A new squaraine and Hg2+-based chemosensor with tunable measuring range for thiol-containing amino acids†
Chao
Luo
,
Qianxiong
Zhou
,
Baowen
Zhang
and
Xuesong
Wang
*
Key Laboratory of Photochemical Conversion and Optoelectronic Materials, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing 100190, P. R. China. E-mail: xswang@mail.ipc.ac.cn; g203@mail.ipc.ac.cn; Tel: +86 10-82543592
First published on 1st November 2010
Abstract
A new squaraine, that undergoes absorption and fluorescence bleaching upon binding Hg2+, can serve as a turn-on colorimetric or fluorescent chemosensor for thiol-containing amino acids, presenting tunable measuring range by changing the concentration of Hg2+.
Thiol-containing amino acids which are known to be the key constituents of many proteins and enzymes such as cysteine or intermediate metabolites such as homocysteine play essential roles in the biological systems.1 They are important indicators of a large variety of diseases such as Alzheimer's, and cardiovascular diseases.1 For instance, the normal concentration of total cysteine (tCys) in plasma is in the range of 250 to 275 μM. Either over 300 μM or less than 225 μM of tCys hints a high risk of vascular diseases.1b Thus, it is of great value to develop rapid, sensitive, and selective methods to quantitatively detect the thiol-containing amino acids. In this regard, both colorimetric and fluorescent sensors are highly pursued by virtue of their sensitive responses, inexpensive instrumentation, simple procedures, and even naked-eye recognition. So far, a number of sensitive and selective chromogenic or fluorogenic probes have been ingeniously developed for sensing thiol-containing amino acids.2 However, a few of them have a wide measuring range spanning from several to hundreds of μM, the concentration region of thiol-containing amino acidsin vivo.3 Generally, to probe an analyte of which the concentrations may vary widely depending on the environments it exists in, a group of sensors with varied sensitivities are in demand. For example, many efforts have been put on the structure tailoring of Zn2+ chemosensors to detect endogenous Zn2+ present on different tissues or cell types.4 In this work, we present an intriguing strategy to modulate the concentration window of thiol-containing amino acids that a squaraine-based chemosenor can respond to.
Squaraines are versatile organic dyes which have been extensively used in photoconductivity, optical data storage, solar cell, low-gap polymers, nonlinear optics, photodynamic therapy (PDT), and chemosensors,5 due to the unique photophysical properties, particularly sharp and intense absorption and fluorescence in the visible to near-infrared (NIR) region. The electron-deficient feature of the central cyclobutene ring of squaraines has been fully utilized to probe nucleophilic analytes such as thiol-containing amino acids by forming addition product accompanied with the absorption and fluorescence bleaching of the squaraine.6 The thiophilic metal ions such as Hg2+ and Pb2+ can react with the addition product, liberating the squaraine and restoring the absorption and fluorescence.7 Inspired by these results, we designed and synthesized a new squaraine derivative bearing sulfur-containing binding units8 at the two terminals of the π conjugation scaffold, as shown in Scheme 1. Chelation of Hg2+ disrupts the π conjugation and leads to absorption and fluorescence turn-off. Then, the thiol-containing amino acids can sequester Hg2+ and restore the absorption and fluorescence of the squaraine. In this way, the sensing of the thiol-containing amino acids becomes a “turn-on” manner. More importantly, the measuring range of the thiol-containing amino acids may be easily controlled by adjusting the excess amount of Hg2+ since the free Hg2+ will be consumed first by the thiol-containing amino acids. Thus, the squaraine dye behaves as a colorimetric/fluorescent indicator to demonstrate the titration end between Hg2+ and the thiol-containing amino acids.
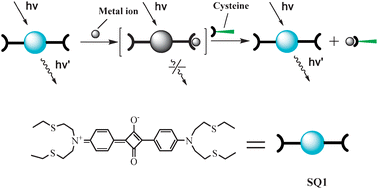 | ||
| Scheme 1 Chemical structure of SQ1 and pictorial demonstration for its sensing of thiol-containing amino acids. | ||
Fig. 1 shows the absorption and fluorescence spectra of SQ1 in acetonitrile as the function of the concentration of Hg2+. Upon addition of Hg2+, the absorption band centered at 636 nm decreased in intensity and a new band centered at 575 nm rose up gradually. When Hg2+ concentration reached 7 equiv. of SQ1, the visible absorption of SQ1 was nearly bleached. The fluorescence of SQ1 was quenched by Hg2+ monotonically, and disappeared completely at 7 equiv. of Hg2+. Such finding may be attributed to the chelation of Hg2+ into the terminal binding units of SQ1, which diminishes the electron-donating ability of nitrogen atom and switches off the visible absorption and emission of SQ1. The recovery of the absorption and emission upon addition of thiol-containing amino acids (vide post) supports our assignment. Similar phenomena were also found in the interactions between squaraines and metal ions.9 Though having two binding sites in SQ1, the formed complex between SQ1 and Hg2+ is consistent with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding mode, and the association constant was calculated to be 5.7 × 104 M−1 by using Bensi–Hildebrand method.10
1 binding mode, and the association constant was calculated to be 5.7 × 104 M−1 by using Bensi–Hildebrand method.10
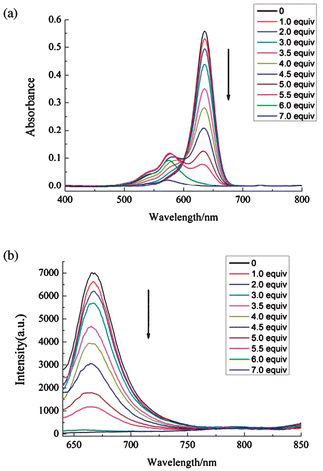 | ||
| Fig. 1 The absorption (a) and fluorescence (b) spectra of SQ1 (2 μM) in acetonitrile in the presence of varied concentrations of Hg(ClO4)2. | ||
Besides Hg2+, SQ1 also experiences remarkable changes in absorption and fluorescence spectra upon interaction with Cu2+ and Fe3+ in acetonitrile (Fig. S1, ESI†). In contrast, the responses are negligible toward other metal ions, including Pb2+, Cd2+, Ag+, Zn2+, Ni2+, Fe2+,Fe3+, Cr3+, and Ca2+. It is very intriguing that SQ1 shows different responses to metal ions, depending on the characters of the solvents. For example, SQ1 specifically recognizes Hg2+ over the other examined metal ions in acetonitrile/water solution (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in volume ratio), as shown in Fig. 2 and Fig. S2, ESI.† In acetone, SQ1 no longer interacts with Hg2+ but responds to Ag+ instead (Fig. S3, ESI†). Though the reasons behind the selectivity dependence on solvent are unclear, the interesting behaviors can obviously be utilized to discriminate Hg2+, Cu2+, Ag+, and Fe3+ from each other and from other metal ions by simply changing the solution of SQ1.
1 in volume ratio), as shown in Fig. 2 and Fig. S2, ESI.† In acetone, SQ1 no longer interacts with Hg2+ but responds to Ag+ instead (Fig. S3, ESI†). Though the reasons behind the selectivity dependence on solvent are unclear, the interesting behaviors can obviously be utilized to discriminate Hg2+, Cu2+, Ag+, and Fe3+ from each other and from other metal ions by simply changing the solution of SQ1.
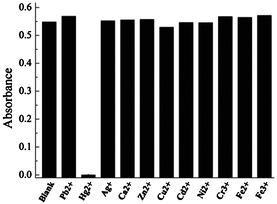 | ||
Fig. 2 Absorbance at 642 nm of SQ1 (2 μM) in acetonitrile/water (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) upon addition of various metal ions (40 μM). 1) upon addition of various metal ions (40 μM). | ||
It is expected that the addition of Hg2+ binding agent to the solution containing both SQ1 and Hg2+ may restore the absorption and fluorescence of SQ1 if the aforementioned interaction mechanism as shown in Scheme 1 is correct. Fig. 3 shows the results of such experiments. Among the examined 21 amino acids and short peptide, cysteine, homocysteine, GSH (glutathione), and histidine can completely restore the visible absorption and emission of SQ1. The absorption change from colorless to green is discernible easily by naked eye. In contrast, the effects of other 17 amino acids are negligible. Fig. S4 (ESI†) shows the absorption and emission spectra changes during the titration of acetonitrile/water (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solutions of SQ1 (1 μM) and Hg2+ (20 μM) by cysteine.
1) solutions of SQ1 (1 μM) and Hg2+ (20 μM) by cysteine.
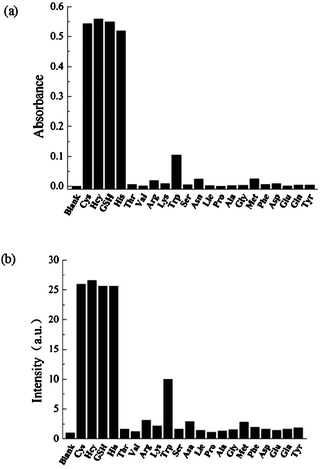 | ||
| Fig. 3 Absorbance at 642 nm (a) and fluorescence intensity at 678 nm (b) of the acetonitrile/water (2:1) solutions of SQ1 (2 μM) and Hg2+ (40 μM) upon addition of various amino acids (40 μM). | ||
Then we examined the effects of Hg2+ concentration on the detection of thiol-containing amino acids, maintaining a constant concentration of SQ1 (1 μM), as shown in Fig. 4. When Hg2+ is 20 μM, the absorption and fluorescence turn-on begins at 4 μM of cysteine and completes at 14 μM of cysteine. The linear range is from 8 μM to 14 μM. In the case of 30 μM of Hg2+, the “turn-on” begins at 12 μM of cysteine and completes at 22 μM of cysteine. Similarly, with the increase of the concentration of Hg2+, the response window shifts to higher concentration levels of cysteine. The ratio between the cysteine concentration at the half recovery of the absorption or emission of SQ1 and the concentration of Hg2+ remains nearly unchanged (ca. 0.6). In other words, a correlation is built up between cysteine and Hg2+, which makes possible the fast determination of the cysteine concentration by simple titration and eqn (1), in which VHg is the volume of the acetonitrile/water solution of SQ1 (1 μM) and Hg2+ (at the concentration cHg), Vcysteine is the volume of cysteine solution by which the SQ1 solution restores the green color, λ ≈ 0.6.
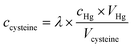 | (1) |
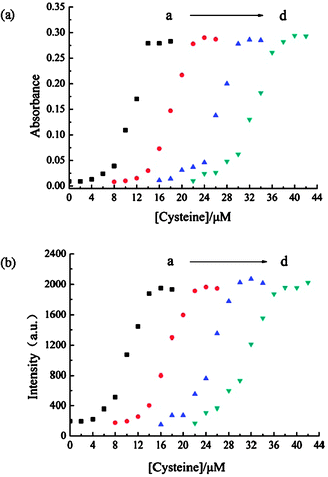 | ||
Fig. 4 Absorbance at 642 nm (a) and fluorescence intensity at 678 nm (b) of the acetonitrile/water solutions (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) of SQ1 (1 μM) and Hg2+ in the presence of varied concentrations of cysteine. The concentrations of Hg2+ are 20 μM (a), 30 μM (b), 40 μM (c), and 50 μM (d), respectively. 1) of SQ1 (1 μM) and Hg2+ in the presence of varied concentrations of cysteine. The concentrations of Hg2+ are 20 μM (a), 30 μM (b), 40 μM (c), and 50 μM (d), respectively. | ||
Though such a titration can only figure out a rough concentration of thiol-containing amino acids, it paves the way for further accurate concentration determination. Once the rough concentration is in hand, a solution of SQ1 containing desired amount of Hg2+ may be prepared and the absorption or fluorescence standard curve can be obtained in a narrow concentration range of analyte, by which the concentration of analyte is measured more precisely. For example, at 50 μM or 120 μM Hg2+ (Fig. 4d and Fig. S5, ESI†), an accurate determination can be achieved on cysteine in the range of 25–35 μM or 80–90 μM, respectively.
In summary, the combination of a new squaraine SQ1 and varied concentrations of Hg2+ was utilized to probe thiol-containing amino acids in an absorption or fluorescence turn-on manner, in which SQ1 plays the role of indicator and Hg2+ serves as the control of measuring range. Such a strategy may be applied to develop new chemosensors requiring wide or tunable measuring ranges.
Experimental
Squaric acid, N-phenyldiethanolamine and ethanethiol were purchased from Alfa Aesar and used without any further purification. Other materials, such as p-toluenesulfonyl chloride, and solvents were purchased from Beijing Chemical Plant and used as received. The target molecule SQ1 was synthesized by the condensation of squaric acid with N,N-bis(2-(ethylthio)ethyl)aniline in n-butanol/toluene (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) under reflux, while N,N-bis(2-(ethylthio)ethyl)aniline was prepared from N-phenyldiethanolamine and ethanethiol using the reported method.8 The 1H NMR and MS(ESI) data are included in the ESI.†
1 v/v) under reflux, while N,N-bis(2-(ethylthio)ethyl)aniline was prepared from N-phenyldiethanolamine and ethanethiol using the reported method.8 The 1H NMR and MS(ESI) data are included in the ESI.†
UV/vis spectra were recorded on an UV-2450 spectrophotometer. Fluorescence spectra were recorded on a F-4500 spectrophotometer.
We are grateful for the financial supports from NNSFC (20772133, 20873170) and CAS (KJCX2.YW.H08).
Notes and references
- (a) S. Seshadri, A. Beiser, J. Selhub, P. F. Jacques, I. H. Rosenberg, R. B. D'Agostino and P. W. F. Wilson, N. Engl. J. Med., 2002, 346, 476 CrossRef CAS; (b) L. El-Khairy, P. M. Ueland, H. Refsum, I. M. Graham and S. E. Vollset, Circulation, 2001, 103, 2544 CAS.
- (a) X. Chen, Y. Zhou, X. Peng and J. Yoon, Chem. Soc. Rev., 2010, 39, 2120 RSC; (b) Y. B. Ruan, A.-F. Li, J.-S. Zhao, J.-S. Shen and Y.-B. Jiang, Chem. Commun., 2010, 46, 4938 RSC; (c) X. Chen, S.-K. Ko, M. J. Kim, I. Shin and J. Yoon, Chem. Commun., 2010, 46, 2751 RSC; (d) W. Jiang, Y. Cao, Y. Liu and W. Wang, Chem. Commun., 2010, 46, 1944 RSC; (e) W. Lin, L. Long and W. Tan, Chem. Commun., 2010, 46, 1503 RSC; (f) L. Xiong, Q. Zhao, H. Chen, Y. Wu, Z. Dong, Z. Zhou and F. Li, Inorg. Chem., 2010, 49, 6402 CrossRef CAS; (g) S. Durocher, A. Rezaee, C. Hamm, C. Rangan, S. Mittler and B. Mutus, J. Am. Chem. Soc., 2009, 131, 2475 CrossRef CAS; (h) L. Yi, H. Li, L. Sun, L. Liu, C. Zhang and Z. Xi, Angew. Chem., Int. Ed., 2009, 48, 4034 CrossRef CAS; (i) W. Lin, L. Yuan, Z. Cao, Y. Feng and L. Long, Chem.–Eur. J., 2009, 15, 5096 CrossRef CAS; (j) H. Li, J. Fan, J. Wang, M. Tian, J. Du, S. Sun, P. Sun and X. Peng, Chem. Commun., 2009, 45, 5904 Search PubMed; (k) X. Zhang, X. Ren, Q. Xu, K. P. Loh and Z. Chen, Org. Lett., 2009, 11, 1257 CrossRef CAS; (l) S. Sreejith, K. P. Divya and A. Ajayaghosh, Angew Chem., Int. Ed., 2008, 47, 7883 CrossRef CAS; (m) W. Jiang, Q. Fu, H. Fan, J. Ho and W. Wang, Angew Chem., Int. Ed., 2007, 46, 8445 CrossRef CAS; (n) B. Tang, Y. Xing, P. Li, N. Zhang, F. Yu and G. Yang, J. Am. Chem. Soc., 2007, 129, 11666 CrossRef CAS; (o) O. Rusin, N. N. St. Luce, R. A. Agbaria, J. O. Escobedo, S. Jiang, I. M. Warner, F. B. Dawan, K. Lian and R. M. Strongin, J. Am. Chem. Soc., 2004, 126, 438 CrossRef CAS.
- (a) J. Liu, C. Bao, X. Zhong, C. Zhao and L. Zhu, Chem. Commun., 2010, 46, 2971 RSC; (b) H.-Y. Shiu, H.-C. Chong, Y.-C. Leung, M.-K. Wong and C.-M. Che, Chem.–Eur. J., 2010, 16, 3308 CrossRef CAS; (c) W. Lin, L. Long, L. Yuan, Z. Cao, B. Chen and W. Tan, Org. Lett., 2008, 10, 5577 CrossRef CAS; (d) M. Zhang, M. Yu, F. Li, M. Zhu, M. Li, Y. Gao, L. Li, Z. Liu, J. Zhang, D. Zhang, T. Yi and C. Huang, J. Am. Chem. Soc., 2007, 129, 10322 CrossRef CAS; (e) H. Chen, Q. Zhao, Y. Wu, F. Li, H. Yang, T. Yi and C. Huang, Inorg. Chem., 2007, 46, 11075 CrossRef CAS.
- E. M. Nolan, J. W. Ryu, J. Jaworski, R. P. Feazell, M. Sheng and S. J. Lippard, J. Am. Chem. Soc., 2006, 128, 15517 CrossRef CAS.
- (a) A. Ajayaghosh, Acc. Chem. Res., 2005, 38, 449 CrossRef CAS; (b) A. Ajayaghosh, Chem. Soc. Rev., 2003, 32, 181 RSC.
- J. V. Ros-Lis, B. García, D. Jiménez, R. Martínez-Máñez, F. Sancenón, J. Soto, F. Gonzalvo and M. C. Valldecabres, J. Am. Chem. Soc., 2004, 126, 4064 CrossRef CAS.
- (a) H. S. Hewage and E. V. Anslyn, J. Am. Chem. Soc., 2009, 131, 13099 CrossRef CAS; (b) E. Climent, M. D. Marcos, R. Martínez-Máñez, F. Sancenón, J. Soto, K. Rurack and P. Amorós, Angew. Chem., Int. Ed., 2009, 48, 8519 CrossRef CAS; (c) J. V. Ros-Lis, M. D. Marcos, R. Martínez-Máñez, K. Rurack and J. Soto, Angew. Chem., Int. Ed., 2005, 44, 4405 CrossRef CAS.
- J. Ishikawa, H. Sakamoto, T. Mizuno and M. Otomo, Bull. Chem. Soc. Jpn., 1995, 68, 3071 CAS.
- J. V. Ros-Lis, R. Martínez-Máñez, K. Rurack, F. Sancenón, J. Soto and M. Spieles, Inorg. Chem., 2004, 43, 5183 CrossRef CAS.
- (a) W. Wang, A. Fu, J. You, G. Gao, J. Lan and L. Chen, Tetrahedron, 2010, 66, 3695 CrossRef CAS; (b) P. Purkayastha, J. Photochem. Photobiol., A, 2010, 212, 43 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedure, 1H NMR and ESI-MS data of SQ1. Spectroscopic responses of SQ1 to metal ions and cysteine. See DOI: 10.1039/c0nj00696c |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2011 |
