Design of a dual sensing highly selective cyanide chemodosimeter based on pyridinium ring chemistry†
Sabir H.
Mashraqui
*a,
Rupesh
Betkar
a,
Mukesh
Chandiramani
a,
Carolina
Estarellas
b and
Antonio
Frontera
b
aDepartment of Chemistry, University of Mumbai, Vidyanagari, Santacruz(E), Mumbai-400098, India. E-mail: sh_mashraqui@yahoo.com; Fax: +91 022-6528547; Tel: +91 022-26526091
bDepartment of Quimica, Universitat de les Illes Balears, Spain. E-mail: toni.frontera@uib.es
First published on 17th November 2010
Abstract
A new chemodosimeter, Quino-P, recognizes the strongly nucleophilic cyanide by dual colorimetric and fluorescence ‘off–on’ signalling responses. Noteworthily, several other anions, even in significantly higher concentrations, induce no detectable photophysical perturbations. The chemodosimeter mechanism involves formation of a C4–cyano adduct, which exhibits an uncommon phenomenon of enhanced internal charge transfer interaction.
The development of optical chemosensors for anions of biological, environmental, and clinical concerns is currently under active investigation.1 Among anions of interest, the trace detection of cyanide is of prime importance because of its lethal effects on livestock, mammals, humans and the environment.2Cyanide contamination in the environment is possible due to its widespread uses in synthetic fibres, resins, herbicides, gold-extraction and various metallurgy processes.3 Moreover, incidences of cyanide poisoning in human and animals can also occur through ingestion of cyanogenic glycosides found in certain vegetables and dietary foodstuffs.4Cyanide exposure through accidental or intentional release in drinking water or food-chains can culminate in environmental damages and in humans, cyanide poisoning can result in convulsion, loss of consciousness, and even death.
A recent review by Yoon et al. summarizes the hitherto reported optical cyanide sensing strategy,5 which includes H-bonding interaction, metal ion displacements, cyano-borane adduct formations, rearrangements and quantum dots. Besides, the strong nucleophilic character of CN− has also been harnessed to design internal charge transfer (ICT) based chemodosimeters carrying receptors, such as oxazine,6pyrylium salts,7 squarane,8acridinium salt,9cyanine dye,10trifluoroacetophenone,11dicyano-vinyl calix[4]pyrrole12 and analogs.13 Typically, these chromophores are specifically designed so as to undergo disruption in their π-conjugations upon irreversible addition of cyanide, offering color bleaching responses. However, ICT-based chemodosimeters capable of executing absorbance red shifts upon exposure to cyanide are indeed rare.6,14
Herein, we present a new chemodosimeter design that carries the requisite structural elements to convert the CN− binding event into an enhanced ICT process, delivering absorbance red shift and a high fluorescence turn-on response. The π-deficient pyridinium ring is well-known to react readily with a variety of carbon and hetero-atom nucleophiles to form the π-rich, 1,4-dihydropyridine (DHP) adducts, often with good regioselectivity.15 Mimicking this aspect of the pyridinium ring chemistry, we have designed a novel CN− selective probe, Quino-P, incorporating a pyridinium ring as a CN−receptor, while a quinoline moiety is appended to serve as an electron withdrawing chromogenic unit.
The synthesis of Quino-P was readily accomplished by implementing Scheme 1. Base catalyzed condensation of isatin 1 with 3-acetylpyridine 2 afforded quinoline–pyridyl conjugate 3, which upon esterification yielded 4. Quaternization of 4 with CH3I in dry CH3CN afforded iodide salt 5, which upon perchlorate anion exchange with Mg(ClO4)2 gave the target, Quino-P as a pale yellow solid in good overall yield. A proposed CN− sensing mechanism is illustrated in Scheme 2. Presuming regioselective reaction, the nucleophilic attack of cyanide on the C4 position of the pyridinium ring would form the corresponding cyano–1,4-DHP adduct. Being π-electron rich, the 1,4-DHP moiety in the resulting cyano-adduct is expected to engage in a push–pull interaction with the conjugated, π-deficient, quinoline ring. This phenomenon would provoke enhanced ICT interaction, thereby forming the basis of a potentially novel cyanide signalling protocol.
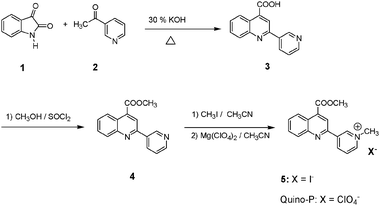 | ||
| Scheme 1 Synthesis of Quino-P. | ||
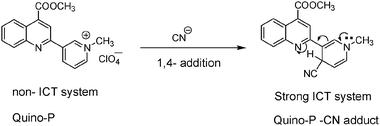 | ||
| Scheme 2 Proposed cyanide sensing mechanism. | ||
The sensitivity of Quino-P towards various anions, as their tetra-n-butylammonium (TBA) salts, was evaluated by optical spectral analysis. The absorption spectrum of Quino-P displayed maxima at 330 and 267 nm, attributable to the local excitations of pyridinium and quinoline rings, respectively. As shown in Fig. 1, the UV-vis profile of the probe (2.8 × 10−5 M) in DMSO–H2O (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v) in tris-HCl buffer pH 7.0 was essentially insensitive to each of F−, AcO−, SCN−, HSO4−, NO3−, Br−, Cl−, I− and H2PO4−, up to 7.5 × 10−2 M, implying the absence of any detectable ground state interactions of these anions with the probe. In contrast, cyanide, at 10-fold lower concentration (7.6 × 10−3 M), induced a significant interaction, as evidenced by an instant color change from the initial colorless to deep yellow, an event that allows selective visual detection of cyanide by the naked eye.
3, v/v) in tris-HCl buffer pH 7.0 was essentially insensitive to each of F−, AcO−, SCN−, HSO4−, NO3−, Br−, Cl−, I− and H2PO4−, up to 7.5 × 10−2 M, implying the absence of any detectable ground state interactions of these anions with the probe. In contrast, cyanide, at 10-fold lower concentration (7.6 × 10−3 M), induced a significant interaction, as evidenced by an instant color change from the initial colorless to deep yellow, an event that allows selective visual detection of cyanide by the naked eye.
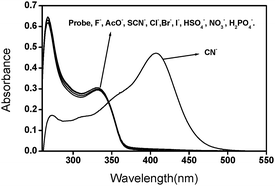 | ||
Fig. 1
Absorption spectra of Quino-P (2.8 × 10−5 M) in DMSO–H2O (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v) in tris-HCl in the presence of TBA salts of F−, AcO−, SCN−, HSO4−, NO3−, Br−, Cl−, I−, H2PO4− at 7.5 × 10−2 M each, whereas CN− is 7.6 × 10−3 M. 3, v/v) in tris-HCl in the presence of TBA salts of F−, AcO−, SCN−, HSO4−, NO3−, Br−, Cl−, I−, H2PO4− at 7.5 × 10−2 M each, whereas CN− is 7.6 × 10−3 M. | ||
Fig. 2a depicts the concentration dependent absorbance profile of Quino-P with the added CN− (0–7.6 × 10−3 M). The spectral response occurred instantly, and the probe's maximum at 330 nm was progressively red shifted to generate a new, more intense absorbance at 406 nm with incremental addition of cyanide. In addition, the higher energy band at 267 nm was also markedly diminished in its absorbance. Absorbance ratiometric analysis of CN− is possible using the ratio, 406 nm/330 nm (Fig. 2b). It may be noted that many known ICT based CN− chemodosimeters exhibit absorbance blue shifts due to the π-conjugation break-down. By contrast, Quino-P delivers a rare phenomenon of absorbance red shift because of the inducement of a dominant ICT transition, as proposed in Scheme 2.
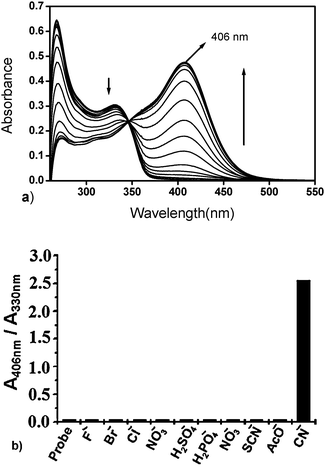 | ||
Fig. 2 (a) Spectrophotometric response of Quino-P (2.8 × 10−5 M) in DMSO–H2O (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v) in tris-HCl at pH 7.0 with increasing TBACN (0–7.6 × 10−3 M). (b) Ratiometric response (406 nm/330 nm) of Quino-P (2.8 × 10−5 M) towards anions. 3, v/v) in tris-HCl at pH 7.0 with increasing TBACN (0–7.6 × 10−3 M). (b) Ratiometric response (406 nm/330 nm) of Quino-P (2.8 × 10−5 M) towards anions. | ||
The emission spectrum of Quino-P (λex = 345 nm) displayed a broad band with a maximum at 435 nm, and the quantum yield, calculated with respect to anthracene (Φ = 0.27), was found to be 0.0041. The weak emission efficiency of Quino-P may be attributable to photoinduced electron transfer (PET) from the excited quinoline fluorophore to the redox active, pyridinium ion.16 This proposition finds support in the fact that quinoline–pyridyl conjugate 4, a neutral analog of the ionic Quino-P, fluoresces 12 times more intensely (Φ = 0.054) than Quino-P.
Evaluation of the fluorescence profile of the probe (1 μM) with 2.5 × 10−3 M each of F−, AcO−, SCN−, HSO4−, NO3−, Br−, Cl−, I− and H2PO4− revealed no evidence of interaction under the excited sate condition as well. Noteworthily, a significant fluorescence ‘on–off’ response was induced by CN− at a significantly lower concentration. Fluorimetric titration (Fig. 3) revealed a linear increase in the emission intensity, until saturating at 2.5 × 10−4 M of CN−, the emission enhancement reached ca. 10 fold (Φ = 0.046) compared to that of the native probe (inset in Fig. 3).
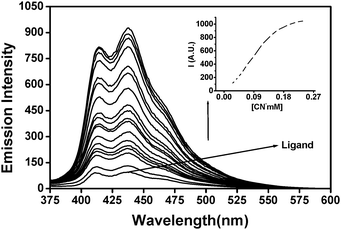 | ||
| Fig. 3 Fluorimetric titration of Quino-P (1 μM) in buffer DMSO with increasing CN−cyanide (0–2.5 × 10−4 M). Inset: emission enhancement as a function of CN− concentration. | ||
The emission off–on response could be attributable to the transformation of a PET-capable pyridinium ring of the free receptor into one of the PET-disabled, electron rich DHP ring within the cyano–Quino-P adduct. The PET nature of the probe is also evident from the observation that the fluorescence amplification is not accompanied by any emission wavelength shift.17 A competitive fluorescence experiment was used to verify the unique chemodosimeter action of Quino-P towards CN− only. Addition of 2.5 × 10−3 M each of F−, AcO−, SCN−, HSO4−, NO3−, Br−, Cl−, I− and H2PO4− did not detectably alter the fluorescence profile of Quino-P recorded in the presence of 2.5 × 10−4 M of CN−. Thus, a potential clearly exists in the probe to selectively discriminate cyanide even when other anions may be present in higher concentrations (ESI†).
The strong nucleophilicity of cyanide and the aqueous measurement capability of the probe ensure that the optical interferences, frequently encountered from the strongly hydrated, weakly nucleophilic or acidic anions, are not observed.18 The Job's plot showed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry and the apparent association constant, log K, was found to be 3.95. The detection limit of CN−, derived from the emission data, was found to be 1.6 μM (ESI†).
1 binding stoichiometry and the apparent association constant, log K, was found to be 3.95. The detection limit of CN−, derived from the emission data, was found to be 1.6 μM (ESI†).
Though we presumed a regioselective 1,4-cyanide addition, competitive reactions on the other reactive C2 and C6 of the pyridinium ring cannot be entirely ruled out. To establish the actual binding mode, we carried out 1H NMR analysis of the probe without and with added five equiv. of KCN in DMSO–d6-D2O. The proton assignments for Quino-P and its cyano-adduct are shown in Fig. 4a and b, respectively (for NMR interpretation, see ESI†). The NMR data indicated the formation of C4–Quino-P–CN− adduct with complete regioselectivity. As shown in Fig. 4b, the cyanide addition causes pronounced upfield shifts (δ 1.34 to 4.05) of the N–CH3 and the pyridinium ring protons because of the charge neutralization.
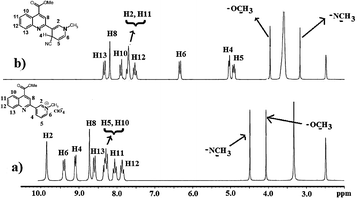 | ||
Fig. 4 300 MHz 1H NMR of (a) Quino-P. (b) 1H NMR Quino-P + 5 equiv. of KCN in 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 (v/v) DMSO–d6-D2O. 30 (v/v) DMSO–d6-D2O. | ||
Though not directly involved in the cyanide reaction, some of the quinoline ring as well as –CO2CH3protons also experience discernible upfield shifts in the range of δ 0.12 to 0.55. These upfield shifts obviously reflect an increase in charge density on the quinoline ring, and thus provide a further confirmation of the presence of the ICT interaction in the Quino-P + CN− adduct. Consistent with the 1H NMR results, the 13C NMR of the probe also displayed prominent upfield shifts of the N–CH3 signal as well as those of the pyridyl ring carbons after adding KCN (ESI†).
In conclusion, we have disclosed a new and efficient chemodosimeter design, which has the in-built structural features to deliver absorbance red shift by undergoing a unique ICT process, and the fluorescence turn-on signalling by cancellation of the PET, post-cyanide addition. The unique selectivity of cyanide is evident since many potentially competing anions pose no detectable optical interferences even in excess concentrations. The NMR analysis confirms the regioselective C4 addition of cyanide, and in agreement with the experimental findings, the theoretical studies (ESI†) support a strong tendency towards π-conjugation in the Quino-P–CN− adduct. The detection limit of 1.6 × 10−6 M is satisfactory for cyanide monitoring up to the toxic threshold of 1.9 μg in drinking water recommended by WHO.
Experimental
Reagents and instrumentation
Reagents and solvents used were purchased from S.D. Fine Chemicals, India, or Sigma–Aldrich. IR spectra were recorded using a Perkin-Elmer FT-IR spectrometer and pellets were made by using KBr. 1H NMR and 13C NMR spectra were recorded on BRUKER model Avance II 300 of 300 MHz. UV–vis spectra were recorded using a Shimadzu UV–vis recording spectrophotometer, model no. UV-2401PC. Fluorescence studies were carried out using a Shimadzu spectrofluorimeter, model no. RF-5301PC. The slit-width was set at 3 nm for both excitation and emission and the PMT detector voltage was 700 V.Synthesis
Compound 3 was prepared by modifying the literature procedure as follows. Isatin 1 (1 mmol, 147 mg) and 3-acetylpyridine 2 (1.5 mmol, 182 mg) were refluxed in 30% aqueous KOH (20 mL) for 10 h. Then, the reaction mixture was cooled in ice-bath and acidified with acetic acid to precipitate acid 3 as a buff-white solid. After filtration, the solid was washed with cold water, then methanol, and air-dried to afford compound 3 in 80% yield (200 mg). mp 315–317 °C [Lit.19 mp 316–318 °C].To a stirred solution of compound 3 (2.5 mmol, 625 mg) in dry MeOH (20 mL) was added an excess of thionyl chloride (3.75 mmol, 0.5 mL) drop-wise at 0 °C. The reaction was allowed to warm to room temperature for 30 min and then refluxed for 5 h. Methanol was distilled out and the residual solid recrystallised from CHCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) pet. ether (1
pet. ether (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) to afford compound 4 in 76% yield (500 mg), mp 95–96 °C, IR (KBr; cm−1): 3057, 2956, 1728, 1590, 1508, 1482, 1459, 1345, 1257, 1203, 1016, 794, 774. 1H NMR (CDCl3): δ 4.1 (s, 3H), 7.57 (m, 2H), 7.69 (t, 1H, J = 8.4), 7.83 (t, 1H, J = 9.3), 8.25 (d, 1H, J = 9.3), 8.42 (s, 1H), 8.67 (d, 1H, J = 9.9), 8.7 (d, 1H, J = 12.9), 9.46 (s,1H). 13C NMR (CDCl3): δ 52.83, 119.70, 123.79, 124.20, 125.49, 128.34, 130.22, 130.32, 134.35, 134.99, 135.922, 148.42, 149.28, 150.23, 153.81, 166.44. Mass m/z value = 265. Anal. Calcd for C16H12N2O2: C, 72.72; H, 4.54; N, 7.69%. Found: C,72.99; H, 4.39; N, 7.48%.
1 v/v) to afford compound 4 in 76% yield (500 mg), mp 95–96 °C, IR (KBr; cm−1): 3057, 2956, 1728, 1590, 1508, 1482, 1459, 1345, 1257, 1203, 1016, 794, 774. 1H NMR (CDCl3): δ 4.1 (s, 3H), 7.57 (m, 2H), 7.69 (t, 1H, J = 8.4), 7.83 (t, 1H, J = 9.3), 8.25 (d, 1H, J = 9.3), 8.42 (s, 1H), 8.67 (d, 1H, J = 9.9), 8.7 (d, 1H, J = 12.9), 9.46 (s,1H). 13C NMR (CDCl3): δ 52.83, 119.70, 123.79, 124.20, 125.49, 128.34, 130.22, 130.32, 134.35, 134.99, 135.922, 148.42, 149.28, 150.23, 153.81, 166.44. Mass m/z value = 265. Anal. Calcd for C16H12N2O2: C, 72.72; H, 4.54; N, 7.69%. Found: C,72.99; H, 4.39; N, 7.48%.
Compound 4 (1 mmol, 264 mg) was dissolved in dry acetonitrile (5 mL) and CH3I (5 mmol, 0.31 mL) was added. The reaction was left at room temperature for 2 days to afford the quaternized salt 5 as a yellow solid in nearly quantitative yield. The obtained iodide 5 was redissolved in warm acetonitrile (5 mL) and Mg(ClO4)2·2H2O (250 mg) dissoved in 2 mL was added. The reaction mixture was vigorously stirred at room temperature for 24 h and then refrigerated overnight. The desired product, Quino-P was obtained as a light yellow solid in 80% yield (291 mg), mp 256–257 °C, IR (KBr; cm−1): 3038, 2951, 1718, 1584, 1503, 1472, 1418, 1258, 1204, 1153, 1017, 777, 666.
1H NMR (DMSO–d6-D2O): δ 4.06 (s, 3H), 4.49 (s, 3H), 7.84 (t, 1H, J = 7.7), 7.97 (t, 1H, J = 7.5), 8.29 (m, 2H), 8.62 (d, 1H, J = 8.7), 8.7 (s, 1H), 9.1 (d, 1H, J = 6), 9.4 (d, 1H, J = 8.1), 9.87 (s, 1H). 13C NMR (DMSO–d6-D2O): δ 48.77, 53.66, 119.96, 124.37, 125.86, 128.26, 130.046, 130.41, 131.76, 137.45, 137.81, 143.08, 145.19, 146.29, 148.60, 150.54, 166.41. Mass m/z = 279 [minus perchlorate ion]. Anal. Calcd for C17H15N2O5Cl: C, 53.89; H, 3.96; N, 7.39; Cl, 9.37%. Found: C, 53.83; H, 3.68; N, 7.62; Cl, 9.13%.
Acknowledgements
Thanks are due to the BRNS, Government of India, and the MICINN, Spain (project CTQ2008-00841/BQU), for generous financial support. C.E. thanks the MEC, Spain, for a fellowship. We also thank the CESCA for computational facilities.Notes and references
- (a) P. D. Beer and P. A. Gale, Angew. Chem., Int. Ed., 2001, 40, 486 CrossRef; (b) R. Martinez-Manez and F. Sancenon, Chem. Rev., 2003, 103, 4419 CrossRef CAS; (c) T. Gunnlaugsson, M. Glynn, G. M. Tocci, P. E. Kruger and F. M. Pfeffer, Coord. Chem. Rev., 2006, 250, 3094 CrossRef CAS; (d) J. L. Sessler, P. A. Gale and W. S. Cho, Anion Receptor Chemistry, Royal Society of Chemistry, Cambridge, 2006 Search PubMed.
- C. Young, L. Tidwell and C. Anderson, Cyanide Social, Industrial, and Economic Aspects, Minerals, Metals, and Materials Society, Warrendale, 2001 Search PubMed.
- (a) C. Baird and M. Cann, Environmental Chemistry, Freeman, New York, 2005 Search PubMed; (b) G. C. Miller and C. A. Pritsos, Cyanide: Soc., Ind. Econ. Aspects, Proc. Symp. Annu. Meet. TMS 2001, 73.
- K. W. Kulig, Cyanide Toxicity, U.S. Department of Health and Human Services, Atlanta, GA, 1991 Search PubMed.
- Z. Xu, X. Chen, H. N. Kim and J. Yoon, Chem. Soc. Rev., 2010, 39, 127 RSC.
- (a) M. Tomasulo and F. M. Raymo, Org. Lett., 2005, 7, 4633 CrossRef CAS; (b) M. Tomasulo, S. Sortino, A. J. P. White and F. M. Raymo, J. Org. Chem., 2006, 71, 744 CrossRef CAS; (c) J. Ren, W. Zhu and H. Tian, Talanta, 2008, 75, 760 CrossRef CAS.
- F. Garcia, J. M. Garcia, B. Garcia-Acosta, R. Martinez-Manez, F. Sancenon and J. Soto, Chem. Commun., 2005, 2790 RSC.
- J. V. Ros-Lis, R. Martinez-Manez and J. Soto, Chem. Commun., 2002, 2248 RSC.
- Y.-K. Yang and J. Tae, Org. Lett., 2006, 8, 5721 CrossRef CAS.
- (a) H.-T. Niu, X. Jiang, J. He and J.-P. Cheng, Tetrahedron Lett., 2009, 50, 6668 CrossRef CAS; (b) P. Kaur, D. Sareen, S. Kaur and K. Singh, Inorg. Chem. Commun., 2009, 12, 272 CrossRef CAS; (c) A. Afkhami and N. Sarlak, Sens. Actuators, B, 2007, 122, 437 CrossRef.
- (a) H. Lee, Y. M. Chung and K. H. Ahn, Tetrahedron Lett., 2008, 49, 5544 CrossRef CAS; (b) H. Miyaji, D.-S. Kim, B.-Y. Chang, E. Park, S.-M. Park and K. H. Ahn, Chem. Commun., 2008, 753 RSC.
- S.-J. Hong, J. Yoo, S.-H. Kim, J. S. Kim, J. Yoon and C.-H. Lee, Chem. Commun., 2009, 189 RSC.
- (a) C.-H. Lee, H. K. Na, D. W. Yoon, D.-H. Won, W. S. Cho, V. M. Lynch, S. V. Shevchuk and J. L. Sessler, J. Am. Chem. Soc., 2003, 125, 7301 CrossRef CAS; (b) D. W. Yoon, H. Hwang and C. H. Lee, Angew. Chem., Int. Ed., 2002, 41, 1757 CrossRef CAS; (c) R. Nishiyabu and P. Anzenbacher, Jr., J. Am. Chem. Soc., 2005, 127, 8270 CrossRef CAS; (d) R. Nishiyabu and P. Anzenbacher, Jr., Org. Lett., 2006, 8, 359 CrossRef CAS.
- (a) Y. Shiraishi, K. Adachi, M. Itoh and T. Hirai, Org. Lett., 2009, 11, 3482 CrossRef CAS; (b) F. Garcia, J. M. Garcia, B. Garcia-Acosta, R. Martinez- Manez, F. Sancenon and J. Soto, Chem. Commun., 2005, 2790 RSC; (c) S. Kumar and S. Kumar, Tetrahedron Lett., 2009, 50, 4463 CrossRef CAS.
- (a) A. Oehlke, A. A. Auer, I. Jahre, B. Walfort, T. Ruffer, P. Zoufala, H. Lang and S. Spange, J. Org. Chem., 2007, 72, 4328 CrossRef CAS; (b) N. A. O. Williams, C. Masdeu, J. L. Diaz and R. Lavilla, Org. Lett., 2006, 8, 5789 CrossRef CAS; (c) R. Lavilla, J. Chem. Soc., Perkin Trans. 1, 2002, 1141 RSC; (d) J. W. Bunting and S. Sindhuatmadja, J. Org. Chem., 1980, 45, 5411 CrossRef CAS; (e) R. Foster and C. A. Fyfe, Tetrahedron, 1969, 25, 1489 CrossRef CAS; (f) A. Klapars and E. Vedejs, Chem. Heterocycl. Compd. (N. Y.), 2004, 40, 759 CrossRef CAS.
- (a) L. Fabbrizzi, F. Gatti, P. Pallavicini and L. Parodi, New J. Chem., 1998, 22, 1403 RSC; (b) A. P. de Silva, H. Q. Gunaratne and P. L. M. Lynch, J. Chem. Soc., Perkin Trans. 2, 1995, 685 RSC.
- A. P. de Silva, H. Q. N. Gunaratne, T. Gunnlaugsson, A. J. M. Huxley, C. P. McCoy, J. T. Rademacher and T. E. Rice, Chem. Rev., 1997, 97, 1515 CrossRef.
- (a) H. Miyaji and J. L. Sessler, Angew. Chem., Int. Ed., 2001, 40, 154 CrossRef CAS; (b) Y.-H. Kim and J.-I. Hong, Chem. Commun., 2002, 512 RSC; (c) D. Jimenez, R. Martinez-Manez, F. Sancenon and J. Soto, Tetrahedron Lett., 2002, 43, 2823 CrossRef CAS; (d) C.-F. Chow, M. H. W. Lam and W.-Y. Wong, Inorg. Chem., 2004, 43, 8387 CrossRef CAS; (e) R. Badugu, J. R. Lakowicz and C. D. Geddes, Dyes Pigm., 2005, 64, 49 CrossRef CAS; (f) R. Badugu, J. R. Lakowicz and C. D. Geddes, J. Am. Chem. Soc., 2005, 127, 3635 CrossRef CAS; (g) K. Parab, K. Venkatasubbaiah and F. Jakle, J. Am. Chem. Soc., 2006, 128, 12879 CrossRef CAS; (h) S. Xu, K. Chen and H. Tian, J. Mater. Chem., 2005, 15, 2676 RSC.
- G. Y. Sarkis, J. Chem. Eng. Data, 1972, 17, 388 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Spectra of compound 4 and Quino-P, competitive fluorescence binding, Job's plot, detection limit, D2O exchange, 1H NMR interpretation of Quino-P and its cyanide adduct, 13C NMR of Quino-P with added KCN and molecular modelling studies. See DOI: 10.1039/c0nj00715c |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2011 |
