Calixarene nanotubes: structural tolerance towards pyridine templates†
Stuart
Kennedy
a,
Christine M.
Beavers
b,
Simon J.
Teat
b and
Scott J.
Dalgarno
*a
aSchool of Engineering and Physical Sciences-Chemistry, Heriot-Watt University, Riccarton, Edinburgh, EH14 4AS, UK. E-mail: S.J.Dalgarno@hw.ac.uk; Fax: +44 (0)131-451-3180
bAdvanced Light Source, Berkeley Laboratory, 1 Cyclotron Road, MS6R2100, Berkeley, CA 94720, USA
First published on 11th October 2010
Abstract
Crystallisation of a tris-p-carboxylatocalix[4]arene from various pyridine derivatives leads, in some cases, to further self-assembly into triply helical nanotubes, demonstrating the degree of tolerance of the structure motif towards the introduction of alkyl groups around the aromatic ring of the guest template.
Controlling the assembly of molecular building blocks into large non-covalent structures is a challenging goal in supramolecular chemistry.1Calixarenes are macrocycles that have been well explored, primarily due to synthetic accessibility, but also the ability to exert control over their conformational properties.2 Typically bowl-shaped, calix[4]arenes have been used as building blocks in the formation of a range of self-assembled structural motifs, including comparatively rare nanometre scale capsules and tubes.1b,3
p-Sulfonatocalix[4]arene (1, Fig. 1A) typically packs in anti-parallel bilayer arrangements.4 Under specific conditions bilayer formation can be disrupted, leading to a parallel back-to-back arrangement, thereby inducing a degree of curvature to the system.3d,e When co-crystallised with specific amounts of pyridine-N-oxide in the presence of a lanthanide salt, the molecular components selectively assemble into either capsules composed of 12 calixarenes at the vertices of an icosahedron, or infinite nanotubes (Fig. 1A).3d The p-carboxylatocalix[4]arenes are relatively unexplored molecular building blocks, and we have recently been mapping out their assembly into nanoscale architectures.3f,g,5p-Carboxylatocalix[4]arene (2, Fig. 1B) assembles into a non-covalent nanotubular array when crystallised from pyridine (Py).3fCalixarene 2 assembles through back-to-back parallel alignment to afford hexameric discs that form the basis of the nanotube. Co-crystallised Py molecules act as guests for the calixarene cavities, and template a head-to-head dimer motif through complementary Py⋯CO2H hydrogen bonding interactions. Based on this result, we reasoned that it would be possible to apply our Py templated head-to-head dimer motif to a known nanotube arrangement in order to modulate the packing between neighbouring assemblies in the solid state.3g–iDi-O-propoxycalix[4]arene (3, Fig. 1C) adopts a cone conformation and forms triply-helical non-covalent nanotubes when crystallised from acetone.3h Neighbouring assemblies are separated by a distance of ∼20 Å, and interlock in a ‘cog-like’ fashion by means of π-stacking and CH⋯π interactions. Introduction of CO2H functionality to the upper-rim of the framework of 3 affords di-O-propoxy-tris-p-carboxylatocalix[4]arene (4, Fig. 1D), and subsequent crystallisation from Py affords the expected triply-helical nanotube arrangement, with neighbouring assemblies linked by the expected hydrogen-bonded dimer motif.3f,g The modulated inter-tubule spacing is found to be ∼27 Å, representing a 35% increase in the distance found between neighbouring nanotubes in 3 (Fig. 1C and D). Given this, we have been interested in the effects of introducing small alkyl groups around the pyridine template so as to (a) explore the tolerance of this nanotube motif towards guest template alteration, and (b) to attempt to reduce disorder in the interstitial regions between neighbouring nanotubes.
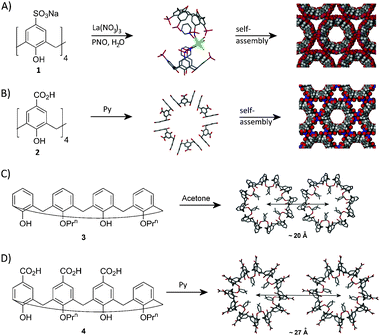 | ||
| Fig. 1 (A) Assembly of 1 into a C-shaped dimer complex, and space filling representation of the nanotube resulting from further self-assembly.3d (B) Assembly of 2 into hexameric discs, and space filling representation of the nanotube resulting from further self-assembly.3f (C) Assembly of 3 into interlocked triply-helical nanotubes with inter-tubule spacing indicated.3h (D) Assembly of 4 into triply helical nanotubes akin to those of 3, with inter-tubule spacing indicated.3g | ||
Compounds 3 and 4 were synthesised according to literature and adapted literature methods respectively.6–8 In order to explore the tolerance of the nanotube motif towards further modulation, we attempted to crystallise 4 from commercially available picolines (2-Pic, 3-Pic and 4-Pic) and ethylpyridines (2-Etpy and 4-Etpy).9 All of these solvents solubilise 4 well, and slow evaporation over a number of days afforded single crystals suitable for X-ray diffraction studies in only two cases (4·2-Pic and 4·2-Etpy).‡ In all other cases, microcrystalline material that was unsuitable for single crystal study was obtained.
Single crystals of 4·2-Pic are in a trigonal cell and structure solution was performed in the space groupR![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) . The asymmetric unit of 4·2-Pic was found to contain one molecule of 4, one 2-Pic guest (disordered over two positions), and diffuse electron density associated with very badly disordered solvent of crystallisation residing in the interstitial voids between neighbouring nanotubes. The calixarene adopts the expected near perfect cone conformation (Fig. 2A) akin to that found in 4·Py due to hydrogen bonding interactions between the hydroxyl protons and the adjacent O-propoxy chain at the lower-rim.
. The asymmetric unit of 4·2-Pic was found to contain one molecule of 4, one 2-Pic guest (disordered over two positions), and diffuse electron density associated with very badly disordered solvent of crystallisation residing in the interstitial voids between neighbouring nanotubes. The calixarene adopts the expected near perfect cone conformation (Fig. 2A) akin to that found in 4·Py due to hydrogen bonding interactions between the hydroxyl protons and the adjacent O-propoxy chain at the lower-rim.
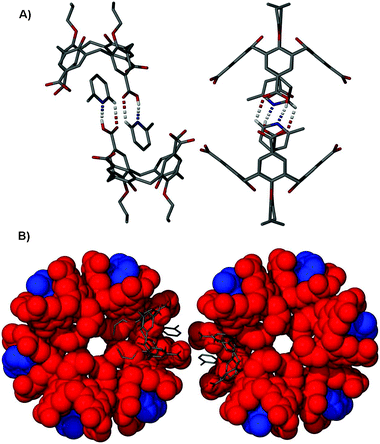 | ||
| Fig. 2 (A) Orthogonal views of the hydrogen-bonded dimer formed by crystallisation of 4 from 2-picoline. Hydrogen bonds are shown as dashed lines, and all other hydrogen atoms omitted for clarity. (B) Space filling view down the centre of two triply helical nanotubes in 4·2-Pic showing the bridging nature of a hydrogen-bonded dimer in stick representation. Space filling colour code: 4—red, 2-Pic—blue. Stick representation colour code: C—grey, N—blue, O—red. Hydrogen atoms omitted for clarity. | ||
The nature of the crystallographically unique 2-Pic occupying the cavity of 4 facilitates the formation of the head-to-head dimer motif in a similar way to the Py H-bonding template observed in our previously reported p-carboxylatocalix[4]arene nanotubes (Fig. 1B and D). Host–guest dimerisation occurs via two CO2H⋯N and two H⋯OCOH interactions with symmetry unique H[6]⋯N[40] and H[41]⋯O[5] distances of 1.86 and 2.59 Å respectively. In addition to this there are two CH⋯π interactions found between the hydrogen atoms meta to the nitrogen in the 2-Pic guest and the aromatic rings of host 4 with H[42]⋯aromatic centroid and H[44]⋯aromatic centroid distances of 2.85 Å and 2.42 Å respectively. Furthermore there is a symmetry unique π-stacking interaction between the aromatic rings of 4 and 2-Pic with an aromatic centroid⋯centroid distance of 3.85 Å. Symmetry expansion reveals that molecules of 4 pack in a back-to-back parallel arrangement resulting in the formation of the triply helical nanotube akin to that of 4·Py (Fig. 2B). Further similarities are evident between 4·Py and 4·2-Pic in that disorder is evident between upper-rim CO2H groups involved, given the symmetry of the structure and the tris-functionality of the calixarene framework. Some of these groups have therefore been modeled at partial occupancies consistent with refined site occupation factors. Examination of the extended structure shows that although space inside the cavities of the head-to-head dimer is somewhat restricted, the introduction of the methyl group on the pyridine template does not significantly affect the packing overall (Fig. 2B and 3), and the observed inter-tubule distance remains at ∼27 Å (Fig. 1D). Diffuse electron density in the interstitial voids between neighbouring nanotubes was indicative of the presence of solvent of crystallisation, but was too poorly disordered to be appropriately resolved. Given this, the routine SQUEEZE10 was applied to the data in order to remove this density, resulting in a marked improvement to the agreement indices. Such interstitial void disorder is a persistent problem with these systems, and we anticipated that extension of the alkyl group in the 2-position of the Py ring (at least) would allow us to reduce the diffuse nature of that region of the resulting structures, and observe key interactions to further understand self-assembly.
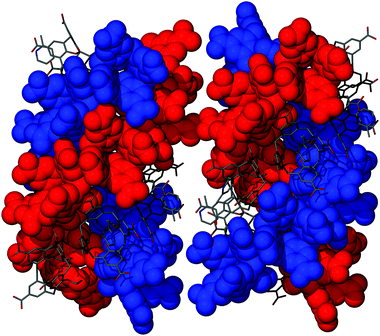 | ||
| Fig. 3 Mixed space filling and stick representation side views of neighbouring self-assembled triply helical nanotubes in 4·2-Pic. 2-Picolines are omitted for clarity in the red and blue space filling strands. | ||
Crystals of 4·2-Etpy are in a triclinic cell and structure solution was performed in the space groupP![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . The asymmetric unit comprises one molecule of 4 and 3.5 2-EtPy molecules (where 0.5 resides around an inversion centre), where one occupies the calixarene cavity (that also adopts a near perfect cone conformation, Fig. 4A). The common head-to-head pyridine templated dimer motif is again observed, and is facilitated by two CO2H⋯N and two H⋯OCOH interactions with symmetry unique H[6]⋯N[38] and H[39]⋯O[5] distances of 1.78 and 2.59 Å respectively. In a similar way to 4·2-Pic there are two CH⋯π interactions found between the hydrogen atoms meta to the nitrogen in the 2-EtPy guest and the aromatic rings of host 4 with H[42]⋯aromatic centroid and H[40]⋯aromatic centroid distances of 2.63 and 2.78 Å respectively. There is also a unique π-stacking interaction between the aromatic rings of 4 and 2-EtPy with an aromatic centroid⋯centroid distance of 4.13 Å. Symmetry expansion reveals that the molecules of 4 do not pack in the desired parallel back-to-back fashion (Fig. 4B), but rather that the introduction of 2-EtPy disturbs the formation of the nanotube motif. Ordering of the upper-rim CO2H groups results in this alternative packing mode, and no disorder is present in the resulting structure. In a similar fashion to Py solvates of the p-carboxylatocalix[4]arene-O-alkyl ethers,11 additional 2-EtPy molecules form H-bonding interactions (H[7]⋯N[54] and H[9]⋯N[46] distances of 1.89 and 1.80 Å respectively) with the remaining CO2H groups that are not involved in dimerisation.
. The asymmetric unit comprises one molecule of 4 and 3.5 2-EtPy molecules (where 0.5 resides around an inversion centre), where one occupies the calixarene cavity (that also adopts a near perfect cone conformation, Fig. 4A). The common head-to-head pyridine templated dimer motif is again observed, and is facilitated by two CO2H⋯N and two H⋯OCOH interactions with symmetry unique H[6]⋯N[38] and H[39]⋯O[5] distances of 1.78 and 2.59 Å respectively. In a similar way to 4·2-Pic there are two CH⋯π interactions found between the hydrogen atoms meta to the nitrogen in the 2-EtPy guest and the aromatic rings of host 4 with H[42]⋯aromatic centroid and H[40]⋯aromatic centroid distances of 2.63 and 2.78 Å respectively. There is also a unique π-stacking interaction between the aromatic rings of 4 and 2-EtPy with an aromatic centroid⋯centroid distance of 4.13 Å. Symmetry expansion reveals that the molecules of 4 do not pack in the desired parallel back-to-back fashion (Fig. 4B), but rather that the introduction of 2-EtPy disturbs the formation of the nanotube motif. Ordering of the upper-rim CO2H groups results in this alternative packing mode, and no disorder is present in the resulting structure. In a similar fashion to Py solvates of the p-carboxylatocalix[4]arene-O-alkyl ethers,11 additional 2-EtPy molecules form H-bonding interactions (H[7]⋯N[54] and H[9]⋯N[46] distances of 1.89 and 1.80 Å respectively) with the remaining CO2H groups that are not involved in dimerisation.
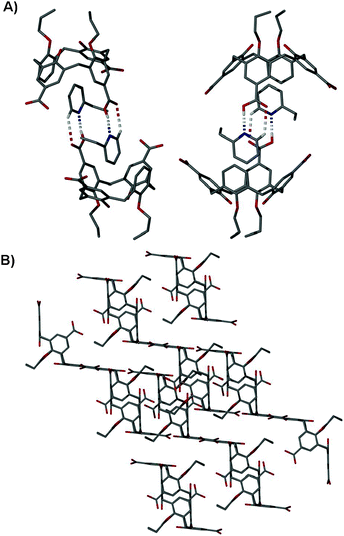 | ||
| Fig. 4 (A) Orthogonal views of the hydrogen-bonded dimer formed by crystallisation of 4 from 2-ethylpyridine. Hydrogen bonds are shown as dashed lines, and all other hydrogen atoms omitted for clarity. (B) Stick representation of the extended structure in 4·2-Etpy showing disruption of nanotube formation. Hydrogen atoms and solvent molecules omitted for clarity. | ||
To conclude, experiments in controlling the self-assembly of di-O-propoxy-tris-p-carboxylatocalix[4]arene using various pyridine derivatives result in major changes in the solid state packing of the target building blocks. Expected dimer formation is observed in the single crystals formed, and in one case (2-Pic) this leads to the formation of the targeted triply helical non-covalent organic nanotube. Even with this small number of results it is apparent that this nanotube motif is only mildly tolerant towards further modification of the templating guest molecule, and the introduction of a methyl group to the aryl ring has made little progress towards resolving the problem of disorder in the interstitial voids presented by the packing of neighbouring nanotubes. The use of more spatially demanding solvents (e.g.2-EtPy) causes disruption to the assembly of the nanotube motif, and subsequently results in a breakdown of the key parallel alignment of calixarene molecules that is required to invoke target nanotube formation. Work to further control the packing of these nanotubes continues with a view to gaining high degrees of control over the formation and packing of rare and challenging nanometre scale assemblies.
We thank the EPSRC for funding. The Advanced Light Source is supported by the Director, Office of Science, Office of Basic Energy Sciences, of the US Department of Energy under contract no DE-AC02-05CH11231.
Notes and references
- For example see: (a) R. S. Meissner, J. Rebek Jr and J. de Mendoza, Science, 1995, 270, 1485 CAS; (b) L. R. MacGillvray and J. L. Atwood, Nature, 1997, 389, 469 CrossRef CAS; (c) J. Rebek Jr, Angew. Chem., Int. Ed., 2005, 44, 2068 CrossRef.
- For examples see: C. D. Gutsche, Calixarenes 2001, Kluwer Academic Publishers, Dordrecht, 2001, ch. 1 and references therein Search PubMed; V. Böhmer, Angew. Chem., Int. Ed. Engl., 1995, 34, 713 Search PubMed.
- (a) L. R. MacGillivary and J. L. Atwood, J. Solid State Chem., 2000, 152, 199 CrossRef CAS; (b) H. Mansikkamäki, M. Nissinen and K. Rissanan, Angew. Chem., Int. Ed., 2004, 43, 1243 CrossRef; (c) S. J. Dalgarno, G. W. V. Cave and J. L. Atwood, Angew. Chem., Int. Ed., 2006, 45, 57; (d) G. W. Orr, L. J. Barbour and J. L. Atwood, Science, 1999, 285, 1049 CrossRef; (e) S. J. Dalgarno, J. L. Atwood and C. L. Raston, Chem. Commun., 2006, 4567 RSC; (f) S. J. Dalgarno, J. E. Warren, J. Antesberger, T. E. Glass and J. L. Atwood, New J. Chem., 2007, 31, 1891 RSC; (g) S. Kennedy and S. J. Dalgarno, Chem. Commun., 2009, 5275 RSC; (h) L. G. Kuz'mina, G. G. Sadikov, J. A. K. Howard, E. A. Shokova and V. V. Kovalev, Kristallografiya, 2003, 48, 272; (i) H. Dvořáková, J. Lang, J. Vlach, J. Sýkora, M. Čajan, M. Himl, M. Pojarová, I. Stibor and P. Lhoták, J. Org. Chem., 2007, 72, 7157 CrossRef CAS.
- J. L. Atwood, A. W. Coleman, H. Zhang and S. G. Bott, J. Inclusion Phenom. Mol. Recognit. Chem., 1989, 5, 203 CrossRef; J. L. Atwood, G. W. Orr, F. Hamada, R. L. Vincent, S. G. Bott and K. D. Robinson, J. Am. Chem. Soc., 1991, 113, 2760 CrossRef CAS; J. L. Atwood, G. W. Orr, N. C. Means, F. Hamada, H. Zhang, S. G. Bott and K. D. Robinson, Inorg. Chem., 1992, 31, 603 CrossRef CAS; J. L. Atwood, G. W. Orr, F. Hamada, R. L. Vincent, S. G. Bott and K. D. Robinson, J. Inclusion Phenom. Mol. Recognit. Chem., 1992, 14, 37 CAS; J. L. Atwood, G. W. Orr, R. K. Juneja, S. G. Bott and F. Hamada, Pure Appl. Chem., 1993, 65, 1471 CrossRef CAS; P. J. Nichols, C. L. Raston and J. W. Steed, Chem. Commun., 2001, 1062 RSC; H. R. Webb, M. J. Hardie and C. L. Raston, Chem.–Eur. J., 2001, 7, 3616 CrossRef CAS; Y. Israeli, G. P. A. Yap and C. Detellier, Supramol. Chem., 2001, 12, 457 CrossRef CAS; B.-T. Zhao, H. Wang, H.-Y. Zhang and Y. Liu, J. Mol. Struct., 2005, 740, 101 CrossRef; J. L. Atwood, T. Ness, P. J. Nichols and C. L. Raston, Cryst. Growth Des., 2002, 2, 171 CrossRef CAS; P. J. Nichols and C. L. Raston, Dalton Trans., 2003, 2923 RSC.
- S. J. Dalgarno, K. M. Claudio-Bosque, J. E. Warren, T. E. Glass and J. L. Atwood, Chem. Commun., 2008, 1410 RSC.
- A. Arduini, M. Fabbi, M. Mantovani, L. Mirone, A. Pochini, A. Secchi and R. Ungaro, J. Org. Chem., 1995, 60, 1454 CrossRef CAS.
- V. Arora, H. M. Chawla and A. Santra, Tetrahedron, 2002, 58, 5591 CrossRef CAS.
- Q. Lin and A. D. Hamilton, C. R. Chim., 2002, 5, 441 CrossRef CAS.
- 3-Ethylpyridine is very costly and is only available in small amounts, and for these reasons we chose not to use this as a crystallisation solvent.
- A. L. Spek, Acta Crystallogr., Sect. A: Fundam. Crystallogr., 1990, 46, C34.
- S. Kennedy, S. J. Teat and S. J. Dalgarno, Dalton Trans., 2010, 39, 384 RSC.
Footnotes |
| † CCDC reference numbers 787577 (4.2-Etpy) and 787578 (4.2-Pic). For crystallographic data in CIF or other electronic format see DOI: 10.1039/c0nj00605j. |
‡ Crystal data for 4·2-Pic: C43H42NO10, M = 732.78, 0.10 × 0.04 × 0.02 mm3, trigonal, space groupR![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) (No. 148), a = b = 46.4651(11), c = 12.0334(3) Å, V = 22499.5(9) Å3, Z = 18, synchrotron radiation, λ = 0.77490 Å, T = 100(2) K, 2θmax = 46.7°, 45 (No. 148), a = b = 46.4651(11), c = 12.0334(3) Å, V = 22499.5(9) Å3, Z = 18, synchrotron radiation, λ = 0.77490 Å, T = 100(2) K, 2θmax = 46.7°, 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 185 reflections collected, 5584 unique (Rint = 0.0548). Final GooF = 2.584, R1 = 0.1118, wR2 = 0.3220. Crystal data for 4·2-Etpy: C123H135N7O20, M = 2031.38, 0.35 × 0.30 × 0.20 mm3, triclinic, space groupP 185 reflections collected, 5584 unique (Rint = 0.0548). Final GooF = 2.584, R1 = 0.1118, wR2 = 0.3220. Crystal data for 4·2-Etpy: C123H135N7O20, M = 2031.38, 0.35 × 0.30 × 0.20 mm3, triclinic, space groupP![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (No. 2), a = 11.5487(7), b = 16.1638(9), c = 16.4446(11) Å, α = 113.729(2)°, β = 92.529(2)°, γ = 95.793(2)°, V = 2783.7(3) Å3, Z = 1, MoKα radiation, λ = 0.71073 Å, T = 100(2) K, 2θmax = 41.6°, 21 (No. 2), a = 11.5487(7), b = 16.1638(9), c = 16.4446(11) Å, α = 113.729(2)°, β = 92.529(2)°, γ = 95.793(2)°, V = 2783.7(3) Å3, Z = 1, MoKα radiation, λ = 0.71073 Å, T = 100(2) K, 2θmax = 41.6°, 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 118 reflections collected, 5696 unique (Rint = 0.0352). Final GooF = 1.033, R1 = 0.0480, wR2 = 0.1235. 118 reflections collected, 5696 unique (Rint = 0.0352). Final GooF = 1.033, R1 = 0.0480, wR2 = 0.1235. |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2011 |
