N. Nakayama-Ratchford, S. Bangsaruntip, X. Sun, K. Welsher and H. Dai, J. Am. Chem. Soc., 2007, 129, 2448 CrossRef CAS.
- A. A. Shvedova, E. R. Kisin, D. Porter, P. Schulte, V. E. Kagan, B. Fadeel and V. Castranova, Pharmacol. Ther., 2009, 121, 192 CrossRef CAS.
- N. W. S. Kam, M. O'Connell, J. A. Wisdom and H. Dai, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 11600 CrossRef CAS.
- R. P. Feazell, N. Nakayama-Ratchford, H. Dai and S. J. Lippard, J. Am. Chem. Soc., 2007, 129, 8438 CrossRef CAS.
- L. Lacerda, G. Pastorin, D. Gathercole, J. Buddle, M. Prato, A. Bianco and K. Kostarelos, Adv. Mater., 2007, 19, 1480 CrossRef CAS.
- B. Kang, D.-C. Yu, S.-Q. Chang, D. Chen, Y.-D. Dai and Y. T. Ding, Nanotechnology, 2008, 19, 375103 CrossRef.
- J. Cheng, K. A. S. Fernando, L. M. Veca, Y.-P. Sun, A. I. Lamond, Y. W. Lam and S. H. Cheng, ACS Nano, 2008, 2, 2085 CrossRef CAS; E. Mooney, P. Dockery, U. Greiser, M. Murphy and V. Barron, Nano Lett., 2008, 8, 2137 CrossRef CAS; A. E. Porter, M. Gass, J. S. Bendall, K. Muller, A. Goode, J. N. Skepper, P. A. Midgley and M. Welland, ACS Nano, 2009, 3, 1485 CrossRef CAS; D. Pantarotto, R. Singh, D. McCarthy, M. Erhardt, J. P. Briand, M. Prato, K. Kostarelos and A. Bianco, Angew. Chem., Int. Ed., 2004, 43, 5242 CrossRef CAS.
- A. Bianco, K. Kostarelos, C. D. Partidos and M. Prato, Chem. Commun., 2005, 571 RSC.
- N. W. S. Kam and H. Dai, J. Am. Chem. Soc., 2005, 127, 6021 CrossRef CAS.
- D. Velluto, D. Demurtas and J. A. Hubbel, Mol. Pharmaceutics, 2008, 5, 632 CrossRef CAS.
- C. P. O'Neil, A. J. van der Vlies, D. Velluto, C. Wandrey, D. Demurtas, J. Dubochet and J. A. Hubbell, J. Controlled Release, 2009, 137, 146 CrossRef CAS.
- M. J. H. Janssen, D. J. A. Crommelin, G. Storm and A. Hulshoff, Int. J. Pharm., 1985, 23, 1 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: (1) Experimental section; (2) Results: DLS analysis (Fig. S1); fluorescence intensity of PEG44PPS20 coated MWNTs/DOX complexes prepared in the presence of different amounts of block copolymer (Fig. S2). See DOI: 10.1039/c0nr00444h |
| This journal is © The Royal Society of Chemistry 2011 |
