Synthesis of 5-arylidene-2,4-thiazolidinediones by Knoevenagel condensation catalyzed by baker's yeast
Umesh R.
Pratap
,
Dhanaji V.
Jawale
,
Rahul A.
Waghmare
,
Dinesh L.
Lingampalle
and
Ramrao A.
Mane
*
Department of Chemistry, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad-431004, India. E-mail: manera2011@gmail.com; Fax: +91 0240-02403113; Tel: +91 0240-02403311
First published on 9th November 2010
Abstract
An ecofriendly baker's yeast catalyzed Knoevenagel condensation of aromatic aldehydes and active methylene compounds has been performed. The developed protocol has been found to be applicable for obtaining 5-arylidene-2,4-thiazolidinediones, the precursors of hypoglycemic agents.
Knoevenagel condensation is one of the classical organic reactions of immense importance in the formation of C–C bonds.1 A wide range of useful products have been synthesized using the condensation.2 It has been used to obtain a range of substituted alkenes, α,β-unsaturated nitriles, esters, acids, drugs, dyes and polymers.3,4
The condensation of 2,4-thiazolidinediones with aldehydes has been a subject of considerable interest. The products 5-arylidene-2,4-thiazolidinediones are important structural elements in medicinal chemistry and are found to possess significant hypoglycemic,5 anti-inflammatory,6aldose reductase inhibitor,7tyrosine phosphate inhibitor,8 antihypertensive9 and anticancer10 activities.
Knoevenagel condensation of 2,4-thiazolidinediones with aldehydes is a key step in the synthesis of some clinically used antidiabetic agents like rosiglitazone, englitazone and netoglitazone.3
In view of the significance of 5-arylidene-2,4-thiazolidinediones, synthetic chemists are taking keen interest in the development of green, rapid and economic protocols for their synthesis.11Catalysts used for Knoevenagel condensations are amines or buffer systems containing an amine and acid.12 These protocols provide the access to 5-arylidene-2,4-thiazolidinediones but suffer from harsh reaction conditions, use of toxic and volatile solvents and corrosive/toxic bases. Therefore, there is an urgent need to provide a more effective and environmental benign procedure for obtaining 5-arylidene-2,4-thiazolidinediones.
Biocatalysis is one of the green methods in organic synthesis which is used to synthesize a wide variety of organic compounds. The method has become a preferable alternative for traditional chemical catalysis.13 Aldolases, transketolase, and hydroxynitrile lyases catalyze the C–C bond forming reactions.14
There is scanty information on the use of enzymes for the acceleration of Knoevenagel condensation. Isolated lipases have been found to catalyze this condensation.15,16 The use of isolated pure enzymes to accelerate organic transformations has several drawbacks such as high cost, narrow substrate specificities and in some cases low performance under nonnatural conditions.
Here, we have attempted the Knoevenagel condensation using a cheaper whole cell biocatalyst, active dry baker's yeast (Saccharomyces cerevisiae). The cell of baker's yeast acts as a mini reactor and produces a variety of enzymes and is known to provide specific enzyme for specific reaction. Baker's yeast has the ability to catalyze various organic transformations.17 It is also used in the formation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds viaacyloin condensation,18,19 and Michael addition reaction.20 Due to this important aspect, baker's yeast is gaining much importance in organic synthesis.21
C double bonds viaacyloin condensation,18,19 and Michael addition reaction.20 Due to this important aspect, baker's yeast is gaining much importance in organic synthesis.21
The active methylene compounds used in the work are malononitrile, ethyl cyanoacetate and 2,4-thiazolidinedione.
The condensations were performed by stirring in ethanol at room temperature (rt) giving moderate to excellent yields of the arylidenyl derivatives.
Initially to optimize the reaction conditions, the condensation of benzaldehyde (1a) and the active methylene compound malononitrile (2a) was carried out using baker's yeast (S. cerevisiae) in water with stirring at room temperature. The product (3a) formation was recorded. However, for its isolation critical extraction with ethyl acetate was needed.
To avoid the tedious extraction procedure and to accelerate the rate, the reaction was carried out in ethanol. Excellent yield of the product 3a was obtained after 3 h of stirring at rt. (Table 1). This protocol did not need extraction and the product isolation was easier. After 3 h of stirring the reaction mass was filtered to remove yeast as a residue. The product was obtained on removal of ethanol from filtrate by vacuum distillation.
| Entry | R | X | Product | Time/h | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: malononitrile or ethyl cyanoacetate (8 mmol), aryl aldehyde (8 mmol) and baker's yeast (2 g) in ethanol (30 mL) stir, rt. b Isolated yields. | |||||
| 1 | H | CN | 3a | 3 | 92 |
| 2 | 3-Cl | CN | 3b | 3 | 86 |
| 3 | 4-Cl | CN | 3c | 3 | 85 |
| 4 | 4-Br | CN | 3d | 3 | 88 |
| 5 | 2-OH | CN | 3e | 3 | 94 |
| 6 | 4-OCH3 | CN | 3f | 3 | 95 |
| 7 | 4-Cl | COOEt | 3g | 5 | 75 |
| 8 | H | COOEt | 3h | 5 | 70 |
| 9 | 4-OCH3 | COOEt | 3i | 5 | 67 |
Then the variety of aryl aldehydes were separately condensed with malononitrile (Scheme 1) under optimized reaction conditions and the products were obtained with excellent yields (Table 1, products 3a–f). In the next attempt we carried out the Knoevenagel condensation of aryl aldehydes and ethyl cyanoacetate (Scheme 1) using baker's yeast in ethanol and obtained the good yields of the products. The time required for the completion of reaction was found to be longer than the condensation with malononitrile (Table 1, products 3g–i). The Knoevenagel condensation of acetyl acetone and ethyl acetoacetate with benzaldehyde was separately performed but we did not observe the desired products even after 2 days of stirring.
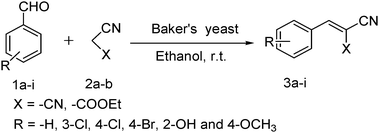 | ||
| Scheme 1 Knoevenagel condensation of aryl aldehydes with malononitrile and ethyl cyanoacetate. | ||
After having these results then we attempted the synthesis of a series of 5-arylidene-2,4-thiazolidinediones by condensing aryl aldehydes with 2,4-thiazolidinedione (Scheme 2) in the presence of baker's yeast (Table 2, products 4a–l) in ethanol at room temperature.
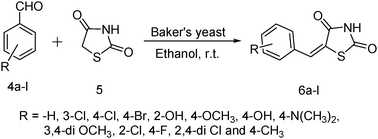 | ||
| Scheme 2 Knoevenagel condensation of 2,4-thiazolidinedione and aryl aldehydes. | ||
| Entry | R | Product | Yieldsb (%) |
|---|---|---|---|
| a Reaction conditions: 2,4-thiazolidinedione (8 mmol), aryl aldehyde (8 mmol) and baker's yeast (2 g) in ethanol (30 mL) stir, rt for 40 h. b Isolated yields. | |||
| 1 | H | 6a | 45 |
| 2 | 4-OCH3 | 6b | 50 |
| 3 | 4-CH3 | 6c | 49 |
| 4 | 2-OH | 6d | 40 |
| 5 | 4-OH | 6e | 42 |
| 6 | 4-N(CH3)2 | 6f | 55 |
| 7 | 3,4-di OCH3 | 6g | 38 |
| 8 | 4-Cl | 6h | 62 |
| 9 | 2-Cl | 6i | 59 |
| 10 | 4-Br | 6j | 80 |
| 11 | 4-F | 6k | 74 |
| 12 | 2,4-di Cl | 6l | 72 |
The geometry of 5-arylidene-2,4-thiazolidinediones may be E or Z. It is well known that the E and Z isomers can be distinguished by the 1H NMR spectral characteristics. Benzylidine proton appears below 7.42 δ ppm in E isomer and above 7.90 δ ppm in Z isomer.22–24 From the spectral data (1H NMR) it was confirmed that all the products obtained are Z isomers.
To examine the catalytical efficiency of baker's yeast, reaction of benzaldehyde and 2,4-thiazolidinedione was performed in the absence of yeast as the control experiment where we found that there was no formation of the product. The result indicates that the baker's yeast is necessary to catalyze the reaction.
Next, we tried to condense 2,4-thiazolidinedione with heteroaryl aldehydesviz.2-chloro 3-formyl quinoline, 3-formyl indole and pyridine 3-carboxaldehyde. We observed no condensations in these experiments.
Recently, Sonawane et al. have reported the lipase catalyzed Knoevenagel condensation and also proposed the catalytical role of lipase in the condensation.16 Literature reveals that baker's yeast does produce lipolytic enzymee.g.lipase.25 Such an enzyme could be responsible for the acceleration of Knoevenagel condensation. A yeast lipase might be abstracting a proton from the active methylene to form the nucleophile. Thus the generated nucleophile would attack the electrophilic carbon of the aldehyde resulting in the aldol adduct which on sequential dehydration leads to Knoevenagel products.
In summary, we have described a novel biocatalytical method for the Knoevenagel condensation of aryl aldehydes and various active methylene compounds at mild reaction conditions in ethanol using baker's yeast as the whole cell biocatalyst. The newly developed protocol is eco-friendly and might be useful in the synthesis of precursors of antidiabetic drugs, 5-arylidene-2,4-thiazolidinediones.
Experimental section
General remarks
All chemicals used were obtained from commercial suppliers and used without further purification. Progress of the reaction was monitored by thin layer chromatography on MERKs silica plates. 1H-NMR spectra were recorded on Bruker DRX FT NMR at 300 and 200 MHz using TMS as internal standard. Mass spectral data were obtained by JEOL AccuTOF DART mass spectrometer. Dry baker's yeast (S. cerevisiae) was procured from Kothari Fermentations and Biochem Ltd, India.General experimental procedure
A mixture of active methylene compound (8 mmol), aryl aldehyde (8 mmol) and dry baker's yeast (2 g) in ethanol (30 mL) was stirred at room temperature for the specified time. The progress of the reaction was monitored by thin layer chromatography using ethyl acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) pet ether (4
pet ether (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) as an eluent. After the specified reaction time the reaction content was filtered through the bed of Celite to remove the yeast. Ethanol was removed from the filtrate by vacuum distillation and crude residues were then purified by crystallization from ethanol (3a–i) and ethanol
6) as an eluent. After the specified reaction time the reaction content was filtered through the bed of Celite to remove the yeast. Ethanol was removed from the filtrate by vacuum distillation and crude residues were then purified by crystallization from ethanol (3a–i) and ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMF (6a–l).
DMF (6a–l).
All the products are well characterized by the comparison of their spectral (1H-NMR, Mass) and physical data (mp) with those reported in literature.23,26
Acknowledgements
Authors are thankful to Professor D. B. Ingle for his valuable suggestions and discussions. One of the authors URP is grateful to University Grants Commission, New Delhi for the award of research fellowship.Notes and references
- (a) Comprehensive Organic Synthesis, ed. B. M. Trost, Pergamon press, Oxford, 1991, vol. 2, p.133 Search PubMed; (b) G. Jones, Org. React., 1967, 15, 204 CAS.
- (a) J. Wang, R. P. Discordia, G. A. Crispino, J. Li, J. A. Grosso, R. Polniaszek and V. C. Truc, Tetrahedron Lett., 2003, 44, 4271 CrossRef CAS; (b) J. K. Gallos and A. E. Koumbis, ARKIVOC, 2003, vi, 135; (c) G. Sabitha, G. S. K. K. Reddy, M. Rajkumar, J. S. Yadav, K. V. S. Ramakrishna and A. C. Kunwar, Tetrahedron Lett., 2003, 44, 7455 CrossRef CAS; (d) S. Marcaccini, R. Pepino, M. C. Pozo, S. Basurto, M. G. Valverda and T. Torroba, Tetrahedron Lett., 2004, 45, 3999 CrossRef CAS; (e) C. Xing and S. Zhu, J. Org. Chem., 2004, 69, 6486 CrossRef CAS.
- (a) J. Cossy, C. Menciu, H. Rakotoarisoa, P. H. Kahn and J. R. Desmurs, Bioorg. Med. Chem. Lett., 1999, 9, 3439 CrossRef CAS; (b) S. L. Gaonkar and H. Shimizu, Tetrahedron, 2010, 66, 3314 CrossRef CAS; (c) D. Lednicer, Strategies for Organic Drug Synthesis and Design, 2nd edn, John Wiley & Sons Inc., 2008 Search PubMed.
- (a) T. Inokuchi and H. Kawafuchi, J. Org. Chem., 2006, 71, 947 CrossRef CAS; (b) R. Chen, X. Yang, H. Tian, X. Wang, A. Hagfeldt and L. Sun, Chem. Mater., 2007, 19, 4007 CrossRef CAS; (c) H. Salim and O. Piva, J. Org. Chem., 2009, 74, 2257 CrossRef CAS; (d) V. Boucard, Macromolecules, 2001, 34, 4308 CrossRef CAS; (e) A. Coelho, E. Ravina, N. Fraiz, M. Yanez, R. Laguna, E. Cano and E. Sotelo, J. Med. Chem., 2007, 50, 6476 CrossRef CAS.
- L. Fernanda, C. C. Leite, R. H. V. Mourao, M. C. A. Lima, S. L. Galdino, M. Z. Hernandes, F. A. R. Neves, S. Vidal, J. Barbe and I. R. Pitta, Eur. J. Med. Chem., 2007, 42, 1239 CrossRef.
- R. Ottana, R. Maccari, M. L. Barreca, G. Bruno, A. Rotondo, A. Rossi, G. Chiricosta, R. D. Paola, L. Sautebin, S. Cuzzocrea and M. G. Vigorita, Bioorg. Med. Chem., 2005, 13, 4243 CrossRef CAS.
- R. Maccari, R. Ottana, R. Ciurleo, M. G. Vigorita, D. Rakowitz, T. Steindl and T. Langer, Bioorg. Med. Chem. Lett., 2007, 17, 3886 CrossRef CAS.
- R. Maccari, P. Paoli, R. Ottana, M. Jacomelli, R. Ciurleo, G. Manao, T. Steindl, T. Langer, M. G. Vigorita and G. Camici, Bioorg. Med. Chem. Lett., 2007, 15, 5137 CrossRef CAS.
- S. V. Bhandari, K. G. Bothara, A. A. Patil, T. S. Chitre, A. P. Sarkate, S. T. Gore, S. C. Dangre and C. V. Khachane, Bioorg. Med. Chem. Lett., 2009, 17, 390 CrossRef CAS.
- D. Kaminsky, B. Zimenkovsky and R. Lesyk, Eur. J. Med. Chem., 2009, 44, 3627 CrossRef.
- (a) O. Yoshitata, M. Teruo, N. Mishiko, J. Motoyuki and K. Norio, Chem. Pharm. Bull., 1992, 40, 907; (b) I. Hitoshi, K. Hirogas, K. Keiko and K. Hirohiko, US Pat. 489706, RZhChim., 1991, 10070 Search PubMed; (c) W. Hanefeld and M. J. Schlietzer, J. Heterocycl. Chem., 1995, 32, 1019 CrossRef CAS; (d) Y. Sluka, S. Kadl and M. Sova, CSFR Pat. 275290, RZhChim., 1994, 2072 Search PubMed; (e) W. Hanefeld, V. Helfrich, M. Jalili and M. Schlitzer, Arch. Pharm., 1993, 326, 359 CrossRef CAS.
- (a) M. Tuncbine, O. B. Dundar, G. Ayhan-Kilcigil, M. Ceylan, A. Waheed, E. J. Verspohl and R. Ertan, Il Farmaco, 2003, 58, 79 CrossRef; (b) D. A. Clark, S. W. Goldstein, R. A. Volkmann, J. F. Eggler, G. F. Holland, B. Hulin, R. W. Stevenson, E. M. Kreutzer, E. M. Gibbs, M. N. Krupp, C. H. Lamphere, F. J. Rajeskas, W. H. Kappeler, W. H. McDermott, N. J. Hutson and M. R. Johnson, J. Med. Chem., 1991, 34, 319 CrossRef CAS; (c) N. B. Levshyn, N. B. Curkan, K. A. V'yunov and A. I. Ginak, Zh. Prikl. Khim., 1983, 56, 1453; (d) K. Popov-Pergal, Z. Chekovich and M. Pergal, Zh. Obshch. Khim., 1994, 61, 2112.
- K. M. Koeller and C. H. Wong, Nature, 2001, 409, 232 CrossRef CAS.
- B. G. Davis and V. Boyer, Nat. Prod. Rep., 2001, 18, 618 RSC.
- X. W. Feng, C. Li, N. Wang, K. Li, W. Zhang, Z. Wang and X. Yu, Green Chem., 2009, 11, 1933 RSC.
- Y. A. Sonawane, S. B. Phadtare, B. N. Borse, A. R. Jagtap and G. S. Shankarling, Org. Lett., 2010, 12, 1456 CrossRef CAS.
- R. Csuk and B. I. Glanzer, Chem. Rev., 1991, 91, 49 CrossRef CAS.
- G. Fronza, C. Fugatti, L. Majori, G. Pedrocchi-Fantoni and F. Spreafico, J. Org. Chem., 1982, 47, 3289 CrossRef CAS.
- G. Fronza, C. Fugatti, P. Grasseli, G. Poli and S. Servi, Biocatalysis, 1990, 3, 51 CrossRef CAS.
- T. Kitazume and N. Ishikawa, Chem. Lett., 1984, 1815 CAS.
- B. Pscheidt and A. Glieder, Microb. Cell Fact., 2008, 7, 25 CrossRef.
- Y. Momose, K. Meguro, H. Ikeda, C. Hatanaka, S. Oi and T. Sodha, Chem. Pharm. Bull., 1991, 39, 1440 CAS.
- Y. Luo, L. Ma, H. Zheng, L. Chen, R. Li, C. He, S. Yang, X. Ye, Z. Chen, Z. Li, Y. Gao, J. Han, G. He, L. Yang and Y. Wei, J. Med. Chem., 2010, 53, 273 CrossRef CAS.
- Z. Xia, C. Knaak, J. Ma, Z. M. Beharry, C. McInnes, W. Wang, A. S. Kraft and C. D. Smith, J. Med. Chem., 2009, 52, 74 CrossRef CAS.
- (a) K. Athenstaedt and G. Daum, J. Biol. Chem., 2003, 278, 23317 CrossRef CAS; (b) SGD pages: Database Copyright 1997–2008, YeastCyc: Saccharomyces cerevisiae Biochemical Pathway Overview, 2008.
- (a) B. M. Reddy, M. K. Patil, K. N. Rao and G. K. Reddy, J. Mol. Catal. A: Chem., 2006, 258, 302 CrossRef CAS; (b) M. Gupta, R. Gupta and M. Anand, Beilstein J. Org. Chem., 2009, 5, 68 Search PubMed; (c) K. F. Shelke, S. B. Sapkal, G. K. Kakade, S. A. Sadaphal, B. B. Shingate and M. S. Shingare, Green Chem. Lett. Rev., 2010, 3, 17 Search PubMed; (d) D. H. Yangt, B. Y. Yangt, B. C. Chentt and S. Y. Chentf, Org. Prep. Proced. Int., 2006, 38, 81 CrossRef.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2011 |
