Regioselective near-equatorial chlorination of Cs-C70(CF3)8†
Alexey A.
Goryunkov
,
Nadezhda A.
Samokhvalova
,
Pavel A.
Khavrel
,
Nikita M.
Belov
,
Vitaliy Yu.
Markov
,
Lev N.
Sidorov
and
Sergey I.
Troyanov
*
Chemistry Department, Moscow State University, Moscow, 119991, Russia. E-mail: stroyano@thermo.chem.msu.ru; Fax: +7 (495) 9391240; Tel: +7 (495) 9395396
First published on 8th October 2010
Abstract
The chlorination of Cs-C70(CF3)8 by ICl occurs at near-equatorial [5,6] bond in agreement with quantum chemical predictions; structure of the synthesized Cs-C70(CF3)8Cl2 was proven by 19F NMR spectroscopy and single crystal X-ray crystallography.
Trifluoromethylated derivatives of [60] and [70]fullerenes (TFMF) have attracted much attention as prospective materials for construction of photoelectron devices due to their high electron affinity, chemical stability and ability to undergo multiple reversible electrochemical reductions.1 More than fifty fullerene derivatives of C60/70(CF3)n, n = 2–18 have already been characterized.2 Thus, the study of the chemical reactivity of the trifluoromethylated fullerenes as well as ways of their regioselective functionalization have become very important. There are rare examples of functionalization of these compounds by chlorination of S6-C60(CF3)123 and regioselective [2+1]- or [4+2]-cycloadditions to C1-C70(CF3)10.4 One of the most promising TFMF's which can be obtained in preparative amounts is Cs-C70(CF3)8.5 Here, we report the first example of regioselective chlorination of Cs-C70(CF3)8 resulting in a mixed chloro(trifluoromethyl)fullerene, Cs-C70(CF3)8Cl2. Its structure was predicted by quantum chemical calculations and has been proven by 19F NMR data and X-ray structure analysis.
A common feature of [70]fullerene derivatives bearing eight or ten addends is their addition pattern in a form of the so-called “equatorial belt”. There are many examples of such compounds, e.g. C70H8,6C70H10,6C70Cl10,7C70Br10,7C70Me8,8C70Me10,8C70Ph8,9C70Ph10,9C70(CF3)8,5C70(OOtBu)8,10 possessing such addition patterns with eight or ten addends attached to carbons atoms of edge-sharing equatorial belt of hexagons (indicated by shadowed region on Schlegel diagram in Fig. 1). In all these molecules, the addition of eight groups, regardless of their size, takes place at para-positions of equatorial hexagons giving para7 motif, p7-Cs-C70X8 (selected by dotted line on Schlegel diagram in Fig. 1). On the contrary, the attachment positions of an additional two groups to p7-Cs-C70X8 is substantially predetermined by the atom/group size. For example, attachment of two additional small-sized atoms like H, Cl, or Br to the corresponding p7-Cs-C70X8 compound results in Cs-symmetrical C70X10 derivatives where two groups are attached to near-equatorial [5,6] bond (so called para9-ortho loop motif).6,7 Addition of bulkier groups, like CF3, OOtBu, occurs in a different way. While Cs-symmetrical C70(CF3)85 and C70(OOtBu)810 demonstrate common para7-addition pattern, additional pairs of CF3 and OOtBu groups attach either to the polar region or to para-positions of unoccupied equatorial hexagon rather than to near-equatorial [5,6] bond, giving respectively p7mp-C1-C70(CF3)1011 and p9-C2-C70(OOtBu)10.10 Formation of patterns other than para9-ortho loop addition pattern in these cases is explained by significant steric repulsion of ortho-positioned bulky groups.
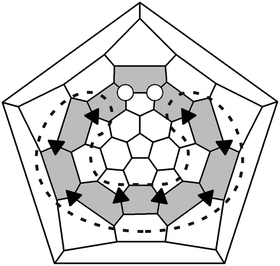 | ||
| Fig. 1 Schlegel diagram of C70(CF3)8Cl2; sites of CF3 and Cl addition are denoted by black triangles and white circles, respectively; para7 addition pattern is selected by dotted line, para9-ortho loop addition pattern is indicated by shadowed region. | ||
Relative DFT energies of C70X10 isomers (X = H, F, Cl, Br, OOtBu, and CF3) with p9o-loop, p9, and p7mp-addition patterns are given in Table 1 (values given after slash; see ESI† for calculation details). One can see that the relative stability of the p9o-loop pattern decreases along with an increase of van der Waals (vdW) radii of the groups. It should be noted that although the p9o-loop-addition pattern in C70(OOtBu)10 was predicted to be energetically more favorable, only C2-p9-pattern has been found experimentally. Thus, the reaction product is under the control of kinetic factors. Obviously, bulky tBu groups surrounding a fullerene cage are the reason for shielding of sp2carbon atom of [5,6] near-equatorial bond and assisting to C2-p9-C70(OOtBu)10 formation.
| Addition pattern | ΔE of C70(CF3)8X2/C70X10, X= | |||||
|---|---|---|---|---|---|---|
| H | F | OOtBu | Cl | Br | CF3 | |
| 1.20a | 1.47a | 1.52b | 1.75a | 1.85a | 2.8c | |
| a Atomic vdW radius, Å. b Oxygen vdW radius. c Sum of fluorine vdW radii and typical C–F bond length (1.33 Å). | ||||||
| C s -p9o(loop) | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 12.0 |
| C 2-p9 | 55.0/55.1 | 49.3/43.7 | 27.6/25.4 | 24.8/25.6 | 9.4/15.4 | 13.0 |
| C 1 -p7mp | 47.2/47.2 | 51.5/52.1 | 33.8/33.1 | 26.0/26.9 | 9.9/16.0 | 0.0 |
A similar situation was established for C70(CF3)8X2 isomers, when two additional Xgroups are attached to Cs-C70(CF3)8. Whereas the addition of bulky CF3 groups leads to energetically preferable p7mp-addition pattern which is experimentally observed for sized C70(CF3)10, outstanding energetic stability of p9o-loop-C70(CF3)8X2 isomers was predicted by quantum chemical calculations for small groupsX like H, F, and Cl. Thus, Cs-p9o-loop-C70(CF3)8X2 isomers (X = H, F, and Cl) are at least 25–47 kJ mol−1 more energetically preferable than any other possible isomer.
Site reactivity of the Cs-C70(CF)8 molecule was evaluated by analysis of frontier molecular orbitals. The highest occupied and lowest unoccupied MOs of the molecule are mostly distributed on near-equatorial [5,6]-bond and contain some percentage of p-orbital component (see ESI†, Fig. S1). Thus, one can expect increased reactivity of this bond toward addition of both small electron donating and electrophilic species. The HOMO−1, which is lower in energy than the HOMO (by ca. 0.36 eV), and the LUMO+1, which is higher in energy than the LUMO (by ca. 0.37 eV), are expected to be less reactive in both types of reactions mentioned above.
These findings prompt us to study the reactivity of near-equatorial [5,6]-bond of Cs-C70(CF3)8 towards addends of small size. Radical chlorination by iodine monochloride was chosen as a test reaction. Irrespective of reactive species (atomic chlorine, weak Lewis acid ICl or their complexes), the reactivity of the Cs-C70(CF3)8, the predominant choice of a reacting bond will be defined by both the HOMO distribution and relative energy of the radical intermediate. It was found that the lowest energy radical intermediate (among all possible radical intermediates of C70(CF3)8Cl˙) is an adduct of chlorine atom at the carbon atoms of near-equatorial [5,6]-bond (see ESI†, Table S4). Both Mulliken and Hirshfeld population analyses of the most energetic preferable radical C70(CF3)8Cl˙ intermediate shows maximal spin density at the [5,6]-neighbour carbon atom (see ESI†, Fig. S2). Thus, following radical addition will occur more readily at the second carbon atom of near-equatorial [5,6]-bond under formation of p9o-C70(CF3)8Cl2.
The isomer Cs-C70(CF3)8 was prepared according to the known two stage method12 and purified by HPLC. Chlorination of Cs-C70(CF3)8 (5 mg, 0.003 mmol) with excess ICl (0.1–0.2 mL, 0.7–1.5 mmol) was carried out at room temperature in o-dichlorobenzene solution for 24 h. After removal of volatile components from the reaction mixture, a yellow colored solid product was obtained.
HPLC analysis (Cosmosil Buckyprep, 4.6 mm i.d. × 25 cm, Nakalai Tesque Inc., toluene as an eluent, 1 ml min−1 flow rate, 290 nm) of the product showed the presence of a major component (ca. 90% of integral HPLC trace area) eluted at 4.72 min, a small amount (ca. 2%) of unreacted Cs-C70(CF3)8 eluted at 4.35 min as well as other minor uncharacterized components at 3.27, 3.50, 3.75, and 5.18 min (see Fig. 2a). UV/Vis spectrum of the main chlorination product (toluene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane = 9
hexane = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; λmax at 310, 397, 421, and 462 nm) differs from that of the starting compound (see inset in Fig. 2). Thus, chlorination resulted in almost quantitative conversion of Cs-C70(CF3)8 and the formation of a product with reaction yield ca. 90%. The reaction product is thermally unstable: it substantially degrades under gentle heating at 70 °C under formation of initial Cs-C70(CF3)8 and other uncharacterized by-products, according to MALDI mass spectrometry and HPLC data (not shown).
1; λmax at 310, 397, 421, and 462 nm) differs from that of the starting compound (see inset in Fig. 2). Thus, chlorination resulted in almost quantitative conversion of Cs-C70(CF3)8 and the formation of a product with reaction yield ca. 90%. The reaction product is thermally unstable: it substantially degrades under gentle heating at 70 °C under formation of initial Cs-C70(CF3)8 and other uncharacterized by-products, according to MALDI mass spectrometry and HPLC data (not shown).
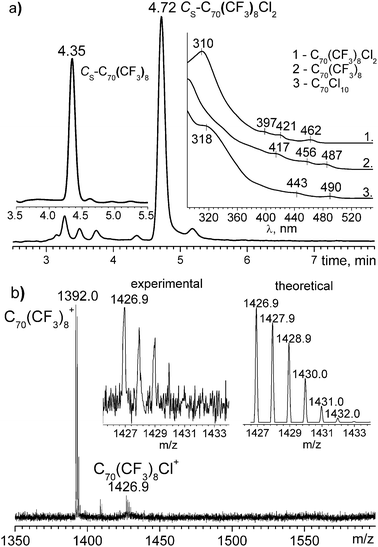 | ||
| Fig. 2 (a) HPLC traces of the reaction product and starting Cs-C70(CF3)8 as well as UV/Vis spectra of C70(CF3)8Cl2, Cs-C70(CF3)8, C70Cl10 (shown on insets); (b) The positive ion MALDI mass spectrum of Cs-C70(CF3)8Cl2, experimental and theoretical isotopic abundance of C70(CF3)8Cl+ are shown on inset. | ||
The negative ion MALDI mass spectrum of the reaction product contains the major peak of C70(CF3)8− along with its oxides and hydroxides. The positive ions MALDI mass spectrum also mainly consists of C70(CF3)8+. However, in the latter, C70(CF3)8Cl+ was observed as a minor peak along with C70(CF3)8(OH)+ (Fig. 2b). The chemical detachment of chlorine atoms under the MALDI conditions is quite usual for chlorofullerenes.13 For negative ions this detachment may occur in a greater extent than for positive ions. Therefore, the low-chlorinated C70 gives chlorine-containing ions only in positive ion mode of MALDI mass spectra, while the peaks corresponding to parent fullerene are solely present in the negative ion mode.14 Thus, the presence of chlorinated C70(CF3)Cl+ species in the MALDI mass spectra indicated the formation of low chlorinated products.
Important information about symmetry and structure of the synthesized compound was obtained from comparison of 19F NMR spectral data of Cs-C70(CF3)8 and its chlorinated product (Fig. 3). Both 19F NMR spectra contain four signals which proves that the molecular symmetry Cs is inherited by chlorinated Cs-C70(CF3)8. Chemical shifts of non-terminal trifluoromethyl groups in the ribbon of C6(CF3)2 edge-sharing hexagons are very close to those of Cs-C70(CF3)8 while the signal of terminal CF3 group of chlorinated compound is shifted ca. 3 ppm downfield compared to Cs-C70(CF3)8. This is apparently due to proximity of chlorine atoms to the terminal CF3 groups. This supposition is confirmed by quantum chemical calculations of 19F NMR chemical shifts in Cs-C70(CF3)8Cl2 and Cs-C70(CF3)8 (see ESI†, Table S4).
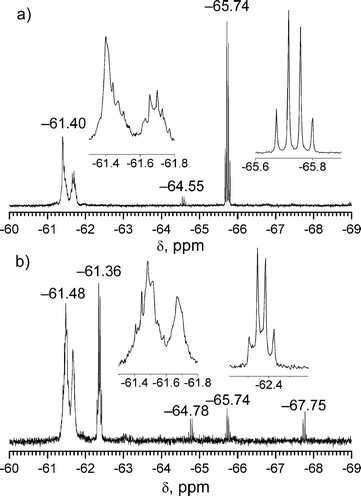 | ||
| Fig. 3 19F NMR spectra of (a) Cs-C70(CF3)8 and (b) Cs-C70(CF3)8Cl2. Zoomed quartet and multiplet regions are shown in insets. | ||
Unambiguous evidence of the structure of chlorinated derivatives of Cs-C70(CF3)8 was obtained by means of X-ray single crystal analysis. X-ray single crystal diffraction study of yellow crystals revealed the structure of Cs-C70(CF3)8Cl2 with a para9-ortho loop addition pattern (Fig. 1 and 4); IUPAC lowest-locant abbreviation is: 11,29-dichloro-1,4,19,41,49,60,66,69-octa(trifluoromethyl)-[70]fullerene. The C70(CF3)8Cl2 molecule has crystallographically imposed mirror symmetry. The attachment of eight CF3 groups and two Cl atoms closely resembles the arrangement of ten addends in C70Cl10.7a (Cl)C–C(Cl) distance is 1.626(10) Å (calculated 1.653 Å), i.e. strongly elongated compared to the same C–C bond in Cs-C70(CF3)8 (1.418(6) Å;5 DFT calculated 1.414 Å).
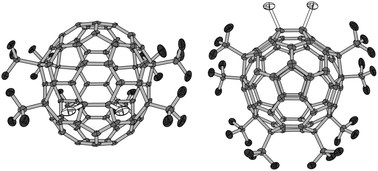 | ||
| Fig. 4 Top and side views of Cs-C70(CF3)8Cl2. | ||
In conclusion, reactivity of near-equatorial [5,6]-bond of trifluoromethylated fullereneCs-C70(CF3)8 to radical addition of a small group was demonstrated by the example of chlorination. It resulted in the high-yield formation of a new mixed compound, Cs-C70(CF3)8Cl2. Its structure was proven by means of 19F NMR spectroscopy and single crystal X-ray crystallography. Such high regioselectivity is a result of both steric and electronic factors, favoring near-equatorial functionalization of Cs-C70(CF3)8 by sterically undemanding groups. It opens a way to near-equatorial conjugation of the molecule with linear electron conducting organic fragments like alkynes and preparation of organic semiconductive materials and molecular switches.
This work was supported in part by the Russian Foundation for Basic Research (grants 09-03-91337 and 09-07-12068).
Experimental
The complete sets of isomers of C70(CF3)8X2 (943 isomers for each X = H, F, Cl, Br, OOtBu, and CF3) and radical intermediates C70(CF3)8Cl˙ (33 isomers) were constructed by attachment of pair of addends X to unsaturated carbon atoms of Cs-C70(CF3)8. Molecular models were initially optimized by molecular mechanics package TINKER 4.2 with MM2 force field set.15 Preliminary geometry optimization was carried out at the AM1 level of theory using Firefly QC package,16 which is partially based on the GAMESS (US) source code.17 Final geometry optimization and NMR chemical shifts calculation were performed at the DFT level of theory using PRIRODA software18 with the implemented original basis set of triple zeta quality with (11s6p2d)/[6s3p2d] contraction scheme for second row elements and PBE exchange-correlation functional.19Mixture of C70(CF3)m, m = 12–18 was synthesized by reaction of C70 with CF3I as described elsewhere.2 This product was mixed with C70, evacuated and sealed in a glass ampoule. The ampoule was heated at 440 °C for 50 h. Cs-C70(CF3)8 was isolated from prepared mixture of lower trifluoromethylated fullerenes by HPLC (Cosmosil Buckyprep column, 10 mm i.d. × 25 cm, equipped with a guardian precolumn 10 mm i.d. × 20 mm,) using toluene as the eluent, 4.6 ml min−1 flow rate, 290 nm.
Mass spectra (negative and positive ions) of matrix-assisted laser desorption/ionization (MALDI) were acquired with the use of Bruker AutoFlex II time-of-flight reflectron device (N2 laser, 337 nm, 1 ns pulse). 2-[(2E)-3-(4-tert-butylphenyl)-2-methylprop-2-enylidene]malononitrile (DCTB, 99%, Fluka) was chosen as a matrix. Sample preparation was performed with use of the matrix and analyte solutions in toluene and 1,2-dichlorobenzene, respectively, the matrix-to-analyte molar ratio being ≥ 1000.
19F NMR spectra were recorded on a Bruker Avance 400 spectrometer operating at 376.5 MHz. The solutions of compounds under investigation in CDCl3 contained C6F6 as an internal standard (δ −162.9 ppm).
19F NMR spectrum of C70(CF3)8: δF (ppm) −61.40–−61.51 (12F, m, CF3), −61.68 (6F, m, CF3), −65.74 (6F, q, J 16.1 Hz, CF3).
19F NMR spectrum of Cs-C70(CF3)8Cl2: δF (ppm) −61.41–−61.51 (12F, m, CF3), −61.67 (6F, m, CF3), −62.36 (6F, q, J 14.9 Hz, CF3).
Crystal data
Synchrotron X-ray data were collected at 100 K at the BL14.2 at the BESSY storage ring (PSF at the Free University of Berlin, Germany) using a MAR225 detector, λ = 0.9050 Å. C70(CF3)8Cl2·2C6H4Cl2: orthorhombic, Pbcm, a = 11.4051(3), b = 19.1550(5), c = 26.914(1) Å, V = 5879.8(3) Å3, Dc = 1.986 g cm−3, Z = 4. Anisotropic refinement with 6222 reflections and 548 parameters yielded a conventional R1(F) = 0.094 for 6089 reflections with I > 2σ(I) and wR2(F2) = 0.213 for all reflections. The C70(CF3)8Cl2 molecule has crystallographically imposed mirror symmetry, whereas o-dichlorobenzene molecule of solvation is situated in a general position in the asymmetric unit. CCDC 768967. See DOI: 10.1039/c0nj00591f for crystallographic data in CIF format.Notes and references
- A. A. Popov, I. E. Kareev, N. B. Shustova, E. B. Stukalin, S. F. Lebedkin, K. Seppelt, S. H. Strauss, O. V. Boltalina and L. Dunsch, J. Am. Chem. Soc., 2007, 129, 11551 CrossRef CAS; A. A. Popov, I. E. Kareev, N. B. Shustova, S. F. Lebedkin, S. H. Strauss, O. V. Boltalina and L. Dunsch, Chem.–Eur. J., 2008, 14, 107 CrossRef CAS.
- N. A. Samokhvalova, P. A. Khavrel, V. Yu. Markov, P. S. Samokhvalov, A. A. Goryunkov, E. Kemnitz, L. N. Sidorov and S. I. Troyanov, Eur. J. Org. Chem., 2009, 2935 CrossRef CAS; D. V. Ignat'eva, T. Mutig, A. A. Goryunkov, N. B. Tamm, E. Kemnitz, S. I. Troyanov and L. N. Sidorov, Russ. Chem. Bull., 2009, 58, 1117 Search PubMed and references therein.
- S. I. Troyanov and E. Kemnitz, Mendeleev Commun., 2008, 18, 27 Search PubMed.
- N. S. Ovchinnikova, D. V. Ignat’eva, N. B. Tamm, S. M. Avdoshenko, A. A. Goryunkov, I. N. Ioffe, V. Yu. Markov, S. I. Troyanov, L. N. Sidorov, M. A. Yurovskaya and E. Kemnitz, New J. Chem., 2008, 32, 89 RSC; Yu. Takano, M. A. Herranz, I. E. Kareev, S. H. Strauss, O. V Boltalina, T. Akasaka and N. Martin, J. Org. Chem., 2009, 74, 6902 CrossRef CAS.
- A. A. Goryunkov, E. I. Dorozhkin, D. V. Ignat'eva, E. Kemnitz, G. M. Sheldrick and S. I. Troyanov, Mendeleev Commun., 2005, 225 Search PubMed.
- H. P. Spielmann, G.-W. Wang, M. S. Meier and B. R. Weedon, J. Org. Chem., 1998, 63, 9865 CrossRef CAS; H. P. Spielmann, B. R. Weedon and M. S. Meier, J. Org. Chem., 2000, 65, 2755 CrossRef CAS.
- P. R. Birkett, A. G. Avent, A. D. Darwish, H. W. Kroto, R. Taylor and D. R. M. Walton, J. Chem. Soc., Chem. Commun., 1995, 683 RSC; S. I. Troyanov, A. A. Popov, N. I. Denisenko, O. V. Boltalina, L. N. Sidorov and E. Kemniz, Angew. Chem., Int. Ed., 2003, 42, 2395 CrossRef CAS.
- H. Al-Matar, A. K. A. Sada, A. G. Avent, R. Taylor and X. W. Wei, J. Chem. Soc., Perkin Trans. 2, 2002, 1251 RSC.
- A. G. Avent, P. R. Birkett, A. D. Darwish, H. W. Kroto, R. Taylor and D. R. M. Walton, Full. Sci. Techn., 1997, 5, 643 Search PubMed; A. G. Avent, P. R. Birkett, A. D. Darwish, H. W. Kroto, R. Taylor and D. R. M. Walton, Tetrahedron, 1996, 52, 5235 CrossRef.
- Z. Xiao, F. D. Wang, S. H. Huang, L. B. Gan, J. Zhou, G. Yuan, M. J. Lu and J. Q. Pan, J. Org. Chem., 2005, 70, 2060 CrossRef CAS.
- I. E. Kareev, I. V. Kuvychko, A. A. Popov, S. F. Lebedkin, S. M. Miller, O. P. Anderson, S. H. Strauss and O. V. Boltalina, Angew. Chem., Int. Ed., 2005, 44, 7984 CrossRef CAS.
- T. Mutig, E. Kemnitz and S. I. Troyanov, Mendeleev Commun., 2008, 18, 73 Search PubMed.
- I. V. Kuvychko, A. V. Streletskii, A. A. Popov, S. G. Kotsiris, T. Drewello, S. H. Strauss and O. V. Boltalina, Chem.–Eur. J., 2005, 11, 5426 CrossRef CAS; L. N. Sidorov, V. Livadaris, N. B. Shustova, I. N. Ioffe, E. Kemnitz and S. I. Troyanov, Russ. Chem. Bull., 2005, 54, 1121 Search PubMed.
- V. Yu. Markov, A. S. Pimenova, G. G. Petukhova, A. A. Kozlov, N. S. Ovchinnikova, V. A. Ioutsi, E. I. Dorozhkin, A. A. Goryunkov, N. B. Tamm, S. I. Troyanov and L. N. Sidorov, Mass-Spectrometriya, 2007, 4, 103 Search PubMed.
- J. W. Ponder, TINKER 4.2, www http://dasher.wustl.edu/tinker.
- A. A. Granovsky, Firefly 7.1.G, www http://classic.chem.msu.su/gran/firefly/index.html.
- M. W. Schmidt, K. K. Baldridge, J. A. Boatz, S. T. Elbert, M. S. Gordon, J. J. Jensen, S. Koseki, N. Matsunaga, K. A. Nguyen, S. Su, T. L. Windus, M. Dupuis and J. A. Montgomery, J. Comput. Chem., 1993, 14, 1347 CrossRef CAS.
- D. N. Laikov, Chem. Phys. Lett., 1997, 281, 151 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Details of 19F NMR simulation, Schlegel diagrams, DFT relative energies of possible C70(CF3)8Cl2 isomers, selected calculated distances and dihedral angles of C70(CF3)8X2, and HOMO/LUMO calculations and spin density analysis. CCDC reference number 768967. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c0nj00591f |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2011 |
