DOI:
10.1039/C0NJ00542H
(Paper)
New J. Chem., 2011,
35, 190-196
Three 3D octamolybdate-based hybrids with 1D–3D CuI/CuII-bis(triazole) motifs: influence of the amount of Et3N†
Received
(in Montpellier, France)
10th July 2010
, Accepted 20th September 2010
First published on 18th October 2010
Abstract
Three three-dimensional (3D) compounds constructed from octamolybdate clusters and 1D–3D CuI/CuII-bis(triazole) motifs, [CuI4(btx)4][β-Mo8O26] (1), [CuI4(btx)2][β-Mo8O26]·H2O (2), and [CuII2(btx)4][α-Mo8O26] (3) [btx = 1,6-bis(1,2,4-triazol-1-yl)hexane], were hydrothermally synthesized in the presence of different amounts of reducing agent Et3N. The crystal structures have been determined by X-ray diffraction. In 1, the copper(I)-btx motif is a 1D meso-helix chain which is further linked by hexadentate β-[Mo8O26]4−clustersvia coordinating to CuI cations to achieve an intricate 3D structure. In 2, the copper(I)-btx motif exhibits a 2D grid sheet, and the hexadentate β-[Mo8O26]4−clusters interact with neighboring sheets to construct a 3D structure. In 3, the copper(II)-btx motif possesses a 3D (4,4)-connected framework with the (65.8)(64.82) topology, which is further connected by the tetradentate α-[Mo8O26]4−clusters forming a 3D self-penetrating (4,6)-connected hybrid network. The thermal stability and luminescent properties of 1–3 are investigated in the solid state.
Introduction
Polyoxometalates (POMs),1 an outstanding class of molecules with structural variety and versatility, have attracted long-lasting research interest in catalysis,2 medicine,3 biology,4 and materials science.5 Currently, a brand-new advance in the POM chemistry is the design and construction of high-dimensional hybrid materials based on the coordination ability of POMs and different transition-metal complexes (TMCs). Such organic–inorganic hybrid materials can combine the unique characteristics of the components and exhibit novel structural motifs, as well as new properties arising from the synergistic interplay of the two components. Many efforts have been devoted to this field by inorganic chemists, which have achieved significant results. Among the reported work, it is a fascinating area for development to rationally design and construct high-dimensional inorganic–organic hybrid materials. To carry out this objective, it is crucial to select felicitous POMs and TMCs.
On the one hand, of the various POMs, octamolybdates present particular cluster structures. Since Schwing-Weill and Arnaud-Neu first proved the existence of isomers of octamolybdates in an infrared (IR) study in 1970,6 eight isomeric forms, α through θ, have been reported one after another. A comprehensive investigation on these isomers has been reported by Zubieta and co-workers.7 The different isomers of octamolybdates can well conform to the coordination environment of various metal–organic motifs, which may offer more potential opportunity to form high-dimensional hybrids.
On the other hand, TMCs not only provide charge compensation, but may also play space-filling, passivating and structure-directing roles. The coordination numbers and geometries of the metal ions and the nature of organic ligand may provide structural flexibility and spatial transmission of structural information. Therefore, we chose copper cations with two oxidation numbers as linkages and flexible bis(triazole) molecules, 1,6-bis(1,2,4-triazol-1-yl)hexane (btx),8 as organic ligands based on the following considerations. (i) As a d9 metal, CuII ions can be easily converted into CuI ions in the presence of different types of reducing agents.9 For example, with a step by step increase of the temperature and the amount of aqueous ammonia, Chen and his co-workers have successfully synthesized a series of polymeric CuII, mixed-valent CuI,II and CuI imidazolates which exhibit intriguing structures10 and Su’s group has achieved this transformation in POMs-based MOFs.11 The changeable oxidation states and versatile coordination geometries make copper ions a prime candidate for a controllable linker. (ii) The 1,2,4-triazole group in btx ligand can provide more potential coordination sites.12 And the two 1,2,4-triazole rings can freely twist around the –(CH2)6– group to meet the requirements of the coordination geometries of metal atoms in the assembly process. Additionally, the longer methylene –(CH2)6– skeleton tends to exhibit more flexible conformations, which is in favor of forming diverse TMCs and final hybrid structures.
As an accurate prediction of the final structures of the hybrid compounds still remains demanding and challenging, we have tried different synthetic conditions and performed many experiments. Fortunately, we isolated three 3D organic–inorganic hybrid compounds, [CuI4(btx)4][β-Mo8O26] (1), [CuI4(btx)2][β-Mo8O26]·H2O (2), and [CuII2(btx)4][α-Mo8O26] (3) with differently structured TMCs and final spacial structures. Their syntheses, structures and the luminescent properties in the solid state are presented and discussed.
Experimental section
Materials and general methods
All reagents were purchased commercially and were used without further purification. Elemental analyses of C, H and N were performed on a Perkin-Elmer 2400 CHN Elemental Analyzer and that of Cu and Mo were carried out with a Leaman inductively coupled plasma (ICP) spectrometer. IR spectra on KBr pellets were recorded on a Nicolet 170SX FT-IR spectrophotometer in the range 400–4000 cm−1. TG analyses were performed with a Perkin–Elmer TGA7 instrument in a N2 atmosphere at a heating rate of 10 °C min−1. The X-ray powder diffraction (XRPD) patterns were recorded on a Siemens D5005 diffractometer with Cu-Kα (λ = 1.5418 Å) radiation. Photoluminescence spectra were measured with pure solid samples under room temperature using a FL–2T2 instrument (SPEX, USA) with a 450 W Xenon lamp monochromatized by double grating (1200).
Synthesis of [CuI4(btx)4][β-Mo8O26] (1)
A mixture of Na2MoO4·2H2O (0.2 g, 0.8 mmol), Cu(Ac)2·H2O (0.08 g, 0.4 mmol), btx (0.11 g, 0.5 mmol), and Et3N (0.2 mL) was dissolved in 12 mL distilled water and stirred at room temperature for 30 min. The pH of the mixture was adjusted to 3.8 with 1 mol L−1HCl, and then sealed in a Teflon-lined autoclave and heated at 160 °C for 4 days. The reactor was slowly cooled to room temperature at a rate of 10 °C h−1. Red block crystals were obtained in 45% yield based on Mo. The crystals were filtered and hand-separated. Anal. calcd for C40H64Cu4Mo8N24O26 (2318.88): C 20.72, H 2.78, N 14.49, Cu 10.99, Mo 33.19 (%); found: C 20.61, H 2.65, N 14.60, Cu 10.91, Mo 33.08 (%). IR (solid KBr pellet, cm−1): 3119 (m), 2937 (w), 2859 (w), 1529 (s), 1459 (m), 1367 (m), 1278 (s), 1214 (m), 1133 (s), 946 (s), 906 (s), 831 (s), 707 (s), 673 (w), 553 (w).
Synthesis of [CuI4(btx)2][β-Mo8O26]·H2O (2)
A similar synthetic procedure to that of 1 was used, except for using 0.1 mL Et3N. Red cuboid crystals were filtered and washed with distilled water (yield: 47% based on Mo). Anal. calcd for C20H34Cu4Mo8N12O27 (1896.31): C 12.67, H 1.81, N 8.86, Cu 13.40, Mo 40.48 (%); found: C 12.59, H 1.74, N 8.97, Cu 13.49, Mo 40.39 (%). IR (solid KBr pellet, cm−1): 3650 (m), 3117 (m), 2910 (w), 2850 (w), 1530 (s), 1457 (m), 1365 (m), 1291 (s), 1210 (m), 1138 (s), 945 (s), 923 (s), 891 (s), 848 (m), 717 (s), 656 (w), 555 (w).
Synthesis of [CuII2(btx)4][α-Mo8O26] (3)
A similar synthetic procedure to that of 1 was used, except for not using Et3N. Blue sheet crystals were filtered and washed with distilled water (yield: 42% based on Mo). Anal. calcd for C40H64Cu2Mo8N24O26 (2191.78): C 21.92, H 2.94, N 15.34, Cu 5.80, Mo 35.02 (%); found: C 21.84, H 2.85, N 15.41, Cu 5.89, Mo 34.89 (%). IR (solid KBr pellet, cm−1): 3133 (m), 2915 (w), 2857 (w), 1528 (s), 1469 (m), 1379 (m), 1286 (s), 1214 (m), 1130 (s), 947 (s), 926 (s), 907 (s), 877 (m), 797 (s), 669 (s), 568 (w).
X-Ray crystallographic study
Single-crystal X-ray diffraction data collections of compounds 1–3 were performed using a Bruker Smart Apex CCD diffractometer with Mo-Ka radiation (λ = 0.71073 Å) at 293 K. Multi-scan absorption corrections were applied. All the structures were solved by the directed methods and refined by full-matrix least-squares on F2 using the SHELXTL crystallographic software package.13 The positions of hydrogen atoms on carbon atoms were calculated theoretically. In 1, one fragment of btx ligand (C17, N2, N3, C4, N1, and C3) is disordered with two possible positions and the occupancies refined to 50%. The hydrogen atoms of water molecules in compounds 2 could not be introduced in the refinement but were included in the structure factor calculation. A summary of the crystal data, data collection, and refinement parameters for 1–3 is listed in Table 1.
Table 1
Crystal data and structure refinements for compounds 1–3
| |
1
|
2
|
3
|
|
R
1 = Σ∥Fo| − |Fc∥/Σ|Fo|. wR2 = Σ[w(Fo2 − Fc2)2]/Σ[w(Fo2)2]1/2. |
| Formula |
C40H64Cu4Mo8N24O26 |
C20H34Cu4Mo8N12O27 |
C40H64Cu2Mo8N24O26 |
|
M
|
2318.88 |
1896.31 |
2191.78 |
| Crystal system |
Triclinic |
Monoclinic |
Triclinic |
| Space group |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
|
C2/m |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
|
|
a/Å |
10.850(1) |
13.295(1) |
12.481(3) |
|
b/Å |
12.843(1) |
20.188(1) |
12.862(4) |
|
c/Å |
14.740(1) |
9.613(1) |
13.332(4) |
|
α/° |
112.112(5) |
90 |
100.001(2) |
|
β/° |
100.830(4) |
120.191(6) |
105.253(2) |
|
γ/° |
98.902(4) |
90 |
111.736(3) |
|
V/Å3 |
1810.7(3) |
2230.1(15) |
1828.1(9) |
|
Z
|
1 |
2 |
1 |
|
D
calcd/g cm−3 |
2.127 |
2.821 |
1.991 |
|
T/K |
293(2) |
293(2) |
293(2) |
|
μ/mm−1 |
2.570 |
4.133 |
1.979 |
| Refl. measured |
12072 |
4393 |
12658 |
| Refl. unique |
6924 |
2202 |
7005 |
|
R
int
|
0.0620 |
0.0279 |
0.0296 |
| GoF on F2 |
1.028 |
1.024 |
0.961 |
|
R
1/wR2 [I≥2σ(I)] |
0.0596/0.0774 |
0.0320/0.0661 |
0.0326/0.0660 |
|
R
1/wR2 (all data) |
0.1646/0.0851 |
0.0542/0.0681 |
0.0533/0.0678 |
Results and discussion
Structure description
Bond valence sum calculations14 for 1–3 show that all Mo atoms are in the +VI oxidation state and Cu atoms are in the +I oxidation state in 1 and 2 and the +II oxidation state in 3. Additionally, the oxidation state of the Cu atoms in compounds 1–3 is further confirmed by their coordination environments and crystal color. The results are consistent with the formulae of 1–3 given by X-ray structure determination.
[CuI4(btx)4][β-Mo8O26] (1)
Single-crystal X-ray diffraction analysis reveals that 1 crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (No. 2) and consists of one β-[Mo8O26]4− (shortened to β-Mo8) anion, four CuI ions, and four btx ligands as shown in Fig. 1. The β-Mo8 anion has an inversion center (0.5, 0.5, 0.5), by which half of the anion are generated. And two of the three btx ligands also lie about other inversion centres [(0.5, 0, 0) and (0.5, −0.5, 0)], through which two whole btx are obtained. The β-Mo8 anion exhibits the most compact structure of eight edge-sharing [MoO6] octahedra with two [Mo4O13] subunits stacking together. Thus, β-Mo8 contains fourteen terminal, six doubly bridging, four triply bridging, and two five-fold bridging oxo groups.15 The bond lengths and angles are in the normal ranges.16 The CuI(1) ion exhibits the [CuN2O2] square configuration, in which two N atoms come from the triazole rings of two btx groups and two O atoms from two β-Mo8 anions. The bond lengths around the CuI(1) are 1.855(9) and 1.880(9) Å for Cu–N bonds and 2.629(7) and 2.782(7) Å for Cu–O bonds. CuI(2) ion displays [CuN2O] T-shaped configuration which is coordinated by two nitrogen atoms from the triazole ring of two btx groups and one oxygen atom from a β-Mo8 anion. The bond lengths around the CuI(2) ions are 1.807(11) and 1.888(9) Å for Cu–N bonds and 2.512(9) Å for Cu–O bonds. Notably, all the three crystallographically unique btx ligands (btx1, btx2 and btx3) served as bidentate ligands linked to each other by bridging CuI ions to generate an extraordinary –Cu1–btx1–Cu1–btx2–Cu2–btx3–Cu2–btx2– meso-helix chain (Fig. 2a and Fig. S1†). To the best of our knowledge, such a meso-helix structure has rarely been reported, especially in POM systems.17 Further, the meso-helix chains with different orientation are connected by hexadentate β-Mo8 anions in a complicated 3D structure (Fig. 1b and Fig. S2†). Therefore, 1 is a meritorious example for the further investigation and synthesis of new meso-helix compounds based on POMs.
(No. 2) and consists of one β-[Mo8O26]4− (shortened to β-Mo8) anion, four CuI ions, and four btx ligands as shown in Fig. 1. The β-Mo8 anion has an inversion center (0.5, 0.5, 0.5), by which half of the anion are generated. And two of the three btx ligands also lie about other inversion centres [(0.5, 0, 0) and (0.5, −0.5, 0)], through which two whole btx are obtained. The β-Mo8 anion exhibits the most compact structure of eight edge-sharing [MoO6] octahedra with two [Mo4O13] subunits stacking together. Thus, β-Mo8 contains fourteen terminal, six doubly bridging, four triply bridging, and two five-fold bridging oxo groups.15 The bond lengths and angles are in the normal ranges.16 The CuI(1) ion exhibits the [CuN2O2] square configuration, in which two N atoms come from the triazole rings of two btx groups and two O atoms from two β-Mo8 anions. The bond lengths around the CuI(1) are 1.855(9) and 1.880(9) Å for Cu–N bonds and 2.629(7) and 2.782(7) Å for Cu–O bonds. CuI(2) ion displays [CuN2O] T-shaped configuration which is coordinated by two nitrogen atoms from the triazole ring of two btx groups and one oxygen atom from a β-Mo8 anion. The bond lengths around the CuI(2) ions are 1.807(11) and 1.888(9) Å for Cu–N bonds and 2.512(9) Å for Cu–O bonds. Notably, all the three crystallographically unique btx ligands (btx1, btx2 and btx3) served as bidentate ligands linked to each other by bridging CuI ions to generate an extraordinary –Cu1–btx1–Cu1–btx2–Cu2–btx3–Cu2–btx2– meso-helix chain (Fig. 2a and Fig. S1†). To the best of our knowledge, such a meso-helix structure has rarely been reported, especially in POM systems.17 Further, the meso-helix chains with different orientation are connected by hexadentate β-Mo8 anions in a complicated 3D structure (Fig. 1b and Fig. S2†). Therefore, 1 is a meritorious example for the further investigation and synthesis of new meso-helix compounds based on POMs.
 |
| | Fig. 1 ORTEP drawing of 1 at the 50% probability level, showing the coordination environment of the CuI centers in 1. Symmetry codes: A: x, y, 1 + z; B: 2 − x, 1 − y, −z; C: 1 − x, 1 − y, −z; D: −1 + x, y, 1 + z; E: 1 − x, 1 − y, 1 − z. | |
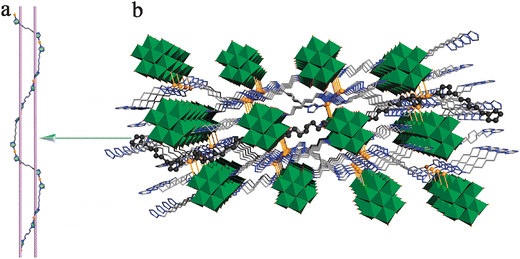 |
| | Fig. 2 (a) View of the 1D meso-helix chain in 1. (b) Illustration of the 3D framework of 1. | |
[CuI4(btx)2][β-Mo8O26]·H2O (2)
Compound 2 crystallizes in the monoclinic space groupC2/m (No. 12). The asymmetric unit in 2 consists of one β-Mo8 anion, four CuI ions, two btx ligands, and one water molecule as shown in Fig. 3. The β-Mo8 anion lies about a site with 2/m symmetry centered at (0.5, 0, 0.5), with the Mo1 and Mo3 atoms on mirror planes and with Mo3 in a general position. The two crystallographically unique CuI ions (CuI(1) and CuI(2)) both lie at sites with imposed two-fold symmetry. The O1W atom lies at another site with 2/m symmetry. CuI(1) and CuI(2) ions are both tetra-coordinated in a “seesaw” geometry by two N atoms from two btx ligands and two O atoms from one β-Mo8 anion for CuI(1) ion and two different β-Mo8 anions for CuI(2) ion. The bond lengths around the CuI(1) ion are 1.949(5) Å for Cu–N bonds and 2.156(5) Å for Cu–O bonds. The bond lengths around the CuI(2) are 1.907(5) Å for Cu–N bonds and 2.443(5) Å for Cu–O bonds. Each CuI ion connects two btx ligands and each btx ligand served as a tetradentate ligand bridging four CuI ions to generate a 2D grid sheet (Fig. 4a). It should be noted that the “grid” in the sheet is a 32-membered macrocycle with the edge distances at ca. 11.9 and 9.6 Å constructed from two CuI(1) ions, two CuI(2) ions, two btx ligands, and two triazoles of other btx ligands. Finally, four neighboring 2D sheets are linked by the hexadentate β-Mo8 anions via coordinating to CuI ions to achieve a 3D framework (Fig. 4b and Fig. S3†).
 |
| | Fig. 3 ORTEP drawing of 2 at the 50% probability level, showing the coordination environment of the CuI centers. Symmetry codes: A: 1 − x, −y, 1 − z; B: 0.5 + x, 0.5 + y, 1 + z; C: 0.5 + x, 0.5 + y, z; D: 0.5 − x, −0.5 − y, −z; E: 0.5 − x, −0.5 − y, 1 − z; F: 0.5 + x, −0.5 − y, 1 + z; G: 1 − x, y, 1 − z; H: x, y, 1 − z. | |
![(a) View and schematic view of the 2D sheet in 2. The inset shows the enlarged view of a 32-membered macrocycle. (b) View of the 3D structure of 2 constructed from 2D sheets and β-[Mo8O26]4−clusters.](/image/article/2011/NJ/c0nj00542h/c0nj00542h-f4.gif) |
| | Fig. 4 (a) View and schematic view of the 2D sheet in 2. The inset shows the enlarged view of a 32-membered macrocycle. (b) View of the 3D structure of 2 constructed from 2D sheets and β-[Mo8O26]4−clusters. | |
[CuII2(btx)4][α-Mo8O26] (3)
Compound 3 crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (No. 2) and is constructed from one α-[Mo8O26]4− (shortened to α-Mo8) anion, two CuII ions, and four btx ligands as shown in Fig. 5. The α-Mo8 anion lies about an inversion centre (1.5, 0.5, 0.5), by which half of the anions are generated. The two independent Cu atoms lie on other independent inversion centres [Cu1 (1.5, 0.5, 1) and Cu2 (2, 0, 0.5)], and two of the three btx ligands also lie about other inversion centres [(1, 0.5, 0.5) and (2, −0.5, 1)], through which two whole btx are obtained. The α-Mo8 anion consists of an equatorial ring of six edge-sharing [MoO6] octahedra and two {MoO4} tetrahedra capped on the poles. Thus, α-Mo8 contains fourteen terminal, six doubly bridging, and six triply bridging oxo groups.18 The bond lengths and angles are in the normal ranges.15 The coordination sphere of both CuII(1) and CuII(2) ions display elongated octahedral geometries achieved by four nitrogen atoms from four btx molecules and two terminal oxygen atoms from two α-Mo8 anions. The bond lengths around the CuII(1) ion are 2.004(3) and 2.015(4) Å for Cu–N bonds and 2.407(3) Å for Cu–O bonds. The bond lengths around the CuII(2) ion are 2.010(4) and 2.036(3) Å for Cu–N bonds and 2.434(3) Å for Cu–O bonds. As shown in Fig. 6a and 6b, the CuII centers are linked together by bidentate btx ligands to yield a 3D (4,4)-connected framework with (65.8)(64.82) topology, taking CuII ions as four-connecting nodes. This framework is highly open, into which the α-Mo8 anions acting as tetradentate ligands are incorporated (Fig. 6c). From the topological view, if each CuII cation is considered as a six-connected node, the α-Mo8 anion acts as a four-connected node, and btx ligands are considered as linkages, the structure of 3 is a novel self-penetrating (4,6)-connected framework with (42.64)(44.610.8)(42.610.83) topology as shown in Fig. 6d and Fig. S4†.
(No. 2) and is constructed from one α-[Mo8O26]4− (shortened to α-Mo8) anion, two CuII ions, and four btx ligands as shown in Fig. 5. The α-Mo8 anion lies about an inversion centre (1.5, 0.5, 0.5), by which half of the anions are generated. The two independent Cu atoms lie on other independent inversion centres [Cu1 (1.5, 0.5, 1) and Cu2 (2, 0, 0.5)], and two of the three btx ligands also lie about other inversion centres [(1, 0.5, 0.5) and (2, −0.5, 1)], through which two whole btx are obtained. The α-Mo8 anion consists of an equatorial ring of six edge-sharing [MoO6] octahedra and two {MoO4} tetrahedra capped on the poles. Thus, α-Mo8 contains fourteen terminal, six doubly bridging, and six triply bridging oxo groups.18 The bond lengths and angles are in the normal ranges.15 The coordination sphere of both CuII(1) and CuII(2) ions display elongated octahedral geometries achieved by four nitrogen atoms from four btx molecules and two terminal oxygen atoms from two α-Mo8 anions. The bond lengths around the CuII(1) ion are 2.004(3) and 2.015(4) Å for Cu–N bonds and 2.407(3) Å for Cu–O bonds. The bond lengths around the CuII(2) ion are 2.010(4) and 2.036(3) Å for Cu–N bonds and 2.434(3) Å for Cu–O bonds. As shown in Fig. 6a and 6b, the CuII centers are linked together by bidentate btx ligands to yield a 3D (4,4)-connected framework with (65.8)(64.82) topology, taking CuII ions as four-connecting nodes. This framework is highly open, into which the α-Mo8 anions acting as tetradentate ligands are incorporated (Fig. 6c). From the topological view, if each CuII cation is considered as a six-connected node, the α-Mo8 anion acts as a four-connected node, and btx ligands are considered as linkages, the structure of 3 is a novel self-penetrating (4,6)-connected framework with (42.64)(44.610.8)(42.610.83) topology as shown in Fig. 6d and Fig. S4†.
 |
| | Fig. 5 ORTEP drawing of 3 at the 50% probability level, showing the coordination environment of the CuII centers. Symmetry codes: A: x, y, −1 + z; B: x, 1 + y, −1 + z; C: −1 + x, y, −1 + z; D: 3 − x, 1–y, 2 − z; E: 4 − x, −y, 3 − z; F: 4 − x, 1 − y, 2 − z; G: x, −1 + y, 1 + z. | |
 |
| | Fig. 6 (a) View of 3D (4,4)-connected copper(II)-btx network. (b) Schematic view of the (4,4)-net. (c) Illustration of the 3D framework of 3. (d) Schematic view of the unique 3D self-penetrating (4,6)-connected net. | |
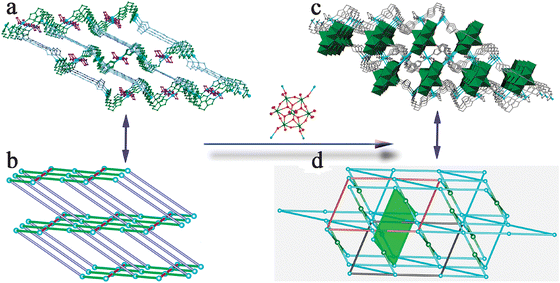
Syntheses of the compounds
As demonstrated in the Experimental Section, compounds 1–3 were obtained under the same conditions, except for using different amount of the reducing agent Et3N. As a result, the oxidation states and coordination geometries (+1; T-shaped and square configuration in 1, +1; “seesaw” geometries in 2, and +2; octahedral geometries in 3), metal–organic motifs (1D meso-helix chain in 1, 2D grid sheet in 2, and 3D (4,4)-connected framework in 3), octamolybdates (β-isomer in 1 and 2 and α-isomer in 3), and the final structures are all different (Scheme 1). We tentatively deduce the process as follows. First of all, Et3N (0.2 mL, 1.386 mol in 1 and 0.1 mL, 0.693 mol in 2) reduces the CuII cations (0.4 mol) to CuI cations. While in 3, Cu cations show the valence of +2 without Et3N. If we assume the stoichiometric ratio of Et3N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CuII cations in the reducing reaction is 1
CuII cations in the reducing reaction is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the amount of Et3N is in excess largely. Especially for 1, the amount of residual Et3N (0.986 mol) already exceeds the amount of ligand btx (0.5 mol). According to previous research, the coordination ability of the nitrogen atom from the organic ligand is stronger than of the polyanion and water molecules.19 So each copper cation with versatile oxidation states in compounds 1–3 is first coordinated by nitrogen atoms. In the synthesis system of 1, due to largely residual Et3N, the weak interaction between Et3N and CuI cations baffles btx ligands to covalently graft CuI cations, which result in btx ligands using part of their N atoms to combine with CuI ion and only forming a 1D meso-helix chain. In the synthesis system of 2, because the amount residual Et3N is low, btx ligands use all their N atoms to interact with CuI cations constructing a 2D grid sheet. CuII cations with high coordination number in 3 benefits the structural extension, therefore giving the 3D (4,4)-connected framework.
1, the amount of Et3N is in excess largely. Especially for 1, the amount of residual Et3N (0.986 mol) already exceeds the amount of ligand btx (0.5 mol). According to previous research, the coordination ability of the nitrogen atom from the organic ligand is stronger than of the polyanion and water molecules.19 So each copper cation with versatile oxidation states in compounds 1–3 is first coordinated by nitrogen atoms. In the synthesis system of 1, due to largely residual Et3N, the weak interaction between Et3N and CuI cations baffles btx ligands to covalently graft CuI cations, which result in btx ligands using part of their N atoms to combine with CuI ion and only forming a 1D meso-helix chain. In the synthesis system of 2, because the amount residual Et3N is low, btx ligands use all their N atoms to interact with CuI cations constructing a 2D grid sheet. CuII cations with high coordination number in 3 benefits the structural extension, therefore giving the 3D (4,4)-connected framework.
 |
| | Scheme 1 Experimental routes for compounds 1–3. | |
On the other hand, imposed by the steric constraint of different metal–organic motifs, the flexible [MoO4]2− ions form two kinds of octamolybdate isomer (β- and α-Mo8) to conform to the stacking environments. Furthermore, the hydrothermal reaction conditions also provide ample energy for fluxional formation of octamolybdate isomers.
The XRPD patterns for 1–3 are presented in Fig. S5†. The diffraction peaks of both simulated and experimental patterns match well, indicating the phase purities of 1–3.
The IR spectra for 1–3 are presented in Fig. S6†. The bands at 1760–1060 cm−1 are attributed to the triazole-ring stretching vibrations (υ(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) + υ(N
N) + υ(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)), and 3180–2850 cm−1 are assigned to the –CH2– stretching vibrations in btx molecules. The bands at 950–482 cm−1 are ascribed to the υ(Mo=O) and υ(Mo–O–Mo) vibrations. In contrast to the band at 707 cm−1 in 1 and 717 cm−1 in 2 for the β-Mo8 isomer, the band at 797 cm−1 in 3 is obviously different, which displays absorption characteristic of the α-Mo8 isomer.20
N)), and 3180–2850 cm−1 are assigned to the –CH2– stretching vibrations in btx molecules. The bands at 950–482 cm−1 are ascribed to the υ(Mo=O) and υ(Mo–O–Mo) vibrations. In contrast to the band at 707 cm−1 in 1 and 717 cm−1 in 2 for the β-Mo8 isomer, the band at 797 cm−1 in 3 is obviously different, which displays absorption characteristic of the α-Mo8 isomer.20
The TG analyses of 1–3 were performed under N2 at a rate of 10 °C min−1 in the range of 45–1000 °C (Fig. S7†). For anhydrous compounds 1 and 3, the weight loss of 38.30% (calc. 38.00%) for 1 and 40.12% (calcd 40.20%) for 3 from 255 to 825 °C corresponds to the loss of btx molecules. The TG curve of compound 2 shows two weight loss steps: the first weight loss step below 220 °C corresponds to the loss of free water molecules, 1.04% (calc. 0.95%) and the second weight loss step in the range of 270–900 °C ascribes to the loss of btx molecules, 23.28% (calcd 23.23%). The TG analysis results of 1–3 support their chemical compositions.
Given a detailed comparison of the thermal behaviors for compounds 1–3, it can be found that the decomposition of btx molecules have a similar pattern, namely, a sharp weight loss followed by a slow one. The weight loss of the sharp part 14.44% in 1, 8.53% in 2, and 15.81% in 3 at about 310 °C is approximately consistent with the release of the methylene –(CH2)6– skeleton (calcd 14.56% in 1, 8.88% in 2, and 15.53% in 3). To further confirm the thermal decomposition process, 2 as an example was heated at 320 °C for 1 h and then was examined using IR. The experimental weight loss matches well with the calculated mass of methylene –(CH2)6– skeleton and the –CH2– stretching vibrations between 3180 and 2830 cm−1 in the IR spectrum disappear (Fig. S8†). So we deduce that the thermal decomposition of ligand btx undergoes two processes, before 310 °C the bond (C6H12)C–N(C2N3H2) broke and the component –(CH2)6– was released and then 1,2,4-triazole ring linking on Cu ion suffered a complicated and slow transformation process.
Luminescent properties.
Recently, inorganic–organic hybrid coordination polymers, especially comprising the d10 metal center and an aromatic-containing system, have been intensively investigated for attractive fluorescence properties and potential applications as new luminescent materials.21 In this work, photoluminescence properties of compounds 1–3 were investigated in the solid state at room temperature. Upon excitation at 200 nm, 1–3 display strong emission with a shoulder band at 359 and 462(sh) nm for 1, 368 and 460(sh) nm for 2, and 373 and 458(sh) nm for 3 (Fig. 7). In order to understand the nature of their luminescence, the emission spectrum of free btx ligand was also recorded under identical experimental conditions. The free btx ligand shows the emission band at 421 nm when excited at 378 nm (Fig. S9†). In comparison with the free btx, the origin of the emission for 1–3 can be tentatively attributable to a joint contribution of ligand-to-metal charge transfer (LMCT) (359–373 nm)22 and intraligand π*→π transitions of the neutral ligand (ca. 460 nm).23 It is, moreover, noteworthy that the strong emission at 359–373 nm is red-shifted from 1 to 3. The results may be that the compound with the high-dimensional metal–organic motif can offer more advantage of energy transfer.24 Additionally, the shoulder bands in 1–3 are also red-shifted compared with the emission spectrum of free btx ligand, which may be due to the coordination of btx ligands to metal ions decreasing the π* → π transition energy.
 |
| | Fig. 7
Emission spectra of compounds 1–3 in the solid state at room temperature. | |
Conclusion
In summary, under hydrothermally reaction conditions, three 3D octamolybdate-based compounds with 1D–3D CuI/CuII-bis(triazole) motifs have been isolated. The structural differences show that the versatile oxidation states and coordination geometries of copper ions controlled by the amount of Et3N have great influences on the metal–organic motifs and final spacial structure. The exploration of such compounds might provide an interesting model for the preparation of new high-dimensional organic–inorganic hybrids with desirable properties.
Acknowledgements
This work was supported by the analysis and testing foundation of Northeast Normal University.
References
-
(a)
M. T. Pope, Heteropoly and Isopoly Oxometalates, Springer-verlag, New York, 1983 Search PubMed;
(b)
Polyoxometalate Chemistry from Topology via Self-Assembly to Applications, ed. M. T. Pope and A. Müller, Kluwer Academic Publishers, Dordrecht, 2001 Search PubMed;
(c)
M. T. Pope and A. Müller, Polyoxometalate Chemistry: From Topology via Self-Assembly to Applications, Kluwer, Dordrecht, The Netherlands, 2001 Search PubMed;
(d)
J. J. Borrys-Almener, E. Coronado, A. Müller and M. T. Pope, Polyoxometalate Molecular Science, Kluwer, Dordrecht, The Netherlands, 2003 Search PubMed;
(e) A. Müller and P. Kögerler, Coord. Chem. Rev., 2000, 199, 335 CrossRef CAS;
(f) D. L. Long, E. Burkholder and L. Cronin, Chem. Soc. Rev., 2007, 36, 105 RSC;
(g) D.-L. Long, R. Tsunashima and L. Cronin, Angew. Chem., Int. Ed., 2010, 49, 1736 CrossRef CAS.
-
(a) V. Artero, A. Proust, P. Herson, F. Villain, C. Moulin and P. Gouzerh, J. Am. Chem. Soc., 2003, 125, 11156 CrossRef CAS;
(b) C. Besson, Z. Q. Huang, Y. V. Geletii, S. Lense, K. I. Hardcastle, D. G. Musaev, T. Q. Lian, A. Proustac and C. L. Hill, Chem. Commun., 2010, 46, 2784 RSC;
(c) K. Kamata, Y. Nakagawa, K. Yamaguchi and N. Mizuno, J. Am. Chem. Soc., 2008, 130, 15304 CrossRef CAS;
(d) D. B. Dang, Y. Bai, C. He, J. Wang, C. Y. Duan and J. Y. Niu, Inorg. Chem., 2010, 49, 1280 CrossRef CAS;
(e) Y. H. Guo and C. W. Hu, J. Mol. Catal. A: Chem., 2007, 262, 136 CrossRef CAS;
(f) C. Y. Sun, S. X. Liu, D. D. Liang, K. Z. Shao, Y. H. Ren and Z. M. Su, J. Am. Chem. Soc., 2009, 131, 1883 CrossRef CAS;
(g) R. D. Gall, C. L. Hill and J. E. Walker, Chem. Mater., 1996, 8, 2523 CrossRef CAS.
-
(a) Special issue on polyoxometalates, C. L. Hill (guest editor), Chem. Rev., 1998, 98, 1;
(b) E. D. Clercq, Rev. Med. Virol., 2000, 10, 255 CrossRef.
-
(a) J. T. Rhule, C. L. Hill and D. A. Judd, Chem. Rev., 1998, 98, 327 CrossRef CAS;
(b) X. Wang, J. Liu and M. Pope, Dalton Trans., 2003, 957 RSC.
-
(a) A. Proust, R. Thouvenot and P. Gouzerh, Chem. Commun., 2008, 1837 RSC;
(b) J. Zhang, Y. F. Song, L. Cronin and T. B. Liu, J. Am. Chem. Soc., 2008, 130, 14408 CrossRef CAS;
(c) J. Zhang, J. Hao, Y. G. Wei, F. P. Xiao, P. H. Yin and L. S. Wang, J. Am. Chem. Soc., 2010, 132, 14 CrossRef CAS;
(d) P. Mialane, A. Dolbecq and F. Sécheresse, Chem. Commun., 2006, 3477 RSC;
(e) B. S. Bassil, S. S. Mal, M. H. Dickman, U. Kortz, H. Oelrich and L. Walder, J. Am. Chem. Soc., 2008, 130, 6696 CrossRef CAS;
(f) X. Fang, P. Kögerler, L. Isaacs, S. Uchida and N. Mizuno, J. Am. Chem. Soc., 2009, 131, 432 CrossRef;
(g) J.-W. Zhao, H.-P. Jia, J. Zhang, S.-T. Zheng and G.-Y. Yang, Chem.–Eur. J., 2007, 13, 10030 CrossRef CAS;
(h) J. Y. Niu, P. T. Ma, H. Y. Niu, J. Li, J. W. Zhao, Y. Song and J. P. Wang, Chem.–Eur. J., 2007, 13, 8739 CrossRef CAS.
-
(a) M. J. Schwing-Weill and F. Arnaud-Neu, Bull. Soc. Chim. Fr., 1970, 853 CAS;
(b) A. J. Bridgeman, J. Phys. Chem. A, 2002, 106, 12151 CrossRef CAS.
-
(a) P. J. Hagrman, D. Hagrman and J. Zubieta, Angew. Chem., Int. Ed., 1999, 38, 2638 CrossRef;
(b) D. Hagrman, C. Zubieta, D. J. Rose, J. Zubieta and R. C. Haushalter, Angew. Chem., Int. Ed. Engl., 1997, 36, 873 CrossRef CAS;
(c) D. Hagrman, C. Sangregorio, C. J. O'Connor and J. Zubieta, J. Chem. Soc., Dalton Trans., 1998, 3707 RSC;
(d) D. G. Allis, E. Burkholder and J. Zubieta, Polyhedron, 2004, 23, 1145 CrossRef CAS;
(e) D. G. Allis, R. G. Rarig Jr., E. Burkholder and J. Zubieta, J. Mol. Struct., 2004, 688, 11 CrossRef CAS;
(f) D. Hagrman, P. J. Zapf and J. Zubieta, Chem. Commun., 1998, 1283 RSC;
(g) R. S. Rarig Jr. and J. Zubieta, Inorg. Chim. Acta, 2001, 312, 188 CrossRef CAS;
(h) R. S. Rarig Jr. and J. Zubieta, Polyhedron, 2003, 22, 177 CrossRef;
(i) R. S. Rarig Jr. and J. Zubieta, J. Chem. Soc., Dalton Trans., 2001, 3446 RSC;
(j) P. J. Hagrman and J. Zubieta, Inorg. Chem., 1999, 38, 4480 CrossRef CAS;
(k) D. Hagrman, P. Hagrman and J. Zubieta, Inorg. Chim. Acta, 2000, 300–302, 212 CrossRef.
-
(a) A.-X. Tian, J. Ying, J. Peng, J.-Q. Sha, Z.-G. Han, J.-F. Ma, Z.-M. Su, N.-H. Hu and H.-Q. Jia, Inorg. Chem., 2008, 47, 3274 CrossRef CAS;
(b) A.-X. Tian, J. Ying, J. Peng, J.-Q. Sha, H.-J. Pang, P.-P. Zhang, Y. Chen, M. Zhu and Z.-M. Su, Cryst. Growth Des., 2008, 8, 3717 CrossRef CAS.
-
(a) C. D. Wu, C. Z. Lu, H. H. Zhuang and J. S. Huang, Inorg. Chem., 2002, 41, 5636 CrossRef CAS;
(b) C. M. Liu, D. Q. Zhang and D. B. Zhu, Cryst. Growth Des., 2005, 5, 1639 CrossRef CAS;
(c) Y. P. Ren, X. J. Kong, X. Y. Hu, M. Sun, L. S. Long, R. B. Huang and L. S. Zheng, Inorg. Chem., 2006, 45, 4016 CrossRef CAS;
(d) K. Pavani, S. E. Lofland, K. V. Ramanujachary and A. Ramanan, Eur. J. Inorg. Chem., 2007, 568 CrossRef CAS.
- X.-C. Huang, J.-P. Zhang, Y.-Y. Lin and X.-M. Chen, Chem. Commun., 2004, 1100 RSC.
- Y.-Q. Lan, S.-L. Li, X.-L. Wang, K.-Z. Shao, D.-Y. Du, H.-Y. Zang and Z.-M. Su, Inorg. Chem., 2008, 47, 8179 CrossRef CAS.
-
(a) U. Beckmann and S. Brooker, Coord. Chem. Rev., 2003, 245, 17 CrossRef CAS;
(b) J. G. Haasnoot, Coord. Chem. Rev., 2000, 200–202, 131 CrossRef CAS;
(c) X.-L. Wang, C. Qin, E.-B. Wang, Z.-M. Su, Y.-G. Li and L. Xu, Angew. Chem., Int. Ed., 2006, 45, 7411 CrossRef CAS;
(d) X.-L. Wang, C. Qin, E.-B. Wang and Z.-M. Su, Chem. Commun., 2007, 4245 RSC;
(e) X. F. Kuang, X. Y. Wu, R. M. Yu, J. P. Donahue, J. S. Huang and C.-Z. Lu, Nat. Chem., 2010, 2, 461 Search PubMed.
-
(a)
G. M. Sheldrick, SHELX97, Program for Crystal Structure Refinement, University of Göttingen, Göttingen, Germany, 1997 Search PubMed;
(b)
G. M. Sheldrick, SHELXL97, Program for Crystal Structure Solution, University of Göttingen, Göttingen, Germany, 1997 Search PubMed.
- I. D. Brown and D. Altermatt, Acta Crystallogr., Sect. B: Struct. Sci., 1985, 41, 244 CrossRef.
- S. M. Chen, C. Z. Lu, Y. Q. Yu, Q. Z. Zhang and X. He, Inorg. Chem. Commun., 2004, 7, 1041 CrossRef CAS.
- J. Fielden, D.-L. Long, L. Cronin and P. Kögerler, Polyhedron, 2009, 28, 2803 CrossRef CAS.
-
(a) G. G. Gao, L. Xu, X. S. Qu, H. Liu and Y. Y. Yang, Inorg. Chem., 2008, 47, 3402 CrossRef CAS;
(b) C. J. Zhang, H. J. Pang, M. X Hu, J. Li and Y. G. Chen, J. Solid State Chem., 2009, 182, 1772 CrossRef CAS.
-
(a) C. Y. Sun, E. B. Wang, D. R. Xiao, H. Y. An and L. Xu, J. Mol. Struct., 2005, 741, 149 CrossRef CAS;
(b) W. B. Yang, C. Z Lu and H. H. Zhuang, J. Chem. Soc., Dalton Trans., 2002, 2879 RSC.
-
(a) S. L. Li, Y. Q. Lan, J. F. Ma, J. Yang, X. H. Wang and Z. M. Su, Inorg. Chem., 2007, 46, 8283 CrossRef CAS;
(b) A. Ramanan and M. S. Whittingham, Cryst. Growth Des., 2006, 6, 2419 CrossRef CAS.
- W. G. Klemperer and W. Shum, J. Am. Chem. Soc., 1976, 98, 8291 CrossRef CAS.
-
(a) C. Seward, W.-L. Jia, R.-Y. Wang, G. D. Enright and S. Wang, Angew. Chem., Int. Ed., 2004, 43, 2933 CrossRef CAS;
(b) S. V. Ganesan and S. Natarajan, Inorg. Chem., 2004, 43, 198 CrossRef CAS.
- L. J. Chen, X. He, C. K. Xia, Q. Z. Zhang, J. T. Chen, W. B. Yang and C. Z. Lu, Cryst. Growth Des., 2006, 6, 2076 CrossRef CAS.
- L.-Y. Zhang, G.-F. Liu, S.-L. Zheng, B.-H. Ye, X.-M. Zhang and X.-M. Chen, Eur. J. Inorg. Chem., 2003, 2965 CrossRef CAS.
- R.-B. Zhang, Z.-J. Li, J.-K. Cheng, Y.-Y. Qin, J. Zhang and Y.-G. Yao, Cryst. Growth Des., 2008, 8, 2562 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2011 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (No. 2) and consists of one β-[Mo8O26]4− (shortened to β-Mo8) anion, four CuI ions, and four btx ligands as shown in Fig. 1. The β-Mo8 anion has an inversion center (0.5, 0.5, 0.5), by which half of the anion are generated. And two of the three btx ligands also lie about other inversion centres [(0.5, 0, 0) and (0.5, −0.5, 0)], through which two whole btx are obtained. The β-Mo8 anion exhibits the most compact structure of eight edge-sharing [MoO6] octahedra with two [Mo4O13] subunits stacking together. Thus, β-Mo8 contains fourteen terminal, six doubly bridging, four triply bridging, and two five-fold bridging oxo groups.15 The bond lengths and angles are in the normal ranges.16 The CuI(1) ion exhibits the [CuN2O2] square configuration, in which two N atoms come from the triazole rings of two btx groups and two O atoms from two β-Mo8 anions. The bond lengths around the CuI(1) are 1.855(9) and 1.880(9) Å for Cu–N bonds and 2.629(7) and 2.782(7) Å for Cu–O bonds. CuI(2) ion displays [CuN2O] T-shaped configuration which is coordinated by two nitrogen atoms from the triazole ring of two btx groups and one oxygen atom from a β-Mo8 anion. The bond lengths around the CuI(2) ions are 1.807(11) and 1.888(9) Å for Cu–N bonds and 2.512(9) Å for Cu–O bonds. Notably, all the three crystallographically unique btx ligands (btx1, btx2 and btx3) served as bidentate ligands linked to each other by bridging CuI ions to generate an extraordinary –Cu1–btx1–Cu1–btx2–Cu2–btx3–Cu2–btx2– meso-helix chain (Fig. 2a and Fig. S1†). To the best of our knowledge, such a meso-helix structure has rarely been reported, especially in POM systems.17 Further, the meso-helix chains with different orientation are connected by hexadentate β-Mo8 anions in a complicated 3D structure (Fig. 1b and Fig. S2†). Therefore, 1 is a meritorious example for the further investigation and synthesis of new meso-helix compounds based on POMs.
(No. 2) and consists of one β-[Mo8O26]4− (shortened to β-Mo8) anion, four CuI ions, and four btx ligands as shown in Fig. 1. The β-Mo8 anion has an inversion center (0.5, 0.5, 0.5), by which half of the anion are generated. And two of the three btx ligands also lie about other inversion centres [(0.5, 0, 0) and (0.5, −0.5, 0)], through which two whole btx are obtained. The β-Mo8 anion exhibits the most compact structure of eight edge-sharing [MoO6] octahedra with two [Mo4O13] subunits stacking together. Thus, β-Mo8 contains fourteen terminal, six doubly bridging, four triply bridging, and two five-fold bridging oxo groups.15 The bond lengths and angles are in the normal ranges.16 The CuI(1) ion exhibits the [CuN2O2] square configuration, in which two N atoms come from the triazole rings of two btx groups and two O atoms from two β-Mo8 anions. The bond lengths around the CuI(1) are 1.855(9) and 1.880(9) Å for Cu–N bonds and 2.629(7) and 2.782(7) Å for Cu–O bonds. CuI(2) ion displays [CuN2O] T-shaped configuration which is coordinated by two nitrogen atoms from the triazole ring of two btx groups and one oxygen atom from a β-Mo8 anion. The bond lengths around the CuI(2) ions are 1.807(11) and 1.888(9) Å for Cu–N bonds and 2.512(9) Å for Cu–O bonds. Notably, all the three crystallographically unique btx ligands (btx1, btx2 and btx3) served as bidentate ligands linked to each other by bridging CuI ions to generate an extraordinary –Cu1–btx1–Cu1–btx2–Cu2–btx3–Cu2–btx2– meso-helix chain (Fig. 2a and Fig. S1†). To the best of our knowledge, such a meso-helix structure has rarely been reported, especially in POM systems.17 Further, the meso-helix chains with different orientation are connected by hexadentate β-Mo8 anions in a complicated 3D structure (Fig. 1b and Fig. S2†). Therefore, 1 is a meritorious example for the further investigation and synthesis of new meso-helix compounds based on POMs.



![(a) View and schematic view of the 2D sheet in 2. The inset shows the enlarged view of a 32-membered macrocycle. (b) View of the 3D structure of 2 constructed from 2D sheets and β-[Mo8O26]4−clusters.](/image/article/2011/NJ/c0nj00542h/c0nj00542h-f4.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (No. 2) and is constructed from one α-[Mo8O26]4− (shortened to α-Mo8) anion, two CuII ions, and four btx ligands as shown in Fig. 5. The α-Mo8 anion lies about an inversion centre (1.5, 0.5, 0.5), by which half of the anions are generated. The two independent Cu atoms lie on other independent inversion centres [Cu1 (1.5, 0.5, 1) and Cu2 (2, 0, 0.5)], and two of the three btx ligands also lie about other inversion centres [(1, 0.5, 0.5) and (2, −0.5, 1)], through which two whole btx are obtained. The α-Mo8 anion consists of an equatorial ring of six edge-sharing [MoO6] octahedra and two {MoO4} tetrahedra capped on the poles. Thus, α-Mo8 contains fourteen terminal, six doubly bridging, and six triply bridging oxo groups.18 The bond lengths and angles are in the normal ranges.15 The coordination sphere of both CuII(1) and CuII(2) ions display elongated octahedral geometries achieved by four nitrogen atoms from four btx molecules and two terminal oxygen atoms from two α-Mo8 anions. The bond lengths around the CuII(1) ion are 2.004(3) and 2.015(4) Å for Cu–N bonds and 2.407(3) Å for Cu–O bonds. The bond lengths around the CuII(2) ion are 2.010(4) and 2.036(3) Å for Cu–N bonds and 2.434(3) Å for Cu–O bonds. As shown in Fig. 6a and 6b, the CuII centers are linked together by bidentate btx ligands to yield a 3D (4,4)-connected framework with (65.8)(64.82) topology, taking CuII ions as four-connecting nodes. This framework is highly open, into which the α-Mo8 anions acting as tetradentate ligands are incorporated (Fig. 6c). From the topological view, if each CuII cation is considered as a six-connected node, the α-Mo8 anion acts as a four-connected node, and btx ligands are considered as linkages, the structure of 3 is a novel self-penetrating (4,6)-connected framework with (42.64)(44.610.8)(42.610.83) topology as shown in Fig. 6d and Fig. S4†.
(No. 2) and is constructed from one α-[Mo8O26]4− (shortened to α-Mo8) anion, two CuII ions, and four btx ligands as shown in Fig. 5. The α-Mo8 anion lies about an inversion centre (1.5, 0.5, 0.5), by which half of the anions are generated. The two independent Cu atoms lie on other independent inversion centres [Cu1 (1.5, 0.5, 1) and Cu2 (2, 0, 0.5)], and two of the three btx ligands also lie about other inversion centres [(1, 0.5, 0.5) and (2, −0.5, 1)], through which two whole btx are obtained. The α-Mo8 anion consists of an equatorial ring of six edge-sharing [MoO6] octahedra and two {MoO4} tetrahedra capped on the poles. Thus, α-Mo8 contains fourteen terminal, six doubly bridging, and six triply bridging oxo groups.18 The bond lengths and angles are in the normal ranges.15 The coordination sphere of both CuII(1) and CuII(2) ions display elongated octahedral geometries achieved by four nitrogen atoms from four btx molecules and two terminal oxygen atoms from two α-Mo8 anions. The bond lengths around the CuII(1) ion are 2.004(3) and 2.015(4) Å for Cu–N bonds and 2.407(3) Å for Cu–O bonds. The bond lengths around the CuII(2) ion are 2.010(4) and 2.036(3) Å for Cu–N bonds and 2.434(3) Å for Cu–O bonds. As shown in Fig. 6a and 6b, the CuII centers are linked together by bidentate btx ligands to yield a 3D (4,4)-connected framework with (65.8)(64.82) topology, taking CuII ions as four-connecting nodes. This framework is highly open, into which the α-Mo8 anions acting as tetradentate ligands are incorporated (Fig. 6c). From the topological view, if each CuII cation is considered as a six-connected node, the α-Mo8 anion acts as a four-connected node, and btx ligands are considered as linkages, the structure of 3 is a novel self-penetrating (4,6)-connected framework with (42.64)(44.610.8)(42.610.83) topology as shown in Fig. 6d and Fig. S4†.


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CuII cations in the reducing reaction is 1
CuII cations in the reducing reaction is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the amount of Et3N is in excess largely. Especially for 1, the amount of residual Et3N (0.986 mol) already exceeds the amount of ligand btx (0.5 mol). According to previous research, the coordination ability of the nitrogen atom from the organic ligand is stronger than of the polyanion and water molecules.19 So each copper cation with versatile oxidation states in compounds 1–3 is first coordinated by nitrogen atoms. In the synthesis system of 1, due to largely residual Et3N, the weak interaction between Et3N and CuI cations baffles btx ligands to covalently graft CuI cations, which result in btx ligands using part of their N atoms to combine with CuI ion and only forming a 1D meso-helix chain. In the synthesis system of 2, because the amount residual Et3N is low, btx ligands use all their N atoms to interact with CuI cations constructing a 2D grid sheet. CuII cations with high coordination number in 3 benefits the structural extension, therefore giving the 3D (4,4)-connected framework.
1, the amount of Et3N is in excess largely. Especially for 1, the amount of residual Et3N (0.986 mol) already exceeds the amount of ligand btx (0.5 mol). According to previous research, the coordination ability of the nitrogen atom from the organic ligand is stronger than of the polyanion and water molecules.19 So each copper cation with versatile oxidation states in compounds 1–3 is first coordinated by nitrogen atoms. In the synthesis system of 1, due to largely residual Et3N, the weak interaction between Et3N and CuI cations baffles btx ligands to covalently graft CuI cations, which result in btx ligands using part of their N atoms to combine with CuI ion and only forming a 1D meso-helix chain. In the synthesis system of 2, because the amount residual Et3N is low, btx ligands use all their N atoms to interact with CuI cations constructing a 2D grid sheet. CuII cations with high coordination number in 3 benefits the structural extension, therefore giving the 3D (4,4)-connected framework.

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) + υ(N
N) + υ(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)), and 3180–2850 cm−1 are assigned to the –CH2– stretching vibrations in btx molecules. The bands at 950–482 cm−1 are ascribed to the υ(Mo=O) and υ(Mo–O–Mo) vibrations. In contrast to the band at 707 cm−1 in 1 and 717 cm−1 in 2 for the β-Mo8 isomer, the band at 797 cm−1 in 3 is obviously different, which displays absorption characteristic of the α-Mo8 isomer.20
N)), and 3180–2850 cm−1 are assigned to the –CH2– stretching vibrations in btx molecules. The bands at 950–482 cm−1 are ascribed to the υ(Mo=O) and υ(Mo–O–Mo) vibrations. In contrast to the band at 707 cm−1 in 1 and 717 cm−1 in 2 for the β-Mo8 isomer, the band at 797 cm−1 in 3 is obviously different, which displays absorption characteristic of the α-Mo8 isomer.20
