A Bodipy-based derivative for selective fluorescence sensing of homocysteine and cysteine†
Ying
Yue
ab,
Yong
Guo
*a,
Jian
Xu
a and
Shijun
Shao
*ac
aKey Laboratory of Chemistry of Northwestern Plant Resources and Key Laboratory for Natural Medicine of Gansu Province, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou 730000, P. R. China. E-mail: guoyong@licp.cas.cn; sjshao@licp.cas.cn
bGraduate School of the Chinese Academy of Sciences, Beijing 100039, P. R. China
cState Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou 730000, P. R. China
First published on 29th November 2010
Abstract
A method for the selective fluorescence sensing of homocysteine (Hcy) and cysteine (Cys) under neutral pH conditions, based on the reaction of the simple aldehyde containing Bodipy dye with Hcy/Cys, has been developed. The location of the aldehyde group at the Bodipy core is important and has an effect on the cyclization with Hcy/Cys.
The design and synthesis of systems which are capable to sense analytes are of major interest for thiol compounds involved in chemical and physiological processes in organisms. As a particular interest, Hcy and Cys play crucial roles in plasma. For instance, Hcy is the risk factor for human diseases such as Alzheimer's, Behcet's, and cardiovascular disease;1 an abnormal level of Cys links to the liver damage, skin lesions, and slowed growth.2 Thus, the search for sensitive and selective sensors for Hcy and Cys has aroused intense attention. A variety of optical probes for thiols, utilizing the strong nucleophilicity of thiols and their high binding affinity towards metal ions, have been reported.3 Recently, a few fluorescent and colorimetric methods for the selective detection of Hcy and Cys have been reported, based on the dyes containing aldehydes that afforded very stable thiazolidine or thiazinane derivatives by the reaction with Hcy or Cys.4
A fluorescent probe, which is composed of a fluorophore and a receptor, used in cells or in vivo detection should be sensitive, selective, stable, and reliable. Among the many known fluorescent dyes, Bodipy dyes have gained recognition and popularity with chemists and biochemists, because of their high extinction coefficient, high quantum yield, narrow emission bandwidth, and thus good signal/noise ratio, insensitivity to pH and solvent polarity, greater chemical and photochemical stabilities in solution and in solid state, and low biological toxicity.5 Applications of Bodipy derivatives have been greatly enlarged in number and range, they are used as biomolecular labels, protein probes, cation sensors, anion chemosensors, etc.6 A probe based on the Bodipy derivative aiming to selectively detect Hcy and Cys, however, has not been developed to date.
By incorporating a Bodipy uorophore within the aldehyde group, herein, we have developed two fluorescent compounds to detect Hcy and Cys (Scheme 1). Compounds 1 and 2 were designed to reveal the reactivity of aldehyde groups located on different positions of Bodipy. The influence of the electronic effect of the Bodipy core on the reactivity of aldehyde groups in the two compounds was discussed.
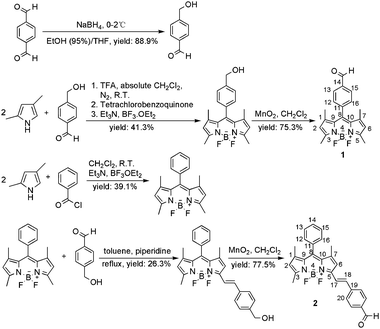 | ||
| Scheme 1 Synthetic routes of target compounds 1 and 2. | ||
Synthetic routes are shown in Scheme 1. 4-Hydroxymethyl-benzaldehyde as the key reagent is obtained by the reduction of dialdehyde.7a Compound 1 was synthesized according to references.7 The second compound, 2, was obtained by the Knoevenagel-type reaction of aldehyde with a known Bodipy derivative, 1,3,5,7-tetramethyl-8-phenyl-Bodipy.
High selectivity is a matter of necessity for an excellent probe in the application of detecting amino acids. The fluorescence response of compound 1 to various amino acids and reduced glutathione (GSH) was investigated by emission spectroscopy in a 0.1 M HEPES buffer of pH 7.4/CH3CN (v/v, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) at ambient temperature (Fig. 1). Probe 1 (2.0 × 10−6 M) was treated with 100 equiv. of a series of amino acids or GSH separately. As shown in Fig. 1, the reaction of Hcy or Cys with the probe results in a significant fluorescence enhancement around 513 nm. In contrast, other amino acids, including Ala, Thr, Arg, Gly and GSH, produce no discernible changes in emission intensities for the probe 1 and do not interfere with their Hcy/Cys response (inset graph in Fig. 1). The emission profiles of 1–Hcy and 1–Cys are unchanged in the presence of various amino acids, GSH or all of them, indicating excellent selectivities for Hcy and Cys. A possible mechanism is proposed to explain these results (Scheme 2). When Hcy or Cys is added, the aldehyde group transforms into a stable ring of thiazinane or thiazolidine (compound 1′), which is formed by thiol and amine together. This is a key element for the selective recognition of Hcy and Cys.
3) at ambient temperature (Fig. 1). Probe 1 (2.0 × 10−6 M) was treated with 100 equiv. of a series of amino acids or GSH separately. As shown in Fig. 1, the reaction of Hcy or Cys with the probe results in a significant fluorescence enhancement around 513 nm. In contrast, other amino acids, including Ala, Thr, Arg, Gly and GSH, produce no discernible changes in emission intensities for the probe 1 and do not interfere with their Hcy/Cys response (inset graph in Fig. 1). The emission profiles of 1–Hcy and 1–Cys are unchanged in the presence of various amino acids, GSH or all of them, indicating excellent selectivities for Hcy and Cys. A possible mechanism is proposed to explain these results (Scheme 2). When Hcy or Cys is added, the aldehyde group transforms into a stable ring of thiazinane or thiazolidine (compound 1′), which is formed by thiol and amine together. This is a key element for the selective recognition of Hcy and Cys.
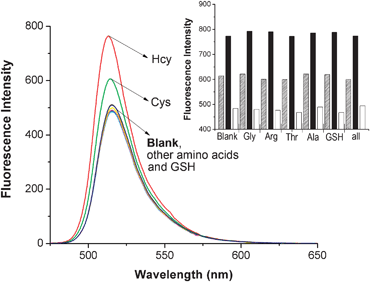 | ||
Fig. 1 Emission spectra (λex = 498 nm) of compound 1 (2.0 × 10−6 M) with or without various amino acids and GSH (100 equiv.) in CH3CN/H2O (v/v, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) at pH 7.4 buffered with 0.1 M HEPES at ambient temperature. (Inset: fluorescence responses of 1 to Hcy/Cys in the presence of various amino acids, GSH or all of them at 513 nm. White bars represent the integrated emission of 1 in the presence of 100 equiv. of other amino acids/GSH/all of them of interest; black and shadow bars represent the changes in integrated emission that occur upon subsequent addition of 100 equiv. of Hcy and Cys to solutions containing 1 and other amino acids/GSH/all of them of interest, respectively.) 2) at pH 7.4 buffered with 0.1 M HEPES at ambient temperature. (Inset: fluorescence responses of 1 to Hcy/Cys in the presence of various amino acids, GSH or all of them at 513 nm. White bars represent the integrated emission of 1 in the presence of 100 equiv. of other amino acids/GSH/all of them of interest; black and shadow bars represent the changes in integrated emission that occur upon subsequent addition of 100 equiv. of Hcy and Cys to solutions containing 1 and other amino acids/GSH/all of them of interest, respectively.) | ||
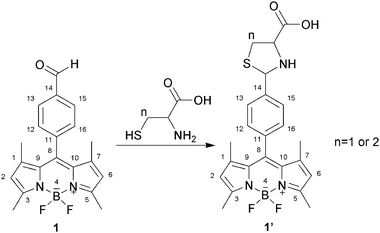 | ||
| Scheme 2 Reaction of compound 1 with Hcy or Cys to form thiazinane (n = 2) or thiazolidine (n = 1). | ||
The characteristics of the reaction between 1 and Hcy/Cys were studied by titration experiments. Upon the addition of an increasing concentration of Hcy (0–1000 equiv.) to the solution of probe 1 (1.0 × 10−6 M), the intensities of the emission bands around 513 nm increase gradually, and the λmax of emission bands shows a hypsochromic shift of about 4 nm to 509 nm (Fig. 2). At the same time, the fluorescence quantum yields of 1 increase from 0.26 to 0.71, which are determined by using fluorescein isothiocyanate as the standard in a 0.1 M NaOH solution (φ = 0.90).8 From the fluorescence titration experiment, the binding constant (Ka) of probe 1 with Hcy is obtained from the variation in the fluorescence intensity at the appropriate wavelength 509 nm (1′) by plotting 1/(I0 − I) against 1/Hcy based on the Benesi–Hildebrand analysis9 (ESI†). The binding constant for probe 1 with Hcy is determined to be 1300 M−1. In addition, the good linearity also indicates the presence of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 probe 1 to Hcy interaction (inset graph in Fig. 2). A similar fluorescence enhancement is also found for Cys (Fig. S2, ESI†). The fluorescence quantum yields of 1 increase from 0.26 to 0.63. The binding constant for probe 1 with Cys is determined to be 741 M−1. No visible changes, however, are observed in the absorbance spectra of 1 + Hcy and 1 + Cys (Fig. S3, ESI†).
1 probe 1 to Hcy interaction (inset graph in Fig. 2). A similar fluorescence enhancement is also found for Cys (Fig. S2, ESI†). The fluorescence quantum yields of 1 increase from 0.26 to 0.63. The binding constant for probe 1 with Cys is determined to be 741 M−1. No visible changes, however, are observed in the absorbance spectra of 1 + Hcy and 1 + Cys (Fig. S3, ESI†).
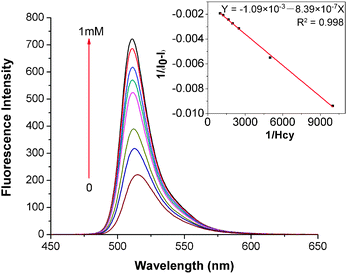 | ||
Fig. 2 Emission spectra (λex = 498 nm) of compound 1 (1.0 × 10−6 M) upon the addition of increasing concentration (0, 0.1, 0.2, 0.4, 0.5, 0.6, 0.8, 1 mM) of Hcy in CH3CN/H2O (v/v, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) at pH 7.4 buffered with 0.1 M HEPES at ambient temperature. (Inset: the Benesi–Hildebrand plot of 1 with Hcy, in which the fluorescence intensity (1/(I0 − I)) at 509 nm is plotted against the concentration of 1/Hcy.) 2) at pH 7.4 buffered with 0.1 M HEPES at ambient temperature. (Inset: the Benesi–Hildebrand plot of 1 with Hcy, in which the fluorescence intensity (1/(I0 − I)) at 509 nm is plotted against the concentration of 1/Hcy.) | ||
We speculate that the enhancement of fluorescence intensity upon the addition of Hcy and Cys is due to the transformation from F–A system 1 (F—Bodipy fluorophore; A—electron acceptor, formylphenyl) to F–D system 1′ (D—electron donator, alkyl substituent with sulfur and amino group) by the reaction of Hcy or Cys with the aldehyde group. The mutation of the pull–push effect leads to the enhancement of the fluorescence yield.4c Besides, the optimized geometries of 1 and 1′ were investigated to reveal the reason why the emission intensity of 1–Hcy is higher than that of 1–Cys. Calculated structures and relevant geometrical parameters are shown in ESI† (Fig. S4 and Table S1). As for 1 or 1–Cys, the formylphenyl group is nearly perpendicular to the Bodipy core, excluding any significant conjugation between 8-phenyl and the Bodipy core. While the dihedral angle C(9)–C(8)–C(11)–C(12) of 1–Hcy is 66.6°, indicating that the planarity and conjugated relationship between 8-phenyl and the Bodipy core of 1–Hcy is better than those of 1 and 1–Cys, so the molecular rigidity and fluorescence intensity of 1–Hcy increase and are higher than those of 1–Cys.
Compound 2 was synthesized with the expectation that the aldehyde group will generate higher reactivity than that in compound 1. Surprisingly, no significant changes were observed in the fluorescence emission spectra (λex = 560 nm) and absorption spectra of 2 in the presence of Hcy or Cys. In order to determine whether or not the reaction has taken place, HPLC-FLD and HPLC-UV/vis were used to compare two sample solutions of 2, one with and one without Hcy. The results showed that no difference appeared in the chromatogram signals. In other words, the reaction of 2 with Hcy or Cys does not take place. To boost the reaction, we tried to increase pH from 3 to 11 and raise the reaction temperature to 60 °C, but without success. These facts indicate that the exact location of the aldehyde group in a Bodipy derivative affects its reactivity.
To find the influence of electronic effect on the reactivity of aldehyde group with Hcy, a comparative experiment was carried out using benzaldehyde, p-hydroxybenzaldehyde and p-nitrobenzaldehyde. Under the same conditions as Hcy with the probe 1, Hcy only reacts with p-nitrobenzaldehyde, indicating that the electron-withdrawing effect could enhance the electropositivity of the aldehyde group and raises the reactivity. Thus, this result illustrates that the Bodipy core shows an electron-withdrawing effect to the aldehyde group in 1.
The conformational properties of compounds 1 and 2 were studied by molecular mechanics and semi-empirical calculations to investigate the influence of the location of the aldehyde group on the reactivity. As shown in Fig. S5 (ESI†), the geometries of 1 and 2 illustrate the almost vertical relationship between 8-formylphenyl and the Bodipy core owing to the presence of the methyl groups at C-1 and C-7 positions, while formylphenyl through ethylene linked at 5-methyl is almost in the same plane as the Bodipy core. Relevant geometrical parameters are compiled in Table S2, ESI†. These data indicate that the Bodipy cores of 1 and 2 have planar geometries. As for 1, the formylphenyl group is nearly perpendicular to the Bodipy core (dihedral angle C(9)–C(8)–C(11)–C(12) 90.2°), excluding any significant conjugation between aldehyde-phenyl and the Bodipy core. The role of the electron-withdrawing effect of the Bodipy core appears and becomes a dominant factor that promotes the reaction of the aldehyde group in 1. While in the structure of 2, formylphenyl is fully conjugated with the Bodipy core. Because of the conjugative effect, the averaging of the electron cloud density depresses the electropositivity of the carbon in the aldehyde group, which results in the failure of the reaction between 2 and Hcy/Cys. 1H-NMR spectra and infrared spectra of compounds 1 and 2 can also confirm this deduction. The singlet peak at 10.10 ppm is the aldehyde group in 1, while the aldehyde group in 2 shifts upfield to 10.01 ppm. Moreover, infrared spectra show that 1699 cm−1 band, assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of the aldehyde group of 1, shifts to higher wave number as compared with that of 2. These data illustrate that carbon of aldehyde in 2 has higher electronic density than that of 1. So, we hold that the electrophilic reactivity of aldehyde in 1 is higher than that in 2.
O stretching of the aldehyde group of 1, shifts to higher wave number as compared with that of 2. These data illustrate that carbon of aldehyde in 2 has higher electronic density than that of 1. So, we hold that the electrophilic reactivity of aldehyde in 1 is higher than that in 2.
Compound 1 (1.2 × 10−5 M) is used as the derivative reagent to the simultaneous detection of Hcy and Cys (2.0 × 10−4 M, respectively) in a mixed solution by the separation of HPLC-UV/Vis-MS. As shown in Fig. 3, the three peaks are separated absolutely. The mass spectra results indicate that the m/z of peaks a and c, 456.2 and 470.2, attributes to 1–Cys and 1–Hcy, respectively, while m/z of peak b is 353.3, corresponding to probe 1. The result also confirms that thiazinane or thiazolidine is formed by the interaction of aldehyde with Hcy or Cys. However, at present this method cannot be used quantitatively to determine Hcy and Cys because the linear ranges of Hcy and Cys have not been found.
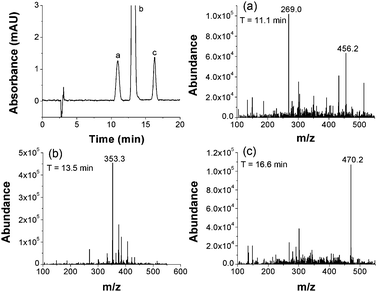 | ||
Fig. 3 Chromatogram of a mixture of Hcy and Cys using probe 1 as the pre-column reagent. Mobile phase: methanol–water (AcOH, pH = 3.5), 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 (v/v). Detection: UV/Vis (500 nm) and MS detector. Flow rate: 1 mL min−1. T: 20 °C. Injection volume: 20 μL. Standard thiols concentration: 0.2 mM for Hcy and Cys. The concentration of 1 is 12 μM. 1 and two amino acids in the mixed solution of CH3CN 30 (v/v). Detection: UV/Vis (500 nm) and MS detector. Flow rate: 1 mL min−1. T: 20 °C. Injection volume: 20 μL. Standard thiols concentration: 0.2 mM for Hcy and Cys. The concentration of 1 is 12 μM. 1 and two amino acids in the mixed solution of CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (3 H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Peaks: a: 1–Cys (11.1 min (a)), b: compound 1 (13.5 min (b)) and c: 1–Hcy (16.6 min (c)). 2). Peaks: a: 1–Cys (11.1 min (a)), b: compound 1 (13.5 min (b)) and c: 1–Hcy (16.6 min (c)). | ||
Thus, in this work, we have shown the utility of the Bodipy chemistry in the design and synthesis of a determination system for Hcy and Cys. The sensors are based on the reaction of the aldehyde group with Hcy or Cys to form a rather stable thiazinane or thiazolidine derivative. The exact location of the aldehyde-phenly group is important, if it is at the meso position, upon the addition of Hcy and Cys, the fluorescence intensities enhance by the mutation of the pull–push effect; if it is in full conjugation with the Bodipy core, however, the averaging of the electron cloud density depresses the electropositivity of carbon in aldehyde and results in a failure of the reaction with Hcy and Cys. These provide a theoretical basis for the development of probes based on Bodipy derivatives and for the application of the reaction between aldehyde and Hcy/Cys.
This work was supported by the National Natural Science Foundation of China (Grant No. 20972170 to S.-J. Shao), the open fund of State Key Laboratory of Oxo Synthesis & Selective Oxidation (Grant No. OSSO2008kjk6 to S.-J. Shao) and by the Natural Science Foundation of Gansu province (No. 096RJ2A033 to Y. Guo).
Experimental section
Synthesis
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1618, 1599, 1541, 1496 (C
O), 1618, 1599, 1541, 1496 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) cm−1. MS (ESI): calcd for C20H19BF2N2O: 352.2, found: m/z 353.3 (M + H)+.
C) cm−1. MS (ESI): calcd for C20H19BF2N2O: 352.2, found: m/z 353.3 (M + H)+.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1617, 1595, 1546, 1497 (C
O), 1617, 1595, 1546, 1497 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) cm−1. MS (ESI): calcd for C27H23BF2N2O: 440.2, found: m/z 441.4 (M + H)+.
C) cm−1. MS (ESI): calcd for C27H23BF2N2O: 440.2, found: m/z 441.4 (M + H)+.
Notes and references
- (a) P. M. Ueland and H. Refsum, J. Lab. Clin. Med., 1989, 114, 473 CAS; (b) Y. Ozkan, S. Yardim-Akaydin, A. Sepici, B. Engin, V. Sepici and B. Simsek, Clin. Chem. Lab. Med., 2007, 45, 73 CrossRef CAS; (c) S. Seshadri, A. Beiser, J. Selhub, P. F. Jacques, I. H. Rosenberg, R. B. D'Agostino, P. W. F. Wilson and P. A. Wolf, N. Engl. J. Med., 2002, 346, 476 CrossRef CAS.
- S. Shahrokhian, Anal. Chem., 2001, 73, 5972 CrossRef CAS.
- (a) X. Q. Chen, Y. Zhou, X. J. Peng and J. Y. Yoon, Chem. Soc. Rev., 2010, 39, 2120 RSC; (b) X. F. Guo, H. Wang, Y. H. Guo, Z. X. Zhang and H. S. J. Zhang, J. Chromatogr., A, 2009, 1216, 3874 CrossRef CAS; (c) T. Matsumoto, Y. Urano, T. Shoda, H. Kojima and T. Nagano, Org. Lett., 2007, 9, 3375 CrossRef CAS; (d) W. Lin, L. Yuan, Z. Cao, F. Feng and L. Long, Chem.–Eur. J., 2009, 15, 5096 CrossRef CAS; (e) H. Maeda, H. Matsuno, M. Ushida, K. Katayama, K. Saeki and N. Itoh, Angew. Chem., Int. Ed., 2005, 44, 29; (f) K. Okabe, R. Wada, K. Ohno, S. Uchiyama, T. Santa and K. Imai, J. Chromatogr., A, 2002, 982, 111 CrossRef CAS; (g) G. Minniti, A. Piana, U. Armani and R. J. Cerone, J. Chromatogr., A, 1998, 828, 401 CrossRef CAS; (h) S. C. Liang, H. Wang, Z. M. Zhang and S. H. Zhang, Anal. Bioanal. Chem., 2005, 381, 1095 CrossRef CAS; (i) H. Wang, W. S. Wang and H. S. Zhang, Talanta, 2001, 53, 1015 CrossRef CAS.
- (a) D. Q. Zhang, M. Zhang, Z. Q. Liu, M. X. Yu, F. Y. Li, T. Yi and C. H. Huang, Tetrahedron Lett., 2006, 47, 7093 CrossRef CAS; (b) O. Rusin, N. N. St. Luce, R. A. Agbaria, J. O. Escobedo, S. Jiang, I. M. Warner, F. B. Dawan, K. Lian and R. M. Strongin, J. Am. Chem. Soc., 2004, 126, 438 CrossRef CAS; (c) L. P. Duan, Y. F. Xu, X. H. Qian, F. Wang, J. W. Liu and T. Y. Cheng, Tetrahedron Lett., 2008, 49, 6624 CrossRef CAS; (d) K. S. Lee, T. K. Kim, J. H. Lee, H. J. Kim and J. I. Hong, Chem. Commun., 2008, 6173 RSC; (e) X. J. Zhang, X. S. Ren, Q. H. Xu, K. P. Loh and Z. K. Chen, Org. Lett., 2009, 11, 1257 CrossRef CAS; (f) W. H. Wang, O. Rusin, X. Y. Xu, K. K. Kim, J. O. Escobedo, S. O. Fakayode, K. A. Fletcher, M. Lowry, C. M. Schowalter, C. M. Lawrence, F. R. Fronczek, I. M. Warner and R. M. Strongin, J. Am. Chem. Soc., 2005, 127, 15949 CrossRef CAS; (g) W. Lin, L. Long, L. Yuan, Z. Cao, Bi. Chen and W. Tan, Org. Lett., 2008, 10, 5577 CrossRef CAS; (h) F. Tanaka, N. Mase and C. F. Barbas III, Chem. Commun., 2004, 1762 RSC; (i) H. Chen, Q. Zhao, Y. Wu, F. Li, H. Yang, T. Yi and C. Huang, Inorg. Chem., 2007, 46, 11075 CrossRef CAS; (j) M. Zhang, M. Li, Q. Zhao, F. Li, D. Zhang, Ji Zhang, T. Yi and C. Huang, Tetrahedron Lett., 2007, 48, 2329 CrossRef CAS; (k) K. Huang, H. Yang, Z. Zhou, H. Chen, F. Li, T. Yi and C. Huang, Inorg. Chim. Acta, 2009, 362, 2577 CrossRef CAS; (l) H. Li, J. Fan, J. Wang, M. Tian, J. Du, S. Sun, P. Sun and X. Peng, Chem. Commun., 2009, 5904 RSC; (m) T.-K. Kim, D.-N. Lee and H.-J. Kim, Tetrahedron Lett., 2008, 49, 4879 CrossRef CAS.
- R. Ziessel, G. Ulrich and A. Harriman, New J. Chem., 2007, 31, 496 RSC.
- (a) J. Karolin, L. B. A. Johansson, L. Strandberg and T. Ny, J. Am. Chem. Soc., 1994, 116, 7801 CrossRef CAS; (b) Y. Gabe, T. Ueno, Y. Urano, H. Kojima and T. Nagano, Anal. Bioanal. Chem., 2006, 386, 621 CrossRef CAS; (c) J. P. Malval, I. Leray and B. Valeur, New J. Chem., 2005, 29, 1089 RSC; (d) Y. Shiraishi, H. Maehara, T. Sugii, D. P. Wang and T. Hirai, Tetrahedron Lett., 2009, 50, 4293 CrossRef CAS; (e) O. A. Bozdemir, F. Sozmen, O. Buyukcakir, R. Guliyev, Y. Cakmak and E. U. Akkaya, Org. Lett., 2010, 12, 1400 CrossRef CAS; (f) Z. Ekmekci, M. D. Yilmaz and E. U. Akkaya, Org. Lett., 2008, 10, 461 CrossRef CAS; (g) A. Coskun and E. U. Akkaya, J. Am. Chem. Soc., 2006, 128, 14474 CrossRef CAS; (h) S. Kolemen, Y. Cakmak, S. Erten-Ela, Y. Altay, J. Brendel, M. Thelakkat and E. U. Akkaya, Org. Lett., 2010, 12, 3812 CrossRef CAS.
- (a) N. M. Loim and E. S. Kelbyscheva, Russ. Chem. Bull., 2004, 53, 2080 Search PubMed; (b) A. Coskun, E. Deniz and E. U. Akkaya, Org. Lett., 2005, 7, 5187 CrossRef CAS; (c) T. Kalai and K. Hideg, Tetrahedron, 2006, 62, 10352 CrossRef CAS.
- J. N. Demas and G. A. Crosby, J. Phys. Chem., 1971, 75, 991 CrossRef.
- (a) H. A. Benesi and J. H. Hildebrand, J. Am. Chem. Soc., 1949, 71, 2703 CrossRef CAS; (b) V. Thiagarajan, P. Ramamurthy, D. Thirumalai and V. T. Ramakrishnan, Org. Lett., 2005, 7, 657 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Instruments and reagents. Spectral data of the two compounds; details concerning the Benesi–Hildebrand analysis; spectra of titration experiments; calculated structural figures and selected data. See DOI: 10.1039/c0nj00720j |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2011 |
