On the multiple B–N bonding in boron compounds using the topological analysis of electron localization function (ELF)
Slawomir
Berski
*a,
Zdzislaw
Latajka
a and
Agnieszka J.
Gordon
ab
aFaculty of Chemistry, University of Wroclaw, 14 F. Joliot-Curie, 54-210, Wroclaw, Poland. E-mail: sberski@wchuwr.pl; Fax: +48 (0)71 3282348; Tel: +48 (0)71 3757246
bSchool of Chemistry, Joseph Black Building, West Mains Road, Edinburgh, EH9 3JJ, Scotland, UK. Fax: +44 (0)131 650 6453; Tel: +44 (0)131 650 7546
First published on 8th October 2010
Abstract
Topological analysis of the Electron Localization Function (ELF) within the framework of Quantum Chemical Topology (QCT) has been applied to study the nature of the boron–nitrogen bonds. A series of 10 compounds have been chosen, with the B–N bond length ranging between 1.698 Å (B–N) and 1.258 Å (B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N). According to the Lewis formula three types of bonds have been recognized. These are: the single B–N bond with a basin population of 1.91 ÷ 2.09e, the double B
N). According to the Lewis formula three types of bonds have been recognized. These are: the single B–N bond with a basin population of 1.91 ÷ 2.09e, the double B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond with a population of 3.78 ÷ 4.28e, and the triple B
N bond with a population of 3.78 ÷ 4.28e, and the triple B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond with a basin population of 5.72 ÷ 5.74e. In the case of partial double bonds (B
N bond with a basin population of 5.72 ÷ 5.74e. In the case of partial double bonds (B![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) N), where formally two or more resonance hybrids have to be considered, our calculations strongly support the concept of double boron–nitrogen bonding (B
N), where formally two or more resonance hybrids have to be considered, our calculations strongly support the concept of double boron–nitrogen bonding (B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N).
N).
1. Introduction
After Quantum Chemical Topology† (QCT) method1 based on the topological analysis of the electron localization function (ELF)8,9 was introduced for modern bond analysis,10–12 the majority of chemists wanted to find a correlation between the mathematical definition of bonding through the ELF-localization basin associated with its attractor,9 and the classical Lewis representation.13 Although the Lewis formula13 was introduced before the quantum chemistry era, a representation of the multiple covalent bond (A–A, A![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) A, A
A, A![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) A) with shared electron pairs was confirmed through the concept of σ and π-orbitals in molecular orbital (MO) and valence-bond (VB) theories.14–16 It is worth mentioning that another QCT method, based on topological analysis of the electron density, AIM17–19 relates the multiple character of the chemical bond through the ellipticity parameter, calculated for the bond critical point.
A) with shared electron pairs was confirmed through the concept of σ and π-orbitals in molecular orbital (MO) and valence-bond (VB) theories.14–16 It is worth mentioning that another QCT method, based on topological analysis of the electron density, AIM17–19 relates the multiple character of the chemical bond through the ellipticity parameter, calculated for the bond critical point.
In general, there is no direct correspondence between the number of valence disynaptic attractors Vi(A,A) representing the multiple bond in topological analysis of ELF and the Lewis structure. For example, in molecules of cylindrical symmetry with a formal triple bond such as acetylene (HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH) or dinitrogen (N
CH) or dinitrogen (N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), only single bonding attractors (the ring V(C,C)9,11,20–22 and the point type V(N,N)9,11,20,23) are observed. Similarly, in formaldehyde (H2C
N), only single bonding attractors (the ring V(C,C)9,11,20–22 and the point type V(N,N)9,11,20,23) are observed. Similarly, in formaldehyde (H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), a molecule of planar symmetry, only a single V(C,O) attractor is found.11,24,25 On the contrary, in ethylene (H2C
O), a molecule of planar symmetry, only a single V(C,O) attractor is found.11,24,25 On the contrary, in ethylene (H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) two disynaptic attractors Vi=1,2(C,C) are localized.11,21 As pointed out by Silvi et al.,11 “the disynaptic basins multiplicity can be explained by symmetry considerations rather than by chemical arguments”.
CH2) two disynaptic attractors Vi=1,2(C,C) are localized.11,21 As pointed out by Silvi et al.,11 “the disynaptic basins multiplicity can be explained by symmetry considerations rather than by chemical arguments”.
The general rule of thumb, which applies to almost all circumstances, is that for a given pair of atoms longer bonds will be weaker, and shorter ones stronger. This can be explained by the fact that more electrons shared by two atoms leads to better attraction between the two nuclei. Since the number of electrons in the chemical bond can easily be obtained from topological analysis of ELF,21,26 it is interesting to study the correlation between the formal bond order or bond length, and the basin population (![[N with combining macron]](https://www.rsc.org/images/entities/i_char_004e_0304.gif) ).
).
Savin et al.21 showed for the first time that such a correlation exists for the ethylene, propene, and trans-butadiene molecules, where formal double C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds were characterised by larger basis populations (3.6 ÷ 3.7e) than the single C–C bonds (1.9 ÷ 2.2e). A comparative study on single, double and triple bonds for the first and second row hydrides, in context of the formal bond order and delocalization, has been undertaken by Chesnut.25 He observed that molecules with increasing bond order displayed higher basin populations.
C bonds were characterised by larger basis populations (3.6 ÷ 3.7e) than the single C–C bonds (1.9 ÷ 2.2e). A comparative study on single, double and triple bonds for the first and second row hydrides, in context of the formal bond order and delocalization, has been undertaken by Chesnut.25 He observed that molecules with increasing bond order displayed higher basin populations.
In a search for a correlation between formal multiple bonding (represented by Lewis structures) and its ELF-topological characterisation, we focus on the very interesting case of boron–nitrogen bonds, important in the area of nanotubes.27 The boron atom contains empty p-orbitals interacting with occupied p-orbitals of nitrogen, so π-bonding (the so called dative pπ–pπ bonding) is responsible for multiple boron–nitrogen bonds. A set of boron compounds (Fig. 1) with the bond length ranging from 1.258 to 1.698 Å has been chosen. As previously reported by Straub,28 they can be characterized as the B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N, B
N, B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, B
N, B![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) N and B–N bonds.
N and B–N bonds.
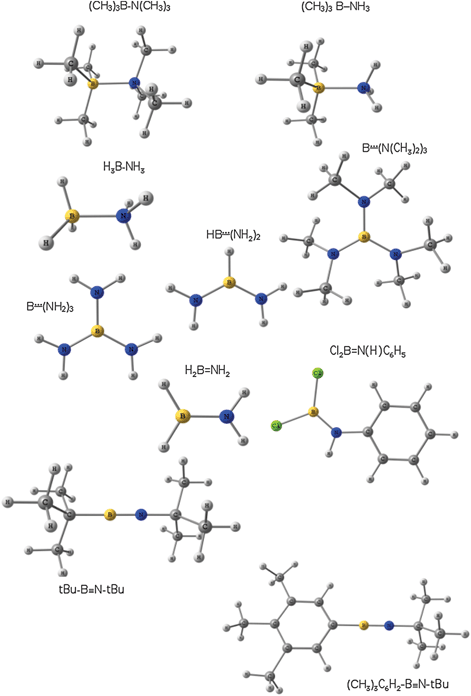 | ||
| Fig. 1 Optimized structures of the molecules with multiple boron–nitrogen bonds. | ||
In this paper we propose to answer the following questions:
1. Is it possible to confirm the concept of multiple boron–nitrogen bond on the grounds of QCT using topological analysis of the ELF function?
2. Can we observe the nitrogen lone pair participating in the donor–acceptor bonds (pπ–pπ bonding) with boronvia the non-bonding, monosynaptic basin V(N)?
3. Is there a correlation between the population of the V(B,N) basin representing the B–N bond and the bond length?
2. Computational details
Full optimization of geometrical structures with the Becke3LYP electron density functional29–31 has been performed using the Gaussian 03 program.32 The pure 5d and 7f atomic orbitals are used. The minima on the potential energy surface have been confirmed by non-imaginary vibrational frequencies. In the case of B(NH2)3 the optimization was carried out with the D3h symmetry, but it converged to a minimum on the PES only for the aug-cc-pVTZ33,34 basis set.Topological analysis of the ELF function has been carried out using the DGrid and DBasin-4.2 programs.35 Graphical representations of molecules have been constructed using the ChemCraft and 3D-representations of ELF and 2D-maps using the VMD36 program.
Comparative analysis of the basin populations has been performed using basis sets from 6-311++G(2d,2p)37,38 upwards and no unexpected attractors have been found.
ELF analysis of the formally triple B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond displays a number of localization basins, which have subsequently been merged into a single V(B,N) basin.
N bond displays a number of localization basins, which have subsequently been merged into a single V(B,N) basin.
3. Results and discussion
For the single type B–N bond, three amine-boranes (X3B–NR3): (CH3)3B–N(CH3)3,(CH3)3B–NH3 and H3B–NH3 (a well known prototype of the dative bond) were chosen. The respective experimental bond lengths (rexp) are: 1.698 Å,39 1.62940 and 1.672 Å.41 Geometry optimizations have been carried out using the 6-311++G(2d,2p), 6-311++G(2df,2pd), 6-311++G(3d,3p), 6-311++G(3df,3pd), aug-cc-pVTZ basis sets, and the calculated B–N bond lengths (ropt) are presented in Table 1. The mean values of ropt are: 1.784, 1.703, and 1.657 Å, differing from the experimental ones by 0.086, -0.074, and -0.015 Å respectively. These results confirm previously observed difficulties in modelling of the dative BN bond.42 This problem increases with the addition of methyl groups. In our opinion such discrepancies do not influence the results of bond analysis using ELF.| Compound | Formal bond order | Bond length (exp)/Å | Bond length (ropt) (calc)/Å | |||||
|---|---|---|---|---|---|---|---|---|
| 6-311++G(2d,2p) | 6-311++G(2df,2pd) | 6-311++G(3d,3p) | 6-311++G(3df,3pd) | aug-cc-pVTZ | Mean value | |||
a The geometrical structure has been optimized under D3h symmetry point group.
b The experimental B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond length determined in solid state for the Cl2BN(C6H5)2 molecule.44 N bond length determined in solid state for the Cl2BN(C6H5)2 molecule.44
|
||||||||
| tBuBNtBu |
B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N N
|
1.258 | 1.241 | 1.241 | 1.240 | 1.240 | 1.241 | 1.241 |
| (CH3)3C6H2BNtBu |
B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N N
|
1.232 | 1.242 | 1.242 | 1.236 | 1.241 | — | 1.240 |
| H2BNH2 | B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N N |
1.391 | 1.388 | 1.389 | 1.389 | 1.388 | 1.390 | 1.389 |
| Cl2BN(H)C6H5 | B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N N |
1.379b | 1.397 | 1.399 | 1.398 | 1.399 | 1.400 | 1.399 |
| HB(NH2)2 |
B![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) N N
|
1.418 | 1.413 | 1.413 | 1.413 | 1.412 | 1.414 | 1.413 |
| B(NH2)3a |
B![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) N N
|
— | 1.430 | 1.430 | 1.430 | 1.429 | 1.431 | 1.430 |
| B(N(CH3)2)3 |
B![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) N N
|
1.439 | 1.442 | 1.443 | 1.442 | 1.442 | 1.443 | 1.442 |
| H3BNH3 | B–N | 1.672 | 1.658 | 1.657 | 1.657 | 1.657 | 1.658 | 1.657 |
| (CH3)3BNH3 | B–N | 1.629 | 1.703 | 1.702 | 1.704 | 1.702 | 1.706 | 1.703 |
| (CH3)3BN(CH3)3 | B–N | 1.698 | 1.780 | 1.787 | 1.783 | 1.785 | 1.786 | 1.784 |
A detailed study of the donor–acceptor bond in the H3B–NH3 molecule has been previously reported by Krokidis et al.,43 who adopted catastrophe theory for a systematic description of the B–N bond evolution during dissociation. It has been shown that dissociation into H3B and NH3 fragments results in change of the disynaptic V(B,N) basin into the monosynaptic V(N) in ammonia. Such behaviour is considered a proof of existence of the dative bond. In the case of “normal” covalent bonds A–B (for example in C2H6), a dissociation process yields two non-bonding basins V(A), V(B) on the separated fragments A, B.
For (CH3)3B–N(CH3)3,(CH3)3B–NH3 and H3B–NH3 molecules, topological analysis of the ELF function reveals the bonding disynaptic basin V(B,N), and an associated single point attractor situated at the interatomic B–N axis. From a topological point of view such attractors and basins characterize the boron–nitrogen bonding as covalent,9 displaying a one-to-one correspondence with the Lewis formula. The V(B,N) localization basin is observed close to the atomic C(N) core, and resembles the distorted lone electron pair V(N) in isolated NH3 or N(CH3)3 molecules.
The basin populations calculated with different basis sets are presented in Table 2. The amount of electron density “concentrated” in the V(B,N) basin does not deviate significantly from 2.0e. It is important to stress that these values are in a good agreement with the classical concept of a single shared electron pair. The largest population is obtained for the (CH3)3B–N(CH3)3 molecule (2.07 ÷ 2.11e), and its value slightly diminishes along with a decrease in the number of methyl groups. The mean values of the basin populations of V(B,N) are as follows: 2.09, 1.94 and 1.92e for (CH3)3B–N(CH3)3,(CH3)3B–NH3 and H3B–NH3, respectively. Therefore, as the number of CH3 groups in the system increases, there is an increase in the population on the B–N bond, thus confirming the electron-releasing character of the CH3 group.
| Compound | Formal bond order |
V(B,N)
![[N with combining macron]](https://www.rsc.org/images/entities/i_char_004e_0304.gif) [e] [e] |
|||||
|---|---|---|---|---|---|---|---|
| 6-311++G(2d,2p) | 6-311++G(2df,2pd) | 6-311++G(3d,3p) | 6-311++G(3df,3pd) | aug-cc-pVTZ | Mean value | ||
| a The total basin population of two bonding disynaptic basins Vi=1,2(B,N) is presented. b The geometrical structure of the B(NH2)3 molecule has been optimized under D3h symmetry point group tBu-tert-butyl. | |||||||
| tBuBNtBu |
B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N N
|
5.77 | 5.75 | 5.75 | 5.72 | 5.73 | 5.74 |
| (CH3)3C6H2BNtBu |
B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N N
|
5.73 | 5.72 | 5.72 | 5.69 | — | 5.72 |
| H2BNH2a | B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N N |
3.86 | 3.74 | 3.78 | 3.74 | 3.80 | 3.78 |
| Cl2BN(H)C6H5 | B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N N |
3.98 | 3.93a | 3.89 | 3.85a | 3.91a | 3.91 |
| HB(NH2)2 |
B![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) N N
|
4.04 | 3.99 | 3.97 | 3.93 | 3.99 | 3.98 |
| B(NH2)3b |
B![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) N N
|
4.13 | 4.09 | 4.07 | 4.03 | 4.09 | 4.08 |
| B(N(CH3)2)3 |
B![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) N N
|
4.31 | 4.28 | 4.26 | 4.24 | 4.29 | 4.28 |
| H3BNH3 | B–N | 1.93 | 1.91 | 1.90 | 1.89 | 1.92 | 1.91 |
| (CH3)3BNH3 | B–N | 1.97 | 1.94 | 1.93 | 1.91 | 1.94 | 1.94 |
| (CH3)3BN(CH3)3 | B–N | 2.11 | 2.08 | 2.08 | 2.07 | 2.09 | 2.09 |
It is worth stressing that analysis of the correlation between the B–N bond lengths, decreasing from (CH3)3B–N(CH3)3 to H3B–NH3 (see Table 1), and the basin population of V(B,N), does not show the evidence to support the rule of “concentrating” a larger amount of electron density on the shorter bond. The B–N bond lengths (mean value) of 1.784, 1.703, 1.657 Å correspond to populations of 2.09, 1.94, 1.91e respectively, a completely opposite effect.
According to Paetzold,44 a boron–nitrogen separation of 1.41 Å may be considered as indicative of the double B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond (in noncyclic aminoboranes), and 1.26 Å as a typical B
N bond (in noncyclic aminoboranes), and 1.26 Å as a typical B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N triple bond. For that reason, the B(N(CH3)2)3, B(NH2)3, HB(NH2)2, H2B
N triple bond. For that reason, the B(N(CH3)2)3, B(NH2)3, HB(NH2)2, H2B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH2, and Cl2B
NH2, and Cl2B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(H)C6H5 molecules (see Fig. 1) will be discussed together, even if B(N(CH3)2)3 and HB(NH2)2 have been considered by Straub28 as molecules with partially double B
N(H)C6H5 molecules (see Fig. 1) will be discussed together, even if B(N(CH3)2)3 and HB(NH2)2 have been considered by Straub28 as molecules with partially double B![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) N bonds. The mean values of the optimized B
N bonds. The mean values of the optimized B![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) N bond lengths in B(N(CH3)2)3, B(NH2)3, HB(NH2)2 are 1.442, 1.430 and 1.413 Å, respectively (see Table 1). These values are in good agreement with the experimental data reported for B(N(CH3)2)344 and HB(NH2)245,46 at 1.439 Å and 1.418 Å, respectively. The mean value of B
N bond lengths in B(N(CH3)2)3, B(NH2)3, HB(NH2)2 are 1.442, 1.430 and 1.413 Å, respectively (see Table 1). These values are in good agreement with the experimental data reported for B(N(CH3)2)344 and HB(NH2)245,46 at 1.439 Å and 1.418 Å, respectively. The mean value of B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N optimized bond lengths in aminoborane H2B
N optimized bond lengths in aminoborane H2B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH2 (an analogue of ethane) is 1.389 Å, and is in very good agreement with the experimental value obtained from microwave spectra (1.391 Å).47–49 In the case of the Cl2B
NH2 (an analogue of ethane) is 1.389 Å, and is in very good agreement with the experimental value obtained from microwave spectra (1.391 Å).47–49 In the case of the Cl2B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(H)C6H5 molecule, the average B
N(H)C6H5 molecule, the average B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond length is 1.399 Å.
N bond length is 1.399 Å.
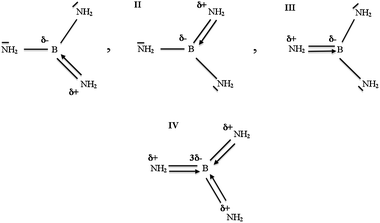
The boranediamine (HB(NH2)2) is molecule with formally partial double B![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) N bond therefore the nature of its bonding will be discussed in detail. Two nitrogen atoms with occupied non-bonding orbitals tend to share one vacant boron p-orbital, so two resonance hybrids with alternating B–N and B
N bond therefore the nature of its bonding will be discussed in detail. Two nitrogen atoms with occupied non-bonding orbitals tend to share one vacant boron p-orbital, so two resonance hybrids with alternating B–N and B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds can be introduced in accordance with the octet rule (see Scheme 1, hybrids I, II). Such an approach predicts the existence of the non-bonding electron density on the nitrogens, which is reflected in topological analysis of ELF by the non-bonding basins V(N). However, another resonance hybrid with two double B
N bonds can be introduced in accordance with the octet rule (see Scheme 1, hybrids I, II). Such an approach predicts the existence of the non-bonding electron density on the nitrogens, which is reflected in topological analysis of ELF by the non-bonding basins V(N). However, another resonance hybrid with two double B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds can be proposed, violating the octet rule (see Scheme 1, hybrid III). In this case, the non-bonding basins V(N) are not present. The link between the Lewis representation of the chemical bonds and the topological partitions of the electron distributions, based on the gradient field analysis of local functions was proposed by Silvi.50 The basin population operators are correlated, therefore the covariance matrix of the basin populations was chosen to be a tool for the description of the electron density delocalisation, in terms of resonant Lewis structures. On the other hand if direct correspondence to the Lewis-style representation can be applied, topological analysis of ELF should reveal two pairs of the Vi =1,2(B,N) attractors, situated both above and below the symmetry plane. If such a correspondence cannot be applied, only two single V(B,N) attractors, located on the interatomic axis should be found. It is worth stressing that regardless of the number of the V(B,N) attractors, unique evidence of the existence of the double B
N bonds can be proposed, violating the octet rule (see Scheme 1, hybrid III). In this case, the non-bonding basins V(N) are not present. The link between the Lewis representation of the chemical bonds and the topological partitions of the electron distributions, based on the gradient field analysis of local functions was proposed by Silvi.50 The basin population operators are correlated, therefore the covariance matrix of the basin populations was chosen to be a tool for the description of the electron density delocalisation, in terms of resonant Lewis structures. On the other hand if direct correspondence to the Lewis-style representation can be applied, topological analysis of ELF should reveal two pairs of the Vi =1,2(B,N) attractors, situated both above and below the symmetry plane. If such a correspondence cannot be applied, only two single V(B,N) attractors, located on the interatomic axis should be found. It is worth stressing that regardless of the number of the V(B,N) attractors, unique evidence of the existence of the double B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond can be obtained by analysing the number of valence electrons “contained” in the V(B,N) or Vi =1,2(B,N) basins. In this case, the basin population value (
N bond can be obtained by analysing the number of valence electrons “contained” in the V(B,N) or Vi =1,2(B,N) basins. In this case, the basin population value (![[N with combining macron]](https://www.rsc.org/images/entities/i_char_004e_0304.gif) ) will be close to the formal value of 4e.
) will be close to the formal value of 4e.
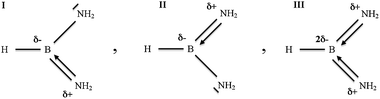 | ||
| Scheme 1 Three resonance hybrids for HB(NH2)2 molecule. | ||
Analysis of the results for HB(NH2)2 shows all attractors localized in the molecular plane, and the vicinity of the boron–nitrogen bond is described by a single V(B,N) attractor. All localised attractors and 3D representation of ELF for HB(NH2)2 are presented in Fig. 2. According to the classification of chemical bonds proposed by Silvi and Savin,9 the boron–nitrogen bonds are covalent. It is remarkable that the non-bonding V(N) attractors are not observed. Thus the resonance hybrids I, II (see Scheme 1) with the lone pairs on nitrogen do not correctly describe the electronic structure of HB(NH2)2. Calculated mean value of the basin population equal to 3.98e is in good correspondence to the expected value of 4e (the B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond). It is evident that the boron–nitrogen bonds have a real double bond character. The hybrid III, one of the three resonance hybrids shown in Scheme 1, is the most dominant. Because the basin population of the H–N bond varies between 1.87 and 1.97e, and the B–H bond population is within the range of 2.05 ÷ 2.09e, the resonance hybrids with H+ N−, H–N bonds and a small negative polarization of the B–H bond must also be taken into consideration.
N bond). It is evident that the boron–nitrogen bonds have a real double bond character. The hybrid III, one of the three resonance hybrids shown in Scheme 1, is the most dominant. Because the basin population of the H–N bond varies between 1.87 and 1.97e, and the B–H bond population is within the range of 2.05 ÷ 2.09e, the resonance hybrids with H+ N−, H–N bonds and a small negative polarization of the B–H bond must also be taken into consideration.
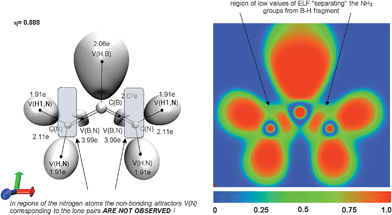 | ||
Fig. 2 The valence and core attractors localized in the gradient field of ELF for HB(NH2)2 molecule. The Lewis structure with double B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds is shown. The 2D plot of the ELF function in molecular plane. The basin populations are calculated at the B3LYP/aug-cc-pVTZ level. Positions of protonated attractors V(H,B), V(H,N) and V(H1,N) are displayed (black dots) in a schematic way. N bonds is shown. The 2D plot of the ELF function in molecular plane. The basin populations are calculated at the B3LYP/aug-cc-pVTZ level. Positions of protonated attractors V(H,B), V(H,N) and V(H1,N) are displayed (black dots) in a schematic way. | ||
The results of topological analysis for HB(NH2)2 are in a good agreement with an infrared and Raman spectroscopy study on the boron–nitrogen bond in boranediamine, which confirms that the BN bond is predominantly of a double bond character.46 Microwave studies also support its partial double bond nature.45
Topological analysis of ELF for B(N(CH3)2)3 and B(NH2)3 reveals single bonding disynaptic basins V(B,N) in the boron–nitrogen bond area. All attractors localized in the B(N(CH3)2)3 molecule are shown in Fig. 3. 2D and 3D representations of ELF for B(NH2)3 are presented in Fig. 4. According to the classification of chemical bonds proposed by Silvi and Savin,9 the boron–nitrogen bonds characterised as formally containing partial double character are covalent. Analysis of ELF-isosurfaces reveals that the bonding basin V(B,N) is contained in a large localization domain surrounding the core nitrogen C(N) basin and the protonated basins V(H,N). However, such a topological feature cannot be considered an indication of dative character.
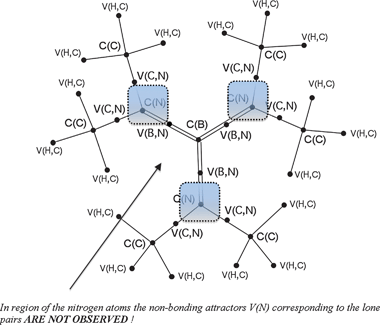 | ||
Fig. 3 The valence and core attractors localized in the gradient field of ELF for B(N(CH3)2)3 molecule. The Lewis structure with double B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds is shown. N bonds is shown. | ||
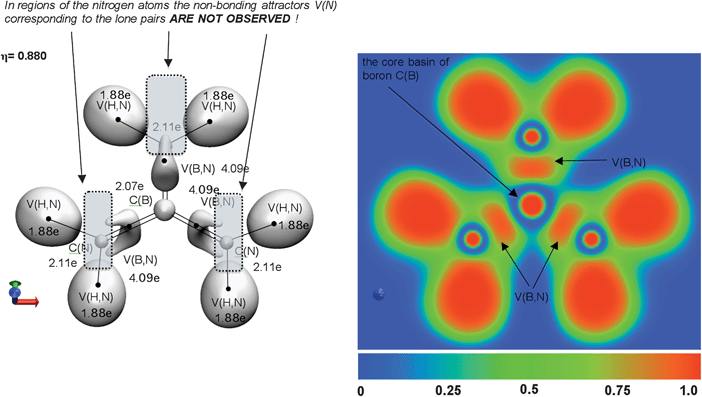 | ||
Fig. 4 The valence and core attractors localized in the gradient field of ELF for B(NH2)3 molecule. The Lewis structure with double B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds is shown. The 2D plot of the ELF function in molecular plane. The basin populations are calculated at the B3LYP/aug-cc-pVTZ level. The positions of the protonated attractors V(H,N) are presented (black dots) in a schematic way. N bonds is shown. The 2D plot of the ELF function in molecular plane. The basin populations are calculated at the B3LYP/aug-cc-pVTZ level. The positions of the protonated attractors V(H,N) are presented (black dots) in a schematic way. | ||
Because each of the lone pairs associated with the three nitrogen atoms of the N(CH3)2 and NH2 groups in B(N(CH3)2)3 and B(NH2)3 interacts with a single vacant orbital on boron, three resonance hybrids (I–III) are proposed (see Scheme 2) to satisfy the octet rule. The resulting covalent-dative B![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) N bonds model assumes the presence of the non-bonding electron density on each nitrogen with population less than 2e. Thus the monosynaptic non-bonding basins V(N) should be observed in the ELF picture. On the other hand, another resonance hybrid (IV), with three covalent-dative B
N bonds model assumes the presence of the non-bonding electron density on each nitrogen with population less than 2e. Thus the monosynaptic non-bonding basins V(N) should be observed in the ELF picture. On the other hand, another resonance hybrid (IV), with three covalent-dative B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds and without non-bonding electron density on the N atom can be proposed (see Scheme 2). These two different models of the bonding, verified by topological analysis of ELF and showing no non-bonding attractors V(N), are presented in Fig. 3 and Fig. 4. It is apparent that topologically the BN bonding in B(N(CH3)2)3 and B(NH2)3 displays properties of the double B
N bonds and without non-bonding electron density on the N atom can be proposed (see Scheme 2). These two different models of the bonding, verified by topological analysis of ELF and showing no non-bonding attractors V(N), are presented in Fig. 3 and Fig. 4. It is apparent that topologically the BN bonding in B(N(CH3)2)3 and B(NH2)3 displays properties of the double B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond.
N bond.
 | ||
| Scheme 2 Four resonance hybrids for B(NH2)3 molecule. | ||
Mean values of the basin populations calculated for V(B,N) using five basis sets (Table 2) range between 4.28e for B(N(CH3)2)3 and 4.08e for B(NH2)3. It is evident that the boron–nitrogen bonds in B(N(CH3)2)3 and B(NH2)3 have double bond character, represented by the B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N formula when the number of electrons “concentrated” in the bond (the disynaptic V(B,N) basin) is considered. Furthermore, this result supports the conclusion drawn on the basis of missing non-bonding basins V(N) in the ELF picture. The large basin population of V(B,N) in B(N(CH3)2)3 (4.28e) can be connected to the polarisation of the C–N bonds, which “contain” only 1.62 ÷ 1.67e (V(C,N) basins), with the remaining electron density presumably transferred to the three B
N formula when the number of electrons “concentrated” in the bond (the disynaptic V(B,N) basin) is considered. Furthermore, this result supports the conclusion drawn on the basis of missing non-bonding basins V(N) in the ELF picture. The large basin population of V(B,N) in B(N(CH3)2)3 (4.28e) can be connected to the polarisation of the C–N bonds, which “contain” only 1.62 ÷ 1.67e (V(C,N) basins), with the remaining electron density presumably transferred to the three B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds.
N bonds.
A comparison between B(NH2)3 and HB(NH2)2 molecules reveals that one of the NH2 groups in B(NH2)3 is replaced by the hydrogen atom. As a result, a smaller basin population of 3.98e for V(B,N) is obtained in comparison to the population of 4.08e for B(NH2)3. This can be associated with the smaller inductive effect of the H atom. It is worth noting that the V(B,N) basin populations around 4e computed for B(NH2)3 and HB(NH2)2, are also an effect of a depopulation of the N–H bonds (1.86 ÷ 1.89e). The “missing” electron density (in respect to the formal value of 2e) is distributed to the B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds. The difference in basin population of V(B,N) in B(N(CH3)2)3 and B(NH2)3 (4.28evs. 4.08e) can be considered as an illustration of the inductive effect of the methyl and amine groups. The smaller basin population (4.08e) of V(B,N) in B(NH2)3 can be explained by smaller electron-donating effect of the NH2 group compared to N(CH3)2. Fourré et al.51 studied the electron withdrawing group (−I) and electron donating group (+I) effects using topological analysis of ELF function. They found that a very short ranged inductive effect was essentially localized on the valence basins related to the carbon-X bonding.
N bonds. The difference in basin population of V(B,N) in B(N(CH3)2)3 and B(NH2)3 (4.28evs. 4.08e) can be considered as an illustration of the inductive effect of the methyl and amine groups. The smaller basin population (4.08e) of V(B,N) in B(NH2)3 can be explained by smaller electron-donating effect of the NH2 group compared to N(CH3)2. Fourré et al.51 studied the electron withdrawing group (−I) and electron donating group (+I) effects using topological analysis of ELF function. They found that a very short ranged inductive effect was essentially localized on the valence basins related to the carbon-X bonding.
The H2B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH2 and Cl2B
NH2 and Cl2B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(H)C6H5 molecules formally contain a B
N(H)C6H5 molecules formally contain a B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond. 2D and 3D plots of the ELF function are presented in Fig. 5 and Fig. 6. Interestingly, the largest basis set aug-cc-pVTZ33,34 yields B
N double bond. 2D and 3D plots of the ELF function are presented in Fig. 5 and Fig. 6. Interestingly, the largest basis set aug-cc-pVTZ33,34 yields B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds described by two bonding disynaptic attractors Vi =1,2(B,N), localized above and below the symmetry plane. This result is in accordance with the Lewis formula. Such topology of ELF is obtained for H2B
N bonds described by two bonding disynaptic attractors Vi =1,2(B,N), localized above and below the symmetry plane. This result is in accordance with the Lewis formula. Such topology of ELF is obtained for H2B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH2, regardless of the basis set used. On the contrary, the results for the Cl2B
NH2, regardless of the basis set used. On the contrary, the results for the Cl2B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(H)C6H5 molecule display dependence on the basis set used. Calculations performed with the 6-311++G(3d,3p) and 6-311++G(2d,2p) basis sets yield only a single V(B,N) attractor. It is worth stressing that the monosynaptic non-bonding basins V(N) are not observed (see Fig. 5 and Fig. 6). Moreover, topological description of B(N(CH3)2)3, B(NH2)3, HB(NH2)2, as well as H2B
N(H)C6H5 molecule display dependence on the basis set used. Calculations performed with the 6-311++G(3d,3p) and 6-311++G(2d,2p) basis sets yield only a single V(B,N) attractor. It is worth stressing that the monosynaptic non-bonding basins V(N) are not observed (see Fig. 5 and Fig. 6). Moreover, topological description of B(N(CH3)2)3, B(NH2)3, HB(NH2)2, as well as H2B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH2 and Cl2B
NH2 and Cl2B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(H)C6H5 molecules, does not show any evidence for B
N(H)C6H5 molecules, does not show any evidence for B![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) N partial double bonds.
N partial double bonds.
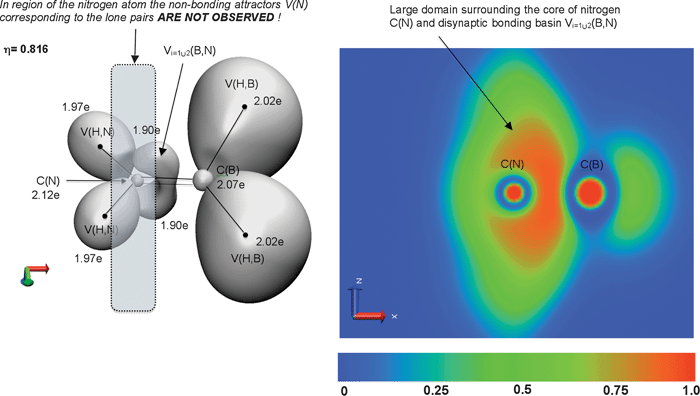 | ||
Fig. 5 The valence and core attractors localized in the gradient field of ELF for H2B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH2 molecule. The Lewis structure with double B NH2 molecule. The Lewis structure with double B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds is shown. The 2D plot of the ELF function in plane perpendicular to molecular plane. The basin populations presented are calculated at the B3LYP/aug-cc-pVTZ level. Positions of protonated attractors V(H,B), V(H,N) are presented (black dots) in a schematic way. N bonds is shown. The 2D plot of the ELF function in plane perpendicular to molecular plane. The basin populations presented are calculated at the B3LYP/aug-cc-pVTZ level. Positions of protonated attractors V(H,B), V(H,N) are presented (black dots) in a schematic way. | ||
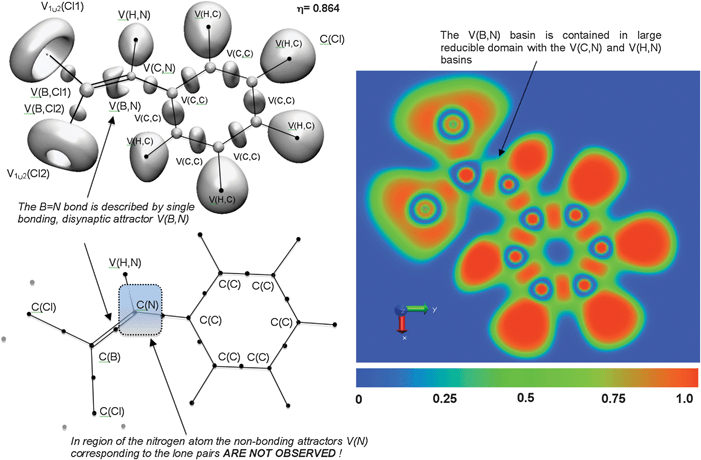 | ||
Fig. 6 The ELF-localization basins and valence and core attractors localized in the gradient field of ELF for Cl2B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(H)C6H5 molecule. The 2D plot of the ELF function in molecular plane. The basin populations presented are calculated at the B3LYP/aug-cc-pVTZ level. N(H)C6H5 molecule. The 2D plot of the ELF function in molecular plane. The basin populations presented are calculated at the B3LYP/aug-cc-pVTZ level. | ||
The sum of basin populations for the B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond [V1(B,N)+V2(B,N)] is 3.74 ÷ 3.86e for H2B
N bond [V1(B,N)+V2(B,N)] is 3.74 ÷ 3.86e for H2B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH2 and 3.85 ÷ 3.98e for Cl2B
NH2 and 3.85 ÷ 3.98e for Cl2B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(H)C6H5. These values confirm that formal characterization of the B
N(H)C6H5. These values confirm that formal characterization of the B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond as a double bond is correct. However, a resonance of equilibrium forms has to be invoked in order to correctly describe basin population values of less than 4.0e.
N bond as a double bond is correct. However, a resonance of equilibrium forms has to be invoked in order to correctly describe basin population values of less than 4.0e.
In the case of single B–N bonds, we have noticed that a shorter bond length does not correspond to a larger amount of electron density in the V(B,N) basin. The same effect is observed for molecules with formal B![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) N and B
N and B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds. Along with decreasing B
N bonds. Along with decreasing B![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) N bond lengths (mean value) of 1.442, 1.430 and 1.413 Å for B(N(CH3)2)3, B(NH2)3 and HB(NH2)2, respectively, the basin populations of V(B,N) decrease from 4.28, 4.08 to 3.98e. Similarly, for B
N bond lengths (mean value) of 1.442, 1.430 and 1.413 Å for B(N(CH3)2)3, B(NH2)3 and HB(NH2)2, respectively, the basin populations of V(B,N) decrease from 4.28, 4.08 to 3.98e. Similarly, for B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds in H2B
N bonds in H2B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH2 and Cl2B
NH2 and Cl2B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(H)C6H5 (mean bond lengths 1.399 and 1.389 Å, respectively), the basin populations of V(B,N) are 3.91 and 3.78e.
N(H)C6H5 (mean bond lengths 1.399 and 1.389 Å, respectively), the basin populations of V(B,N) are 3.91 and 3.78e.
The formal triple B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond has been investigated using two exemplar iminoborane molecules, (CH3)3C6H2B
N bond has been investigated using two exemplar iminoborane molecules, (CH3)3C6H2B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) NtBu and tBuB
NtBu and tBuB![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) NtBu (see Fig. 1), where the experimental (solid state) B
NtBu (see Fig. 1), where the experimental (solid state) B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond lengths are 1.232 Å for (tBu)3C6H2B
N bond lengths are 1.232 Å for (tBu)3C6H2B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) NtBu52 and 1.258 Å53 for tBuB
NtBu52 and 1.258 Å53 for tBuB![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) NtBu. The mean optimized values (see Table 1) are 1.240 and 1.241 Å.
NtBu. The mean optimized values (see Table 1) are 1.240 and 1.241 Å.
Topological analysis of the ELF function shows a torus-like V(B,N) disynaptic basin situated between the C(B) and C(N) cores (see Fig. 7), with basin populations in the range 5.69 ÷ 5.73e for (CH3)3C6H2B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) NtBu, and 5.72 ÷ 5.77e for tBu-B
NtBu, and 5.72 ÷ 5.77e for tBu-B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) NtBu (Table 2). There is no direct correspondence with the Lewis description since only one bonding basin V(B,N) is observed. Calculated values of the basin population (5.72e, 5.74e) are smaller than the value of 6e formally expected for the triple bond, but the topological bond orders 2.86 and 2.87 obtained from the mean values of
NtBu (Table 2). There is no direct correspondence with the Lewis description since only one bonding basin V(B,N) is observed. Calculated values of the basin population (5.72e, 5.74e) are smaller than the value of 6e formally expected for the triple bond, but the topological bond orders 2.86 and 2.87 obtained from the mean values of ![[N with combining macron]](https://www.rsc.org/images/entities/i_char_004e_0304.gif) strongly support the triple bond character.
strongly support the triple bond character.
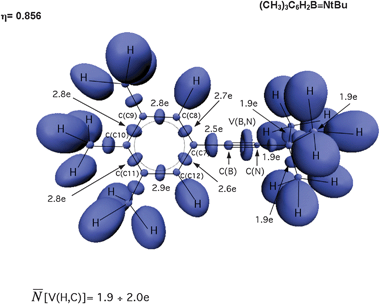 | ||
Fig. 7 The ELF-localization basins in the (CH3)3C6H2–B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–tBu molecule. The basin populations presented are calculated at the B3LYP/6-311++G(2d,2p) computational level. N–tBu molecule. The basin populations presented are calculated at the B3LYP/6-311++G(2d,2p) computational level. | ||
4. Conclusions
Topological analysis of the ELF within the QCT framework confirms that the ELF is a powerful tool for investigation of the multiple boron–nitrogen bonding character.
1. All studied molecules show covalent boron–nitrogen bonds described by the bonding basin V(B,N). Topological analysis of the ELF functions confirms the existence of the multiple boron–nitrogen bonds. Three different types can be distinguished, notably single B–N, double B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and triple B
N and triple B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bonds with the disynaptic valence basins V(B,N), corresponding to chemical bonds containing 1.91 ÷ 2.09e, 3.78 ÷ 4.28e and 5.72 ÷ 5.74e, respectively. However, from a topological point of view there is no difference between the B
N bonds with the disynaptic valence basins V(B,N), corresponding to chemical bonds containing 1.91 ÷ 2.09e, 3.78 ÷ 4.28e and 5.72 ÷ 5.74e, respectively. However, from a topological point of view there is no difference between the B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bonds in H2B
N double bonds in H2B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH2, Cl2B
NH2, Cl2B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(H)C6H5 and B
N(H)C6H5 and B![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) N partial double bonds in B(N(CH3)2)3, B(NH2)3, HB(NH2)2 distinguished by Straub.28
N partial double bonds in B(N(CH3)2)3, B(NH2)3, HB(NH2)2 distinguished by Straub.28
2. The lone pair of nitrogen participating in the donor–acceptor bonds (pπ–pπ bonding) is not confirmed within the topological analysis of ELF framework. Even for the molecules with a single, empty p-orbital, shared by two or three lone pairs, for example HB(NH2)2, B(N(CH3)2)3 or B(NH2)3, the non-bonding basins V(N), expected on a basis of the resonance hybrids, are not observed. Only single bonding basins V(B,N), are localized in the vicinity of the boron–nitrogen bonds
3. The results obtained in this study clearly show the correlation between the basin population, V(B,N), and the bond length. For different B–N bond types (e.g. single, double, triple), the shorter bond lengths usually correspond to a larger number of electrons in the V(B,N) basin. The opposite effect applies for the same type bonds (e.g. B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), where the shorter bond length is related to the smaller number of electrons in the basin.
N), where the shorter bond length is related to the smaller number of electrons in the basin.
4. In general, there is no direct correspondence between the multiplicity of the BN bond and the number of localisation basins Vi(B,N). The exceptions are H2B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH2 and Cl2B
NH2 and Cl2B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(H)C6H5, where the formally double B
N(H)C6H5, where the formally double B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond is described by two Vi =1,2(B,N) basins. The presence of the Vi =1,2(B,N) basins depends on molecular symmetry and computational level.
N bond is described by two Vi =1,2(B,N) basins. The presence of the Vi =1,2(B,N) basins depends on molecular symmetry and computational level.
Acknowledgements
The authors would like to thank the The Wroclaw Networking and Supercomputing Centre for generous allocation of computer time. Dr Charles M. Gordon is thanked for editing and proofreading the manuscript.References
- N. O. J. Malcolm and P. L. A. Popelier, Faraday Discuss., 2003, 124, 353 RSC.
- R. F. W. Bader, S. G. Anderson and A. J. Duke, J. Am. Chem. Soc., 1979, 101, 1389 CrossRef CAS.
- R. F. W. Bader and T. T. Y. Nguyen-Dang, J. Chem. Phys., 1979, 70, 4316 CrossRef CAS.
- R. F. W. Bader, Y. Tal, S. G. Anderson and T. T. Nguyen-Dang, Isr. J. Chem., 1980, 19, 8 CAS.
- R. F. W. Bader, J. Chem. Phys., 1980, 73, 2871 CrossRef CAS.
- Y. Tal, R. F. W. Bader, T. T. Nguyen-Dang, M. Ojha and S. G. Anderson, J. Chem. Phys., 1981, 74, 5162 CrossRef CAS.
- “Quantum Topology (Series on Knot and everything—Vol. 3)”, ed. L.H. Kauffman, World Scientific Publishing Company, Singapore, 1993 Search PubMed.
- A. D. Becke and K. E. Edgecombe, J. Chem. Phys., 1990, 92, 5379.
- B. Silvi and A. Savin, Nature, 1994, 371, 683 CrossRef CAS.
- H. Chevreau, F. Fuster and B. Silvi, L'Actualité Chimique, 2001, 240, 15 Search PubMed.
- B. Silvi, I. Furré and M. E. Alikhani, Monatsh. Chem., 2005, 136, 855 CrossRef CAS.
- R. Llusar, A. Beltrán J. Andrés, S. Noury and B. Silvi, J. Comp. Chem., 1517, 20, 1999 Search PubMed.
- G. N. Lewis, J. Am. Chem. Soc., 1916, 38, 762 CrossRef CAS.
- A. Shurki, Theor. Chem. Acc., 2006, 116, 253 CrossRef CAS.
- G. Frenking and S. Shaik, J. Comput. Chem., 2007, 28, 1 CrossRef CAS.
- P. C. Hiberty and S. Shaik, J. Comput. Chem., 2007, 28, 137 CrossRef CAS.
- R. F. W. Bader, Atoms in Molecules—A Quantum Theory, Oxford University Press, Oxford, 1990 Search PubMed.
- R. F. W. Bader, J. Phys. Chem. A, 2010, 114, 7431 CrossRef CAS.
- R. F. W. Bader, J. Mol. Struct. THEOCHEM, 2010, 943, 2 CrossRef CAS.
- A. Savin, J. Mol. Struct THEOCHEM, 2005, 727, 127 CrossRef CAS.
- A. Savin, B. Silvi and F. Colonna, Can. J. Chem., 1996, 74, 1088 CAS.
- J. Santos, V. Polo and J. Andrés, Chem. Phys. Lett., 2005, 406, 393 CrossRef CAS.
- J. Pilme, B. Silvi and M. E. Alikhani, J. Phys. Chem. A, 2005, 109, 10028 CrossRef CAS.
- S. Berski, G. Gajewski and Z. Latajka, J. Mol. Struct. (Studies In Hydrogen-Bonded Systems—A collection of Invited Papers in honour of Professor Lucjan Sobczyk, on the occasion of his 80th Birthday), 2007, 844–845, 278 Search PubMed.
- D. B. Chesnut, Chem. Phys., 2001, 271, 9 CrossRef CAS.
- M. Kohout and A. Savin, Int. J. Quantum Chem., 1998, 60, 875.
- N. G. Chopra, R. J. Luyken, K. Cherrey, V. H. Crespi, M. L. Cohen, S. G. Louie and A. Zettl, Science, 1995, 269, 966 CrossRef CAS.
- D. K. Straub, J. Chem. Educ., 1995, 72, 494 CrossRef CAS.
- A. D. Becke, J. Chem. Phys., 1993, 98, 1372 CrossRef CAS.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648 CrossRef CAS.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter, 1988, 37, 785 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery, Jr., T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez and J. A. Pople, GAUSSIAN 03, Revision D.02, Gaussian, Inc., Wallingford CT, 2004 Search PubMed.
- T. H. Dunning, Jr., J. Chem. Phys., 1989, 90, 1007 CrossRef CAS.
- R. A. Kendall, T. H. Dunning, Jr. and R. J. Harrison, J. Chem. Phys., 1992, 96, 6796 CrossRef CAS.
- M. Kohout, program DGrid, versions 4.2 and 4.3, program Basin, versions 4.2 and 4.3, 2008.
- VMD for MAC OS X X86, version 1.8.6 (April 6, 2007), W. Humphrey, A. Dalke and K. Schulten, J. Mol. Graphics, 1996, 14, 33 Search PubMed.
- R. Krishnan, J. S. Binkley, R. Seeger and J. A. Pople, J. Chem. Phys., 1980, 72, 650 CrossRef CAS.
- M. J. Frisch, J. A. Pople and J. S. Binkley, J. Chem. Phys., 1984, 80, 3265 CrossRef CAS.
- P. M. Kuznesof, Inorg. Chem., 1978, 17, 2308 CrossRef CAS.
- M. D. Harmony, V. W. Laurie, R. L. Kuczkowsky, R. H. Schwendeman, D. A. Ramsay, F. J. Lovas, W. J. Lafferty and A. G. Maki, J. Phys. Chem. Ref. Data, 1979, 8, 3.
- L. R. Thorne, R. D. Suenram and F. J. Lovas, J. Chem. Phys., 1983, 78, 167 CrossRef CAS.
- J. A. Plumley and J. D. Evanseck, J. Phys. Chem. A, 2007, 111, 13472 CrossRef CAS.
- X. Krokidis, S. Noury and B. Silvi, J. Phys. Chem. A, 1997, 101, 7277 CrossRef CAS.
- P. Paetzold, Adv. Inorg. Chem., 1987, 31, 123 CAS.
- L. R. Thorne and W. D. Gwinn, J. Am. Chem. Soc., 1982, 104, 3822 CrossRef CAS.
- D. C. Reuter, L. R. Thorne and W. D. Gwinn, J. Phys. Chem., 1982, 86, 4737 CrossRef CAS.
- M. Sugie, H. Takeo and C. Matsumura, J. Mol. Spectrosc., 1987, 123, 286 CrossRef CAS.
- M. Sugie, H. Takeo and C. Matsumura, Chem. Phys. Lett., 1979, 64, 573 CrossRef CAS.
- M. C. L. Gerry, W. Lewis-Bevan, A. J. Merer and N. P. C. Westwood, J. Mol. Spectrosc., 1985, 110, 153 CrossRef CAS.
- B. Silvi, Phys. Chem. Chem. Phys., 2004, 6, 256 RSC.
- I. Fourré, H. Gérard and B. Silvi, J. Mol. Struct. THEOCHEM, 2007, 811, 69 CrossRef CAS.
- G. Elter, M. Neuhaus, A. Meller and D. Schmidt-Bäse, J. Organomet. Chem., 1990, 381, 299 CrossRef CAS.
- P. Klaeboe, D. Bougeard, B. Schrader, P. Paetzold and C. von Plotho, Spectrochim. Acta, Part A, 1985, 41, 53 CrossRef.
Footnote |
| † The beginning of Quantum Chemical Topology (QCT) is associated with studies on topological properties of the electron density by R. Bader, who in the years 1979–1981 published a series of papers in which he used the term “quantum topology”.2–6 However, the term “quantum topology” is also a branch of mathematical physics, exploring topological knot theory, quantum groups and quantum field theory.7 |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2011 |
