Insulated molecular wires of polyaniline pseudopolyrotaxane†
Souhaira
Hbaieb
*a,
Noureddine
Amdouni
a,
Hélène
Parrot-Lopez
b,
Rafik
Kalfat
c and
Yves
Chevalier
d
aUR Physico-Chimie des Matériaux Solides, Faculté des Sciences de Tunis, Université El Manar Manar II, 2092 Tunis, Tunisia
bUniversité de Lyon, UMR 5246, Institut de Chimie et Biochimie Moléculaires et Supramoléculaires, Université Lyon 1, 69622 Villeurbanne, France
cInstitut National de Recherche et d’Analyse Physico-chimique, 2020 Sidi Thabet, Tunisia
dUniversité de Lyon, Laboratoire d'Automatique et de Génie des Procédés, CNRS UMR 5007, Université Lyon 1, 69622 Villeurbanne, France
First published on 11th October 2010
Abstract
High density threading of cyclodextrins to conducting poly(aniline) as its emeraldine salt was achieved. Such insulated molecular wires were obtained using a strongly complexing chemically modified β-cyclodextrin. Inclusion complexation of the aniline monomer, polymerization of the inclusion complex, physicochemical characterizations of the polyaniline pseudopolyrotaxane and the electrical properties of its thin films are reported.
Insulated molecular wires (IMWs) are made of single chains of conducting polymer encapsulated in an insulating host. Such encapsulation allows control of the conductive properties of polyconjugated polymers for the manufacturing of new polymeric materials as thin films that find applications in electronic devices such as photovoltaic cells, batteries, sensors and light-emitting diodes. The manufacture and properties of IMWs have recently been reviewed by Frampton and Anderson,1 from which we took our terminology and nomenclature.
New conductive materials are prepared by means of confinement and/or the orientation of conjugated polymer chains in template materials;2 polyaniline (PANI) is widely used in such instances.3 The fabrication of IMWs is feasible either by confining conducting polymers inside linear cavities of porous materials or by threading macrocyclic molecules onto conducting polymers. As an example, cyclodextrins (CD) are threaded onto the linear chain of a conjugated polymer by passing it through the CD's cavity, forming a necklace-like supramolecule with the conducting polymer chain surrounded by insulating CDs. Such complex species are called pseudopolyrotaxanes. End-capping polymer chains with bulky substituents gives polyrotaxanes, preventing macrocyclic molecules from leaving the threads. Polyrotaxanes made of conducting polymers are presented as insulated molecular wires, although most electrical measurements have shown that they are not electrically insulated. IMWs of polyrotaxanes require a high linear density of threaded molecules. This has been reached in several instances, such as in poly(para-phenylene)⊂β-CD4 or poly(azomethine)⊂β-CD.5 Polythiophene6,7 and polyaniline8–11 appear difficult to thread due to a high linear density; NMR spectroscopy analyses6 and small-angle neutron scattering investigations7 lead to linear densities between 0.1 and 0.6 CD per repeat unit, below the theoretical limiting value of 1. Most effort has been devoted to mechanistic studies of IMWs, meaning that preparation methods have often implemented tedious repeated purification schemes in order to isolate the right compound for academic research on well-characterized materials.
The present work deals with IMWs of PANI that could not be prepared until now. PANI is a very popular conducting polymer12 because of its easy synthesis by the oxidative polycondensation of aniline in aqueous media, excellent environmental stability and the possible control of electrical properties by suitable doping with acids. The preparation of PANI polyrotaxanes has previously been attempted using aniline polymerization in the presence of CD. However, it was not successful at reaching a high enough linear density of threaded CDs. The formation of IMWs was claimed because electric birefringence has been observed and scanning tunneling microscopy images have shown pieces of rigid threads having the expected size range.8 However, such observations were made at temperatures below 275 K. None of these effects could be seen at higher temperatures, although the linear density should have been the same; this showed that the actual linear density of CD was not at its closest packing. The behaviour seen at low temperature is reminiscent of a one-dimensional phase separation of the CD along the polymer chain, giving rise to dense domains coexisting with low density domains; the pieces of dense phase were observed as rigid rods and caused birefringence. A low linear CD density has been measured from chemical analyses by two independent teams in same range (0.07 CD per repeat unit);9 a low threading density of per-methylated β-CD (0.2 CD per repeat unit) has been measured by 1H NMR.11
The present communication reports on the preparation and properties of densely threaded CD on conducting PANI in its emeraldine salt form doped with hydrochloric acid (Fig. 1). The key point was the utilization of a chemically modified β-CD that forms strong inclusion complexes with the aniline monomer in place of the native β-CD (non-modified) that has been used until now. The full preparation process was conducted in 1 M HCl, which ensures the formation of doped PANI that is ready to use in applications. The process was efficient (high yield), meaning that the purification process was minimal. We report data on the formation of the inclusion complex of heptakis(amino)-β-CD with aniline, its oxidative polycondensation into PANI together with several physicochemical characterizations that show the formation of dense threading (IMW), and finally its processing as thin films and their electrical conductivity measurements.
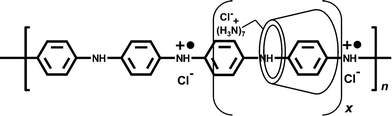 | ||
| Fig. 1 The proposed structure of the pseudopolyrotaxane of polyaniline emeraldine salt and cyclodextrin. | ||
The dense threading of CDs and sustainable encapsulation requires the formation of strong inclusion complexes of the guest monomer inside the host cavity of CD. Native β-CD and its common commercial derivatives do not form inclusion complexes of high affinity with aniline; this is the origin of the failure of the preparation of PANI IMWs. The chemically modified β-CD, heptakis(6-amino-6-deoxy)-cyclomaltoheptaose (β-CDNH2), has been used in place of native β-CD as a host for the complexation of the aniline monomer and its subsequent polymerization into PANI⊂β-CDNH2 pseudopolyrotaxane. β-CDNH2 has seven primary amino groups at its lower rim. Substitution of the seven primary hydroxyls of β-CD by amino groups was done by means of a three step synthesis, as reported previously.13 The formation of an inclusion complex with aniline in 1 M HCl has been investigated by means of classical UV-vis absorbance and 1H NMR experiments.14,15 Typical UV-vis spectra recorded for mixed aniline/β-CDNH2 solutions in 1 M HCl are shown in Fig. 2A. The stoichiometry and stability constant of the inclusion complex were determined by the Benesi–Hildebrand method.16 The variation of the 1H NMR chemical shifts of aniline and the β-CDNH2 protons as a function of the aniline/β-CDNH2 ratio allowed the characterization of the inclusion complex in 1 M DCl (Fig. 2B). The results from both methods agreed quite well, with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry and a large stability constant K11 = 1100 mol−1 L.
1 stoichiometry and a large stability constant K11 = 1100 mol−1 L.
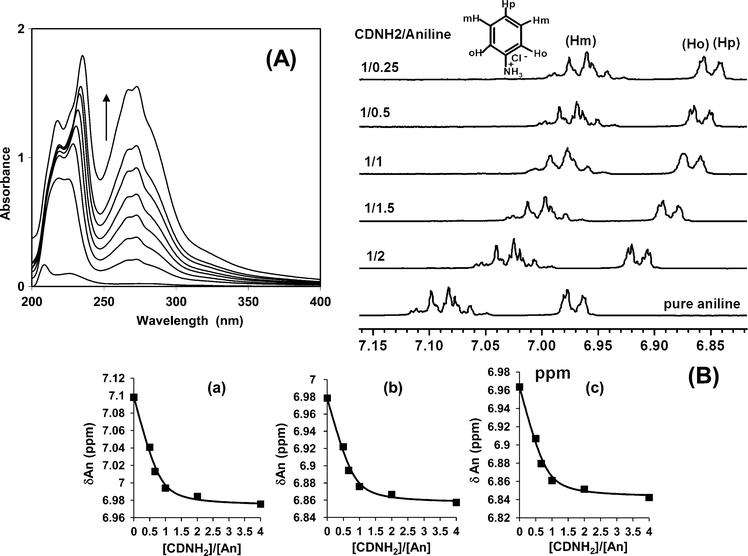 | ||
Fig. 2 (A) UV-vis absorption spectra of aniline at constant concentration (4 × 10−5 M) in a 1 M HCl aqueous solution with increasing concentration of β-CDNH2 ranging from 5 × 10−4 to 5 × 10−3 M. (B) 1H NMR spectra of the aromatic region showing the shifted peaks for the (a) meta, (b) ortho and (c) para protons for different β-CDNH2/aniline molar ratios, and the best fit of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexation equilibrium under fast exchange conditions with K11 = 1100 mol−1 L. 1 complexation equilibrium under fast exchange conditions with K11 = 1100 mol−1 L. | ||
Oxidative polymerization of aniline as its inclusion complex was carried out in 1 M HCl by the addition of the ammonium persulfate oxidant (NH4)2S2O8 at 0 °C. A bluish color appeared, which progressively turned dark blue and finally dark green as the consequence of the apparition of the emeraldine salt. The precipitated PANI⊂β-CDNH2 salt was collected by filtration, washed with 1 M HCl and methanol, and dried in a vacuum at 50 °C. The same process was used with the solid substrate immersed in the reaction medium for thin film deposition. This was a very simple process that did not involve tricky purification steps, allowing easy scale-up or implementation into a manufacturing process.
IR spectroscopy of powders and thin films gave evidence of the formation of PANI in its doped emeraldine salt form, in particular, the characteristic N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Q
Q![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching band of the quinoid rings at 1568 cm−1, the N–B–N stretching band of the benzenoid rings at 1488 cm−1, the C–N stretching band for the benzenoid ring in the polaron structure at 1250 cm−1, and the C–H in plane bending vibration of N
N stretching band of the quinoid rings at 1568 cm−1, the N–B–N stretching band of the benzenoid rings at 1488 cm−1, the C–N stretching band for the benzenoid ring in the polaron structure at 1250 cm−1, and the C–H in plane bending vibration of N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Q
Q![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, Q
N, Q![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N+H–B and B–N+H–B species formed by protonation at 1146 cm−1.17 The chemical composition of polyaniline pseudopolyrotaxane determined by elemental analysis gives it a threading density of 0.93 β-CDNH2 per aromatic ring of PANI (Table 1). The low proportion of sulfur present in the material comes from the sulfuric acid produced during the oxidation process of PANI.
N+H–B and B–N+H–B species formed by protonation at 1146 cm−1.17 The chemical composition of polyaniline pseudopolyrotaxane determined by elemental analysis gives it a threading density of 0.93 β-CDNH2 per aromatic ring of PANI (Table 1). The low proportion of sulfur present in the material comes from the sulfuric acid produced during the oxidation process of PANI.
1H NMR characterization of the PANI⊂β-CDNH2 salt in DMSO-d6 solution (Fig. 3) showed the aromatic protons of PANI at 6.62, 6.75 and 6.95 ppm. The H3 and H5 protons of β-CDNH2 located inside the cavity appeared at 3.82 and 3.68 ppm, upfield shifted with respect to pure β-CDNH2. Conversely, the H2 and H4 protons located at the external surface of the β-CDNH2 remained relatively unaffected. Such shifts are very characteristic of inclusion complexation.15 The density of β-CDNH2 was inferred from the areas under the NMR lines of the aromatic protons of PANI between 6.50 and 7.00 ppm, and the H1 protons of CDNH2 at 5.05 ppm. Let us recall that the compound is a pure pseudopolyrotaxane because it has been thoroughly washed with 1 M HCl and methanol after the polymerization; washing steps were repeated until the UV-vis analysis showed that no more material was extracted. The threading density was 0.94 β-CDNH2 per aromatic ring of PANI (x/4n in Fig. 1), which corresponded to close packing along the PANI chain. These results are in good agreement with those obtained from the elemental analysis of the material. This ratio is within the range expected on the basis of the crystal structure of head-to-head packed β-CD (0.65)1 and Molecular Dynamics simulations of PANI⊂β-CD (1.0).18 NMR spectra were unchanged over time, meaning that the CD remained threaded as an inclusion complex in DMSO solution. IR spectra showed the characteristic bands of HCl-doped PANI and β-CDNH2, in which some bands were slightly shifted with respect to the pure compounds, indicating the complete formation of PANI⊂β-CDNH2. It is presumed that the strong complexation properties of β-CDNH2 towards aniline still operate for PANI. End-capping of the pseudopolyrotaxane into the polyrotaxane was not useful for preventing CD release into solution.
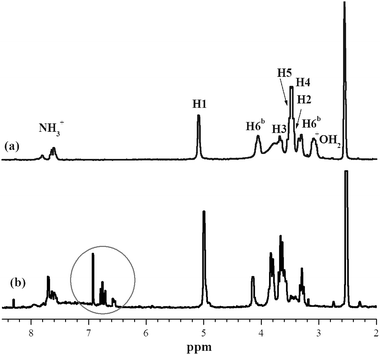 | ||
| Fig. 3 1H NMR spectra of (a) β-CDNH2·HCl salt and (b) HCl-doped PANI⊂β-CDNH2 in DMSO-d6. PANI aromatic protons are circled. | ||
The X-ray diffraction of PANI⊂β-CDNH2 in powder form did not show Bragg reflections of the emeraldine salt ES-I crystalline form19 observed in the absence of β-CDNH2. Instead a Debye–Scherrer halo was recorded, showing the amorphous structure of the material. Transmission electron microscopy pictures of the powder showed submicronic particles of anisotropic shape (Fig. 4). Such particles precipitated from the reaction medium during the polymerization. From an image analysis of 100 particles, the average rod length was 170 nm and the small dimension was 50 nm, close to the diameter of the fibers of the HCl-doped emeraldine salt formed in the early stage of aniline polymerization or by the interfacial polymerization of aniline.20 As a general rule, the precipitation of amorphous materials gives spherical particles because their growth is isotropic. The strongly anisotropic shape of amorphous particles at the 100 nm scale reveals the rod-like shape of the rigid PANI⊂β-CDNH2 IMWs that comprise them; this is an indirect indication of the rigid nature of the densely threaded pseudopolyrotaxanes.
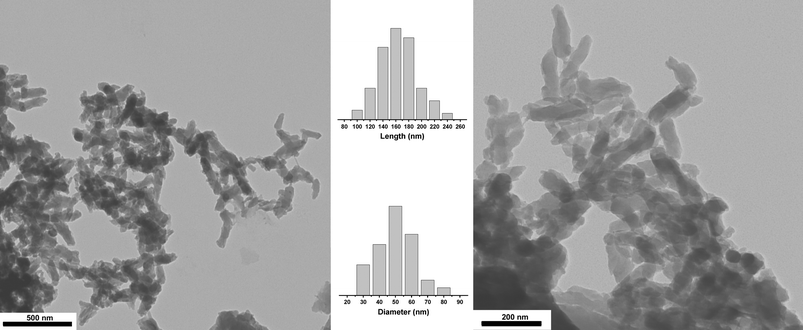 | ||
Fig. 4 TEM pictures of PANI⊂β-CDNH2 precipitated particles. Left: ×25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000; right: ×53 000; right: ×53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000; center: the distribution of rod lengths and diameters. 000; center: the distribution of rod lengths and diameters. | ||
The UV-vis absorption spectra of thin film materials (Fig. 5) indicated that PANI was in its HCl-doped emeraldine salt form. The band at 320 nm corresponds to a π–π* transition of the benzenoid rings, while the bands at 415 and 832 nm are characteristic of the localized polarons of the protonated PANI.21 The absorption bands of PANI⊂β-CDNH2 exhibited a blue shift and a decrease of intensity with respect to a pure PANI film. The electrical conductivity of PANI⊂β-CDNH2 thin films was 0.10 S cm−1, which was not very low, although significantly lower than films of pure PANI (0.35 S cm−1). The encapsulation by a dense cladding sheath of cyclodextrins indeed decreased the conductivity; the molecular wire was not electrically insulated, however.
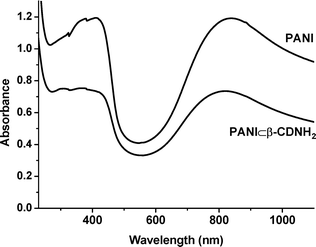 | ||
| Fig. 5 UV-vis spectra of PANI films deposited on quartz plates. | ||
As a final summary, the preparation of a PANI pseudopolyrotaxane with close-packed threaded cyclodextrins was achieved by a simple method that requires neither difficult purification steps nor end-capping of the polyrotaxane. This was made possible by the choice of a modified CD that forms a strong inclusion complex with the aniline monomer and the PANI polymer under the polymerization conditions (1 M HCl). The process is simple enough, enabling scale-up to the manufacturing of large batches required by applications. Preparation in 1 M HCl directly affords the conductive emeraldine salt material in its doped form, which can be cast as a thin film. The electrical conductivity of the thin film is lower than for doped PANI, although the IMW material is not an insulator.
Experimental
β-CD (Roquette Frères, Lestrem, France) was dried at 120 °C overnight under a vacuum. Aniline (Merck) was distilled under reduced pressure. Ammonium peroxydisulfate (Aldrich) was used as received. β-CDNH2 was prepared from β-CD by means of a three-step synthesis.13Mass E. S. (m/z): 1151.1 for [M + Na]+; calc. for C42H77O28N7: M = 1128.1 g mol−1.
1H NMR δH (300 MHz, D2O, ppm): 3.25 (dd, 7H, H6a), 3.44 (dd, 14H, H2, H4), 3.72 (m, 14H, H3, H5), 3.88 (m, 7H, H6b) and 4.14 (d, 7H, J = 3 Hz, H1).
13C NMR δC (60 MHz, D2O, ppm): 51.3 (C6), 70.4–72.6 (C2, C3, C5), 83.2 (C4) and 102.1–102.9 (C1).
PANI⊂β-CDNH2 was prepared by the oxidative polymerization of aniline (0.9 mL) in a 1 M HCl solution (180 mL) containing a stoichiometric amount of β-CDNH2 (11.12 g). Ammonium peroxydisulfate (2.25 g) oxidant was slowly added to the reaction medium while cooling in an ice bath and stirring with a magnetic bar.22 The fine green powder precipitate was collected by filtration, washed with 1 M HCl and methanol, and dried under a vacuum at 50 °C. Thin films of PANI⊂β-CDNH2 were formed in situ on the surface of quartz plates immersed in the reaction medium. The film thickness was estimated from optical absorption at a 400 nm wavelength according to the method of Stejskal et al.23
IR spectra of KBr pellets and PANI films were recorded in the range 600–4000 cm−1 by a Perkin-Elmer 1000 and a Bruker IFS 55 Equinox spectrometer, respectively. Elemental analyses were performed on a Perkin-Elmer 2400 series II instrument. 1H NMR spectra were run on an AV500 Bruker spectrometer. Absorbance spectra were recorded using a Perkin-Elmer Lambda 2 UV-vis spectrophotometer. Wide-angle X-ray diffraction powder patterns were measured using a Philips PW 3040 diffractometer working with the Cu-Kα line at a 0.154 nm wavelength and at a 0.02° min−1 angular scan rate. TEM pictures of particle dispersions dried on a microscope grid were taken using a Philips CM120 microscope at the “Centre Technologique des Microstructures” (CTμ, University Lyon 1, France). The DC conductivity of samples was measured at room temperature by the standard collinear four-point van der Pauw technique.24
References
- M. J. Frampton and H. L. Anderson, Angew. Chem., Int. Ed., 2007, 46, 1028–1064 CrossRef CAS.
- D. J. Cardin, Adv. Mater., 2002, 14, 553–563 CrossRef CAS.
- (a) S. Palaniappan and V. Nivasu, New J. Chem., 2002, 26, 1490–1494 RSC; (b) S. Palaniappan and C. A. Amarnath, New J. Chem., 2002, 26, 1671–1674 RSC; (c) L. Pan, L. Pu, Y. Shi, S. Song, Z. Xu, R. Zhang and Y. Zheng, Adv. Mater., 2007, 19, 461–464 CrossRef CAS; (d) Y.-G. Wang, W. Wu, L. Cheng, P. He, C.-X. Wang and Y.-Y. Xia, Adv. Mater., 2008, 20, 2166–3170 CrossRef CAS.
- (a) P. N. Taylor, M. J. O'Connell, L. A. McNeill, M. J. Hall, R. T. Aplin and H. L. Anderson, Angew. Chem., Int. Ed., 2000, 39, 3456–3460 CrossRef CAS; (b) F. Cacialli, J. S. Wilson, J. J. Michels, C. Daniel, C. Silva, R. H. Friend, N. Severin, P. Samorì, J. P. Rabe, M. J. O'Connell, P. N. Taylor and H. L. Anderson, Nat. Mater., 2002, 1, 160–164 CrossRef CAS; (c) J. J. Michels, M. J. O'Connell, P. N. Taylor, J. S. Wilson, F. Cacialli and H. L. Anderson, Chem.–Eur. J., 2003, 9, 6167–6176 CrossRef CAS; (d) M. H. Chang, M. J. Frampton, H. L. Anderson and L. M. Herz, Appl. Phys. Lett., 2006, 89, 232110 CrossRef; (e) G. Latini, L.-J. Parrott, S. Brovelli, M. J. Frampton, H. L. Anderson and F. Cacialli, Adv. Funct. Mater., 2008, 18, 2419–2427 CrossRef CAS; (f) M. J. Frampton, G. Sforazzini, S. Brovelli, G. Latini, E. Townsend, C. C. Williams, A. Charas, L. Zalewski, N. S. Kaka, M. Sirish, L. J. Parrott, J. S. Wilson, F. Cacialli and H. L. Anderson, Adv. Funct. Mater., 2008, 18, 3367–3376 CrossRef CAS.
- (a) D. Nepal, S. Samal and K. E. Geckeler, Macromolecules, 2003, 36, 3800–3802 CrossRef CAS; (b) A. Farcas and M. Grigoras, Polym. Int., 2003, 52, 1315–1320 CrossRef CAS.
- (a) C. Lagrost, J. C. Lacroix, S. Aeiyach, M. Jouini, K. 1. Chane-Ching and P. C. Lacaze, Chem. Commun., 1998, 489–490 RSC; (b) C. Lagrost, K. I. Chane Ching, J.-C. Lacroix, S. Aeiyach, M. Jouini, P.-C. Lacaze and J. Tanguy, J. Mater. Chem., 1999, 9, 2351–2358 RSC; (c) P. Hapiot, C. Lagrost, S. Aeiyach, M. Jouini and J.-C. Lacroix, J. Phys. Chem. B, 2002, 106, 3622–3628 CrossRef CAS; (d) J. Storsberg, H. Ritter, H. Pielartzik and L. Groenendaal, Adv. Mater., 2000, 12, 567–569 CrossRef CAS; (e) I. Yamaguchi, K. Kashiwagi and T. Yamamoto, Macromol. Rapid Commun., 2004, 25, 1163–1166 CrossRef CAS; (f) Y. Takashima, Y. Oizumi, K. Sakamoto, M. Miyauchi, S. Kamitori and A. Harada, Macromolecules, 2004, 37, 3962–3964 CrossRef CAS.
- M. van den Boogaard, G. Bonnet, P. van't Hof, Y. Wang, C. Brochon, P. van Hutten, A. Lapp and G. Hadziioannou, Chem. Mater., 2004, 16, 4383–4385 CrossRef CAS.
- (a) K.-i. Yoshida, T. Shimomura, K. Ito and R. Hayakawa, Langmuir, 1999, 15, 910–913 CrossRef CAS; (b) T. Shimomura, T. Akai, T. Abe and K. Ito, J. Chem. Phys., 2002, 116, 1753–1756 CrossRef CAS; (c) T. Akai, T. Shimomura and K. Ito, Synth. Met., 2003, 135–136, 777–778 CrossRef CAS; (d) T. Shimomura, T. Akai, M. Fijumori, S. Heike, T. Hashizume and K. Ito, Synth. Met., 2005, 153, 497–500 CrossRef CAS.
- (a) E. Subramanian, G. Anitha, M. K. Selvam and M. I. A. Badusha, Bull. Mater. Sci., 2005, 28, 55–61 Search PubMed; (b) G. Anitha and E. Subramanian, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 281–294 CrossRef CAS; (c) M. Grigoras and D. G. Conduruta, J. Inclusion Phenom. Macrocyclic Chem., 2006, 54, 101–107 CrossRef CAS.
- Y. Liu, J. Shi, Y. Chen and C.-F. Ke, Angew. Chem., Int. Ed., 2008, 47, 7293–7296 CrossRef.
- J. Shi, Y. Chen, Q. Wang and Y. Liu, Adv. Mater., 2010, 22, 2575–2578 CrossRef CAS.
- (a) A. G. MacDiarmid, Angew. Chem., Int. Ed., 2001, 40, 2581–2590 CrossRef CAS; (b) A. J. Heeger, Angew. Chem., Int. Ed., 2001, 40, 2591–2611 CrossRef CAS; (c) W.-S. Huang, A. G. MacDiarmid and A. J. Epstein, J. Chem. Soc., Chem. Commun., 1987, 1784–1786 RSC; (d) M. Angelopoulos, A. Ray, A. G. MacDiarmid and A. J. Epstein, Synth. Met., 1987, 21, 21–30 CrossRef CAS; (e) W. R. Salanch, I. Lundstrom, T. Hjertberg, C. B. Duke, E. Conwell, A. Paton, A. G. MacDiarmid, N. L. D. Somasiri, W. S. Huang and A. F. Richter, Synth. Met., 1987, 18, 291–296 CrossRef; (f) A. Yue, A. J. Epstein, Z. Zhong, P. K. Gallagher and A. G. MacDiarmid, Synth. Met., 1991, 41, 765–768 CrossRef CAS.
- (a) H. Parrot-Lopez, C. C. Ling, P. Zhang, A. Baszkin, G. Albrecht, C. De Rango and A. W. Coleman, J. Am. Chem. Soc., 1992, 114, 5479–5480 CrossRef CAS; (b) S. Hbaieb, R. Kalfat, Y. Chevalier, N. Amdouni and H. Parrot-Lopez, Mater. Sci. Eng., C, 2008, 28, 697–704 CrossRef CAS.
- (a) M. V. Rekharsky and Y. Inoue, Chem. Rev., 1998, 98, 1875–1917 CrossRef CAS; (b) K. A. Connors, Chem. Rev., 1997, 97, 1325–1357 CrossRef CAS.
- H.-J. Schneider, F. Hacket, V. Rüdiger and H. Ikeda, Chem. Rev., 1998, 98, 1755–1785 CrossRef CAS.
- H. A. Benesi and J. H. Hildebrand, J. Am. Chem. Soc., 1949, 71, 2703–2707 CrossRef CAS.
- E. T. Kang, K. G. Neoh and K. L. Tan, Prog. Polym. Sci., 1998, 23, 277–324 CrossRef CAS.
- R. V. Belosludov, H. Mizuseki, K. Ichinoseki and Y. Kawazoe, Jpn. J. Appl. Phys., 2002, 41, 2739–2741 CrossRef CAS.
- J. P. Pouget, M. E. Jozefowicz, A. J. Epstein, X. Tang and A. G. MacDiarmid, Macromolecules, 1991, 24, 779–789 CrossRef CAS.
- (a) J. Huang and R. B. Kaner, Angew. Chem., Int. Ed., 2004, 43, 5817–5821 CrossRef CAS; (b) J. Huang and R. B. Kaner, J. Am. Chem. Soc., 2004, 126, 851–855 CrossRef CAS.
- (a) R. P. McCall, J. M. Ginder, J. M. Leng, H. J. Ye, S. K. Manohar, J. G. Masters, G. E. Asturias, A. G. MacDiarmid and A. J. Epstein, Phys. Rev. B: Condens. Matter, 1990, 41, 5202–5213 CrossRef CAS; (b) J. Ryu and C. B. Park, Angew. Chem., Int. Ed., 2009, 48, 4820–4823 CrossRef CAS.
- D. C. Trivedi, Polyanilines, in Conductive molecules and electric properties, Handbook of organic conductive molecules and polymers, ed. H. S. Nalwa, Wiley, Chichester, 1997, vol. 2, pp. 505–572 Search PubMed.
- J. Stejskal, I. Sapurina, J. Proke and J. Zemek, Synth. Met., 1999, 105, 195–202 CrossRef CAS.
- L.J. van der Pauw, Philips Res. Rep., 1958, 13, 1–9 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H NMR spectra. See DOI: 10.1039/c0nj00559b |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2011 |
