Synthesis, characterisation and ethylene oligomerization behaviour of N-(2-substituted-5,6,7-trihydroquinolin-8-ylidene)arylaminonickel dichlorides†
Jiangang
Yu
ac,
Xinquan
Hu
b,
Yanning
Zeng
c,
Liping
Zhang
c,
Caihua
Ni
*a,
Xiang
Hao
c and
Wen-Hua
Sun
*c
aSchool of Chemical and Material Engineering, Jiangnan University, Wuxi 214122, China. E-mail: nicaihua2000@163.com
bCollege of Chemical Engineering and Materials Science, Zhejiang University of Technology, Hangzhou 310014, China
cKey Laboratory of Engineering Plastics, Beijing National Laboratory for Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China. E-mail: whsun@iccas.ac.cn; Fax: +86-10-62618239
First published on 11th October 2010
Abstract
A series of N-(2-substituted-5,6,7-trihydroquinolin-8-ylidene)-arylaminonickel(II) dichlorides were synthesized by the one-pot stoichiometric reaction of nickel dichloride, 2-chloro- or 2-phenyl-substituted 5,6,7-trihydroquinolin-8-one, and the corresponding anilines. All nickel complexes were characterized by elemental and spectroscopic analysis. The molecular structures of representative nickel complexes, determined by the single-crystal X-ray diffraction, indicate the different coordination numbers around nickel either four with more bulky ligands or five with less bulky ligands. All nickel complexes, activated with ethylaluminium sesquichloride (Et3Al2Cl3), showed high activities (up to 9.5 × 106 g mol−1 h−1) in ethylene oligomerization for dimer and trimers.
Introduction
Ethylene oligomerization is one of most important industrial processes for producing linear alpha-olefins, in which a well-known process is the Shell Higher Olefin Process (SHOP) employing the nickel catalyst (A) (Scheme 1).1 The interest in nickel catalysts for ethylene reactivity was resurrected with the observation that highly active ethylene polymerization catalyst of diiminonickel complexes (B)2 and self-activating catalyst of neutral salicylaldiminonickel complexes (C).3 In the past decade, papers of nickel complexes acting as catalysts in ethylene oligomerization and polymerization have mushroomed with extensive works of nickel complexes bearing bidentate ligands such as N⁁N,4,5N⁁P,6N⁁O,7P⁁O,8 and tridentate ligands such as N⁁N⁁N,4e,9N⁁N⁁O,4e,5e,10N⁁P⁁N.11 In general, N,N-bidentate Ni(II) complexes are more attractive due to their easy syntheses and better catalytic performances.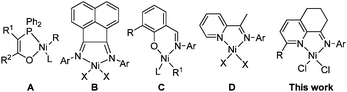 | ||
| Scheme 1 Model catalysts. | ||
Within N,N-bidentate Ni(II) complexes, the 2-iminopyridinyl nickel halides (D) showed good activities in ethylene polymerization,5a,b and their derivatives showed activities for both oligomerization and polymerization.5d,e However, there is no research on the fused-cycloalkanonylpyridine for such 2-iminopyridines as ligands. In this work, the 2-chloro- and 2-phenyl-substituted 5,6,7-trihydroquinolin-8-ones12 are used to form N-(2-substituted-5,6,7-trihydroquinolin-8-ylidene)arylaminonickel dichlorides. The molecular structures of representative complexes are determined by single-crystal X-ray crystallography analysis, and indicate that the five-coordinated number is preferred for nickel complexes bearing N-(2-chloro-5,6,7-trihydroquinolin-8-ylidene)arylamines, whereas the four-coordinated number is found for nickel complexes ligating N-(2-phenyl-5,6,7-trihydroquinolin-8-ylidene)arylamines. All nickel catalysts are highly active in ethylene oligomerization in the presence of ethylaluminium sesquichloride (Et3Al2Cl3). Herein the synthesis and characterisation of the title nickel complexes are reported along with their performance in ethylene oligomerisation.
Results and discussion
Synthesis and characterisation
The reaction of 2-chloro/phenyl-5,6,7-trihydroquinolin-8-one with anilines gave two compounds due to partial migration of the double bonding of Schiff-base into inner cyclic 4,5-dihydroquinolin-8-arylamines, therefore the stepwise procedure of preparing ligands and then forming nickel complexes is not suitable for the current work. To overcome the problem in synthesizing unstable ligands11,13 or forming ligands in low yields,14 one-pot reaction is an efficient methodology to form metal complex due to cation-induced assembly. Therefore, the title nickel complexes are formed in acceptable yields through the one-pot reactions of 2-chloro- (or phenyl-)5,6,7-trihydroquinolin-8-one, the corresponding anilines and NiCl2·6H2O in acetic acid (Scheme 2). All complexes were consistent with their elemental analyses, and their IR spectra with a strong band in the range 1550–1600 cm−1 which can be ascribed to the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N. The unambiguous molecular structures of Ni2, Ni3, Ni8 and Ni10 are confirmed by single-crystal X-ray crystallography.
N. The unambiguous molecular structures of Ni2, Ni3, Ni8 and Ni10 are confirmed by single-crystal X-ray crystallography.
 | ||
| Scheme 2 Synthetic procedure of bidentate nickel complexes. | ||
Molecular structures
Crystals of Ni2, Ni3, Ni8 and Ni10 suitable for single-crystal X-ray crystallography have been obtained by laying diethyl ether on their ethanol solution at room temperature. The molecular structures indicate two kinds of coordination numbers around nickel atoms. The five-coordination around nickel atom is observed within complexes Ni2 and Ni3, which are a chloro-bridged dimer or a monomeric nickel complex containing a coordinated ethanol, respectively. Within complexes Ni8 and Ni10, they adapt four-coordination numbers with bidentate ligands and two chlorides. The coordination numbers are dependent on the steric hindrances associated with their ligands. The ligands with a bulky aryl group occupy more space around nickel and induce the lower coordination numbers for complexes Ni8 and Ni10; the ligands bearing chloro-substituent form nickel complexes with five-coordination number, forming the chloro-bridged dimeric Ni2 and monomer Ni3 having an additional solvent. Such phenomena are commonly observed within other nickel complexes in the literature.15 The molecular structures of Ni2, Ni3, Ni8 and Ni10 are shown in Fig. 1–4, and their selected bond lengths and angles are listed in Table 1. | ||
| Fig. 1 ORTEP drawing of complex Ni2 with thermal ellipsoids at 30% probability level. Hydrogen atoms have been omitted for clarity. | ||
 | ||
| Fig. 2 ORTEP drawing of complex Ni3 with thermal ellipsoids at 30% probability level. Hydrogen atoms have been omitted for clarity. | ||
 | ||
| Fig. 3 ORTEP drawing of complex Ni8 with thermal ellipsoids at 30% probability level. Hydrogen atoms have been omitted for clarity. | ||
 | ||
| Fig. 4 ORTEP drawing of complex Ni10 with thermal ellipsoids at 30% probability level. Hydrogen atoms have been omitted for clarity. | ||
| Ni2 | Ni3 | Ni8 | Ni10 | |
|---|---|---|---|---|
| Bond lengths/Å | ||||
| Ni1–N1 | 2.077(5) | 2.077(9) | 2.044(6) | 2.018(5) |
| Ni1–N2 | 2.030(4) | 2.054(8) | 2.012(6) | 2.011(5) |
| Ni1–Cl1 | 2.2766(16) | 2.278(3) | 2.202(3) | 2.2166(17) |
| Ni1–Cl2 | 2.3789(17) | 2.324(3) | 2.234(2) | 2.1992(18) |
| N2–C9 | 1.289(7) | 1.292(13) | 1.293(9) | 1.276(7) |
| N2–C10 | 1.464(7) | 1.460(13) | 1.451(9) | 1.440(7) |
| N1–C1 | 1.327(7) | 1.327(13) | 1.367(10) | 1.345(8) |
| N1–C5 | 1.352(7) | 1.379(12) | 1.362(10) | 1.360(8) |
| Ni1–O1 | — | 2.038(8) | — | — |
| Bond angles (°) | ||||
| N2–Ni1–N1 | 79.10(18) | 79.8(3) | 81.6(3) | 82.0(2) |
| N2–Ni1–Cl1 | 110.73(14) | 102.5(3) | 106.79(19) | 104.65(15) |
| N1–Ni1–Cl1 | 93.77(13) | 89.4(3) | 137.8(2) | 123.12(14) |
| N2–Ni1–Cl2 | 97.11(14) | 107.6(3) | 110.2(2) | 112.34(14) |
| N1–Ni1–Cl2 | 171.25(13) | 89.6(2) | 96.68(19) | 100.04(14) |
| Cl1–Ni1–Cl2 | 94.95(6) | 149.20(12) | 117.29(13) | 126.21(7) |
| O1–Ni1–N2 | — | 99.0(3) | — | — |
| O1–Ni1–N1 | — | 176.1(3) | — | — |
| O1–Ni1–Cl1 | — | 87.3(2) | — | — |
The five-coordinated complexes Ni2 and Ni3 display the distorted bipyramidal coordination environment around nickel atom, whereas the four-coordinated complexes Ni8 and Ni10 adopt the distorted tetrahedral sphere. As shown in Table 1, the ligands embrace nickel atoms stronger in complexes Ni8 and Ni10 with shorter bond lengths of Ni–N and larger bond angles of N1–Ni–N2 than the analogous complexes Ni2 and Ni3 show. Regarding dimeric Ni2, there is no direct bonding between two nickel atoms with intramolecular distance 3.481 Å, which is quite similar to the data 3.475 Å observed in the analogous di-μ-chloro-bis(2-iminopyridinyl)dinickel dichlorides.16
Ethylene oligomerization
Various alkylaluminiums such as MAO, MMAO, AlEt2Cl and ethylaluminium sesquichloride (Et3Al2Cl3, EASC) have been evaluated as activators to activate the complex Ni3 in ethylene oligomerization (entries 1–4 in Table 2). The system employing EASC as cocatalyst exhibits highest activity (entry 4 in Table 2), which is consistent to its analogues of iminopyridinylnickel catalysts.17 Therefore, the EASC is used as activator for selecting optimum reaction parameters such as ratios of aluminium to nickel (entries 4–8 in Table 2), reaction temperatures (entry 6, entries 9–11 in Table 2) and lifetime of the active species (entry 6, entries 12–15 in Table 2).| Entry | Cocatalyst | Al/Ni | T/°C | t/min | Activityb | Product distribution (%)c | ||
|---|---|---|---|---|---|---|---|---|
| C4/∑ | α-C4/C4 | C6/∑ | ||||||
| a Reaction conditions: 5 μmol of Ni; 10 atm of ethylene; 100 mL of toluene. b Activity, 106 g mol−1(Ni) h−1. c Determined by GC. ∑ donates the total amount of oligomers. | ||||||||
| 1 | MAO | 1000 | 20 | 30 | 0.31 | 87.1 | 98.0 | 12.9 |
| 2 | MMAO | 1000 | 20 | 30 | 0.93 | 86.9 | 89.0 | 13.1 |
| 3 | AlEt2Cl | 200 | 20 | 30 | 0.94 | 84.3 | 84.3 | 15.7 |
| 4 | EASC | 200 | 20 | 30 | 4.54 | 92.5 | >99.0 | 7.5 |
| 5 | EASC | 300 | 20 | 30 | 5.1 | 89.4 | 97.1 | 10.6 |
| 6 | EASC | 400 | 20 | 30 | 8.7 | 89.6 | >99.0 | 10.4 |
| 7 | EASC | 500 | 20 | 30 | 5.9 | 88.0 | >99.0 | 12.0 |
| 8 | EASC | 600 | 20 | 30 | 5.4 | 85.1 | >99.0 | 14.9 |
| 9 | EASC | 400 | 40 | 30 | 7.6 | 77.9 | 94.2 | 22.1 |
| 10 | EASC | 400 | 60 | 30 | 6.5 | 81.2 | 87.4 | 18.8 |
| 11 | EASC | 400 | 80 | 30 | 5.8 | 73.9 | 76.7 | 26.1 |
| 12 | EASC | 400 | 20 | 10 | 9.5 | 91.5 | >99.0 | 8.5 |
| 13 | EASC | 400 | 20 | 20 | 9.0 | 86.7 | >99.0 | 13.3 |
| 14 | EASC | 400 | 20 | 40 | 5.8 | 87.5 | >99.0 | 12.5 |
| 15 | EASC | 400 | 20 | 50 | 4.7 | 86.8 | >99.0 | 13.2 |
The Ni3/EASC is studied with changing Al/Ni molar ratios from 200 to 600 (entries 4–8 in Table 2) at 20 °C, shows best value of 8.7 × 106 g mol−1(Ni) h−1 with the molar ratio of Al/Ni = 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (entry 6 in Table 2). The molar ratio of Al/Ni = 400
1 (entry 6 in Table 2). The molar ratio of Al/Ni = 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, both catalytic activity and α-olefin and butuene selectivity are observed greatest at 20 °C (entry 6 in Table 2); and the catalytic system behave a lower activity and worse α-olefin selectivity along with increasing reaction temperature (entries 9–11 in Table 2), such phenomena were also observed by other nickel catalysts.4e,9b,18 Prolonging reaction time (entries 6, 12–13 in Table 2), the activity is slightly decreased meanwhile the amount of hexenes are mainly increased. These results indicate no inducing period of ethylene oligomerization, and the active species are slowly deactivated. Therefore, other nickel procatalysts are investigated at 20 °C with the molar ratio of Al/Ni = 400
1, both catalytic activity and α-olefin and butuene selectivity are observed greatest at 20 °C (entry 6 in Table 2); and the catalytic system behave a lower activity and worse α-olefin selectivity along with increasing reaction temperature (entries 9–11 in Table 2), such phenomena were also observed by other nickel catalysts.4e,9b,18 Prolonging reaction time (entries 6, 12–13 in Table 2), the activity is slightly decreased meanwhile the amount of hexenes are mainly increased. These results indicate no inducing period of ethylene oligomerization, and the active species are slowly deactivated. Therefore, other nickel procatalysts are investigated at 20 °C with the molar ratio of Al/Ni = 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and all catalytic performances are tabulated in Table 3.
1, and all catalytic performances are tabulated in Table 3.
| Entry | Cat. | Activityb | Product distribution (%)c | ||
|---|---|---|---|---|---|
| C4/∑ | α-C4/C4 | C6/∑ | |||
| a Reaction conditions: 5 μmol of Ni; Al/Ni = 400; 10 atm of ethylene; 30 min; 20 °C; 100 mL of toluene. b Activity, 106 g mol−1(Ni) h−1. c Determined by GC. ∑ donates the total amount of oligomers. | |||||
| 1 | Ni1 | 3.7 | 88.5 | 98.0 | 11.5 |
| 2 | Ni2 | 7.1 | 87.0 | 90.1 | 13.0 |
| 3 | Ni3 | 8.7 | 89.6 | >99.0 | 10.4 |
| 4 | Ni4 | 2.4 | 79.3 | >99.0 | 20.7 |
| 5 | Ni5 | 2.5 | 89.1 | 94.4 | 10.9 |
| 6 | Ni6 | 3.1 | 84.5 | 90.2 | 15.5 |
| 7 | Ni7 | 3.4 | 84.5 | 87.1 | 15.5 |
| 8 | Ni8 | 4.1 | 85.8 | 85.6 | 14.2 |
| 9 | Ni9 | 2.5 | 79.5 | 89.7 | 20.5 |
| 10 | Ni10 | 4.0 | 87.7 | 83.1 | 12.3 |
Even though the complex Ni2 is dinuclear as a solid, the active species of all nickel catalysts are generally considered as their monomeric species. Therefore, two series of active species are formed regarding to the differences of their ligands with R1 substituent. Two sets of data are comparable on the base of ligands with R1 substituent (chloro for the nickel complexes Ni1–Ni5 and Ph for the nickel complexes Ni6–Ni10). Their activities decrease in the order of 2,6-di(i-Pr) > 2,6-di(Et) > 2,6-di(Me) > 2,6-di(Et)-4-Me > 2,4,6-tri(Me) with R1 = Cl; meanwhile, with R1 = Ph, the activities decrease in the order of 2,6-di(i-Pr) > 2,6-di(Et) > 2,6-di(Me), and 2,6-di(Et)-4-Me > 2,4,6-tri(Me). Such phenomena are consistent with observations in literature that bulky alkyl substituents help solubility of procatalysts for better activity.18b,19 Interestingly, catalysts Ni4 and Ni9 (R2 and R3 = Me) which showed the lowest activities with producing more hexene. This phenomena were caused by the various substituents of R1 and R2. As shown in Table 3, the activities by Ni6–Ni10 (R1 = Ph, entries 6–10 in Table 3) were much smaller than those by Ni1–Ni5 (R1 = Cl, entries 1–5 in Table 3) due to steric influence of R1 around nickel centre.20
Experimental
General considerations
All manipulations of air and/or moisture sensitive compounds were performed under nitrogen atmosphere using standard Schlenk techniques. All solvents were routinely purified and distilled before use. Methylaluminoxane (MAO, 1.46 M solution in toluene) and modified methylaluminoxane (MMAO, 1.93 M in heptane, 3A) were purchased from Akzo Nobel Corp. Diethylaluminium chloride (Et2AlCl2, 0.79 M in toluene) and ethylaluminium sesquinochroride (EASC, 0.87 M in toluene) were purchased from Beijing Yansan Petrochemical Co. Elemental analysis is conducted on a Flash EA 1112 microanalyzer. IR spectra are recorded on a Perkin-Elmer System 2000 FT-IR spectrometer using KBr discs in the range 4000–400 cm−1. Gas chromatography (GC) analysis was performed with a VARIAN CP-3800 gas chromatograph equipped with a flame ionization detector and a 30 m (0.2 mm i.d., 0.25 μm film thickness).Synthesis and characterisation of complexes Ni1–Ni10
All nickel complexes are prepared in the same manner. In typically synthesizing the complex Ni1, the mixture of 2-chloro-5,6,7-trihydroquinolin-8-one (1.0 mmol), 2,6-dimethylaniline (1.0 mmol) and NiCl2·6H2O (1.0 mmol) in glacial acetic acid (10 mL) is refluxed for 3 h. Acid was removed under reduced pressure, and the residue was dissolved in 10 mL methanol. Unreacted NiCl2 was removed by filtration. 50 mL of diethyl ether was added to precipitate Ni1. After filtrated and washed with diethyl ether (3 × 5 mL), the collected solid was dried under vacuum.
[2,6-Dimethyl-N-(2-chloro-5,6,7-trihydroquinolin-8-ylidene) phenylamino]nickel(II) dichloride (Ni1) was obtained as green powder in 51.1% yield. IR (KBr; cm−1): 3345, 2931, 1567 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1488, 1409, 1341, 1239, 1123, 1028, 866, 783, 675, 616, 502, 403. Anal. calcd for C17H17Cl3N2Ni(414): C, 49.27; H, 4.14; N, 6.76%. Found: C, 49.21; H, 4.41; N, 6.49%. MS-ESI: calcd for C17H17Cl3N2Nim/z 411.9, found m/z 377.0 (M − Cl)+.
N), 1488, 1409, 1341, 1239, 1123, 1028, 866, 783, 675, 616, 502, 403. Anal. calcd for C17H17Cl3N2Ni(414): C, 49.27; H, 4.14; N, 6.76%. Found: C, 49.21; H, 4.41; N, 6.49%. MS-ESI: calcd for C17H17Cl3N2Nim/z 411.9, found m/z 377.0 (M − Cl)+.
[2,6-Diethyl-N-(2-chloro-5,6,7-trihydroquinolin-8-ylidene) phenylamino]nickel(II) dichloride (Ni2) was obtained as green powder in 55.0% yield. IR (KBr; cm−1): 3049, 2963, 2930, 2875, 1581 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1449, 1262, 1237, 1190, 1156, 1124, 864, 817, 781, 676, 641, 521. Anal. calcd for C19H21Cl3N2Ni(442): C, 51.58; H, 4.78; N, 6.33%. Found: C, 51.53; H, 5.12; N, 6.05%. MS-ESI: calcd for C19H21Cl3N2Nim/z 440.0, found m/z 405.0 (M − Cl)+.
N), 1449, 1262, 1237, 1190, 1156, 1124, 864, 817, 781, 676, 641, 521. Anal. calcd for C19H21Cl3N2Ni(442): C, 51.58; H, 4.78; N, 6.33%. Found: C, 51.53; H, 5.12; N, 6.05%. MS-ESI: calcd for C19H21Cl3N2Nim/z 440.0, found m/z 405.0 (M − Cl)+.
[2,6-Bis(1-methylethyl)-N-(2-chloro-5,6,7-trihydroquinolin-8-ylidene)phenylamino]nickel(II) dichloride (Ni3) was obtained as green powder in 62.9% yield. IR (KBr; cm−1): 3350, 2961, 2927, 2867, 1618, 1581 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1453, 1268, 1238, 1189, 1127, 1038, 928, 877, 816, 779, 550. Anal. calcd for C21H25Cl3N2Ni(470): C, 53.61; H, 5.36; N, 5.95%. Found: C, 53.25; H, 5.21; N, 6.03%. MS-ESI: calcd for C21H25Cl3N2Nim/z 468.0, found m/z 433.0 (M − Cl)+.
N), 1453, 1268, 1238, 1189, 1127, 1038, 928, 877, 816, 779, 550. Anal. calcd for C21H25Cl3N2Ni(470): C, 53.61; H, 5.36; N, 5.95%. Found: C, 53.25; H, 5.21; N, 6.03%. MS-ESI: calcd for C21H25Cl3N2Nim/z 468.0, found m/z 433.0 (M − Cl)+.
[2,4,6-Trimethyl-N-(2-chloro-5,6,7-trihydroquinolin-8-ylidene) phenylamino]nickel(II) dichloride (Ni4) was obtained as green powder in 65.2.0% yield. IR (KBr; cm−1): 2923, 1575 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1412, 1235, 1210, 1122, 1035, 1011, 817, 680, 562, 505. Anal. calcd for C18H19Cl3N2Ni(428): C, 50.46; H, 4.47; N, 6.54%. Found: C, 50.34; H, 4.76; N, 6.91%. MS-ESI: calcd for C18H19Cl3N2Nim/z 426.0, found m/z 391.0 (M − Cl)+.
N), 1412, 1235, 1210, 1122, 1035, 1011, 817, 680, 562, 505. Anal. calcd for C18H19Cl3N2Ni(428): C, 50.46; H, 4.47; N, 6.54%. Found: C, 50.34; H, 4.76; N, 6.91%. MS-ESI: calcd for C18H19Cl3N2Nim/z 426.0, found m/z 391.0 (M − Cl)+.
[2,6-Diethyl-4-methyl-N-(2-chloro-5,6,7-trihydroquinolin-8-ylidene)phenylamino]nickel(II) dichloride (Ni5) was obtained as green powder in 71.0% yield. IR (KBr; cm−1): 2956, 2933, 2872, 2854, 1626, 1578 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1444, 1341, 1243, 1142, 1121, 922, 858, 646, 501. Anal. calcd for C20H23Cl3N2Ni(456): C, 52.63; H, 5.08; N, 6.14%. Found: C, 52.38; H, 5.29; N, 6.02%. MS-ESI: calcd for C20H23Cl3N2Nim/z 454.0, found m/z 419.0 (M − Cl)+.
N), 1444, 1341, 1243, 1142, 1121, 922, 858, 646, 501. Anal. calcd for C20H23Cl3N2Ni(456): C, 52.63; H, 5.08; N, 6.14%. Found: C, 52.38; H, 5.29; N, 6.02%. MS-ESI: calcd for C20H23Cl3N2Nim/z 454.0, found m/z 419.0 (M − Cl)+.
[2,6-Dimethyl-N-(2-phenyl-5,6,7-trihydroquinolin-8-ylidene) phenylamino]nickel(II) dichloride (Ni6) was obtained as green powder in 60.5% yield. IR (KBr; cm−1): 3272, 2928, 1558 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1401, 1341, 1162, 1122, 1034, 1009, 815, 763, 679, 614, 482. Anal. calcd for C23H22Cl2N2Ni(456): C, 60.58; H, 4.86; N, 6.14%. Found: 60.76; H, 5.02; N, 6.33%. MS-ESI: calcd for C23H22Cl2N2Nim/z 454.0, found m/z 419.0 (M − Cl)+.
N), 1401, 1341, 1162, 1122, 1034, 1009, 815, 763, 679, 614, 482. Anal. calcd for C23H22Cl2N2Ni(456): C, 60.58; H, 4.86; N, 6.14%. Found: 60.76; H, 5.02; N, 6.33%. MS-ESI: calcd for C23H22Cl2N2Nim/z 454.0, found m/z 419.0 (M − Cl)+.
[2,6-Diethyl-N-(2-phenyl-5,6,7-trihydroquinolin-8-ylidene) phenylamino]nickel(II) dichloride (Ni7) was obtained as green powder in 66.9% yield. IR (KBr; cm−1): 3272, 2933, 1555 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1400, 1341, 1166, 1122, 1034, 1009, 679, 614, 494. Anal. calcd for C25H26Cl2N2Ni(484): C, 62.03; H, 5.41; N, 5.79%. Found: 62.21; H, 5.64; N, 5.91%. MS-ESI: calcd for C25H26Cl2N2Nim/z 482.0, found m/z 447.1 (M − Cl)+.
N), 1400, 1341, 1166, 1122, 1034, 1009, 679, 614, 494. Anal. calcd for C25H26Cl2N2Ni(484): C, 62.03; H, 5.41; N, 5.79%. Found: 62.21; H, 5.64; N, 5.91%. MS-ESI: calcd for C25H26Cl2N2Nim/z 482.0, found m/z 447.1 (M − Cl)+.
[2,6-Bis(1-methylethyl)-N-(2-phenyl-5,6,7-trihydroquinolin-8-ylidene)phenylamino]nickel(II) dichloride (Ni8) was obtained as green powder in 71.0% yield. IR (KBr; cm−1): 3358, 2969, 1557 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1406, 1341, 1027, 762, 677, 616, 508, 403. Anal. calcd for C27H30Cl2N2Ni(442): C, 63.32; H, 5.90; N, 5.47%. Found: C, 63.63; H, 5.97; N, 5.54%. MS-ESI: calcd for C27H30Cl2N2Nim/z 510.1, found m/z 475.1.1 (M − Cl)+.
N), 1406, 1341, 1027, 762, 677, 616, 508, 403. Anal. calcd for C27H30Cl2N2Ni(442): C, 63.32; H, 5.90; N, 5.47%. Found: C, 63.63; H, 5.97; N, 5.54%. MS-ESI: calcd for C27H30Cl2N2Nim/z 510.1, found m/z 475.1.1 (M − Cl)+.
[2,4,6-Trimethyl-N-(2-phenyl-5,6,7-trihydroquinolin-8-ylidene)phenylamino]nickel(II) dichloride (Ni9) was obtained as green powder in 78.0% yield. IR (KBr; cm−1): 2247, 2927, 1566 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1406, 1342, 1214, 1124, 1034, 1010, 681, 615, 480. Anal. calcd for C24H24Cl2N2Ni(470): C, 61.32; H, 5.15; N, 5.96%. Found: C, 61.44; H, 5.39; N, 6.20%. MS-ESI: calcd for C24H24Cl2N2Nim/z 468.0, found m/z 433.1 (M − Cl)+.
N), 1406, 1342, 1214, 1124, 1034, 1010, 681, 615, 480. Anal. calcd for C24H24Cl2N2Ni(470): C, 61.32; H, 5.15; N, 5.96%. Found: C, 61.44; H, 5.39; N, 6.20%. MS-ESI: calcd for C24H24Cl2N2Nim/z 468.0, found m/z 433.1 (M − Cl)+.
[2,6-Diethyl-4-methyl-N-(2-phenyl-5,6,7-trihydroquinolin-8-ylidene)phenylamino]nickel(II) dichloride (Ni10) was obtained as green powder in 81.6% yield. IR (KBr; cm−1): 3305, 2601, 1567 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1417, 1344, 1234, 1120, 1033, 764, 682, 504. Anal. calcd for C26H28Cl2N2Ni(498): C, 62.69, H, 5.67; N, 5.62%. Found: C, 62.76, H, 5.54; N, 5.69%. MS-ESI: calcd for C26H28Cl2N2Nim/z 496.1, found m/z 461.1 (M − Cl)+.
N), 1417, 1344, 1234, 1120, 1033, 764, 682, 504. Anal. calcd for C26H28Cl2N2Ni(498): C, 62.69, H, 5.67; N, 5.62%. Found: C, 62.76, H, 5.54; N, 5.69%. MS-ESI: calcd for C26H28Cl2N2Nim/z 496.1, found m/z 461.1 (M − Cl)+.
General procedure for ethylene oligomerization at 10 atm of ethylene pressure
Ethylene oligomerization was performed in a stainless steel autoclave (300 mL capacity) equipped with gas ballast through a solenoid clave for continuous feeding of ethylene at constant pressure. 50 mL of toluene was added into the autoclave under ethylene atmosphere. The catalyst was dissolved in 20 mL toluene in a Schlenk tube with stirring. When the desired reaction temperature was reached, the catalyst dissolved in 20 mL toluene, the desired amount of cocatalyst and the remained toluene (total volume was 100 mL) were added in turn by syringes. Ethylene at the desired pressure was introduced to start the reaction. After stirred for the desired period of time, the reaction was stopped with ceasing the ethylene inputting. The autoclave was cooled in an ice-water bath, and then the pressure was released. 2 mL the reaction solution was collected and terminated by addition of 4 mL 10% aqueous hydrogen chloride. The organic was collected and analyzed by gas chromatography (GC) to determine the composition and mass distribution of the oligomers. The remaining reaction solution was quenched with 5% hydrogen chloride ethanol.X-Ray crystallographic studies
Single crystals of Ni2, Ni3·CH22CH33OH, Ni8 and Ni10 suitable for X-ray diffraction analysis were obtained by laying diethyl ether on their ethanol solution at room temperature. With graphite-monochromatic Mo Ka radiation (k = 0.71073 Å) at 173(2) K, cell parameters were obtained by global refinement of the positions of all collected reflections. Intensities were corrected for Lorentz and polarization effects and empirical absorption. The structures were solved by direct methods and refined by full-matrix least squares on F2. All hydrogen atoms were placed in calculated positions. Structure solution and refinement were performed by using the SHELXL-97 package.21 Details of the X-ray structure determinations and refinements are provided in Table 4. Crystallographic data have been deposited with the Cambridge Crystallographic Data Centre, CCDC 779268 (Ni2), 779269 (Ni3·CH22CH33OH), 779270 (Ni8) and 779271 (Ni10). For crystallographic data in CIF or other electronic format see DOI: 10.1039/c0nj00516a| 2Ni2 | Ni3·CH22CH33OH | Ni8 | Ni10 | |
|---|---|---|---|---|
| Empirical formula | C38H42Cl6N4Ni2 | C23H31Cl3N2NiO | C27H30Cl2N2Ni | C26H28Cl2N2Ni |
| Formula weight | 884.88 | 516.56 | 512.14 | 498.11 |
| T/K | 173(2) | 173(2) | 293(2) | 173(2) |
| Wavelength/Å | 0.71073 | 0.71073 | 0.71073 | 0.71073 |
| Crystal system | Monoclinic | Monoclinic | Orthorhombic | Monoclinic |
| Space group | P21/n | P21/c | Pbca | C2/c |
| a/Å | 9.4118(19) | 11.597(2) | 15.896(3) | 31.488(6) |
| b/Å | 18.517(4) | 17.402(4) | 16.770(3) | 10.033(2) |
| c/Å | 11.063(2) | 12.326(3) | 18.790(4) | 15.549(3) |
| α (°) | 90 | 90 | 90 | 90 |
| β (°) | 93.02(3) | 95.83(3) | 90 | 102.20(3) |
| γ (°) | 90 | 90 | 90 | 90 |
| V/Å3 | 1925.3(2) | 2450.9(9) | 5009.0(18) | 4801.2(17) |
| Z | 2 | 4 | 8 | 8 |
| D calcd/g cm−3 | 1.526 | 1.400 | 1.358 | 1.378 |
| μ/mm−1 | 1.428 | 1.136 | 1.005 | 1.047 |
| F(000) | 912 | 1080 | 2144 | 2080 |
| Crystal size/mm | 0.30 × 0.20 × 0.04 | 0.30 × 0.13 × 0.08 | 0.20 × 0.24 × 0.10 | 0.17 × 0.13 × 0.07 |
| θ range [°] | 2.15–27.47 | 1. 78–27.42 | 2.07–27.44 | 2.13–25.50 |
| Limiting indices | −12 ≤ h ≤ 11 | −15 ≤ h ≤ 12 | −18 ≤ h ≤ 20 | −38 ≤ h ≤ 38 |
| −24 ≤ k ≤ 19 | −22 ≤ k ≤ 22 | −21 ≤ h ≤ 17 | −12 ≤ k ≤ 12 | |
| −14 ≤ l ≤ 14 | −12 ≤ l ≤ 15 | −24 ≤ h ≤ 24 | −18 ≤ l ≤ 18 | |
| No. of rflns collected | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 557 557 |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 496 496 |
37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 551 551 |
27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 177 177 |
| No. unique rflns [R(int)] | 4403 (0.0672) | 5525 (0.0983) | 5709(0.0906) | 4466(0.0755) |
| No. of params | 262 | 303 | 289 | 327 |
| Completeness to θ [%] | 99.8% | 99.1% | 99.8% | 99.9% |
| Goodness of fit on F2 | 1.347 | 1.412 | 1.463 | 1.377 |
| Final R indices [I > 2∑(I)] | R 1 = 0.0845 | R 1 = 0.1587 | R 1 = 0.1399 | R 1 = 0.0908 |
| wR2 = 0.1972 | wR2 = 0.3174 | wR2 = 0.2849 | wR2 = 0.1570 | |
| R indices (all data) | R 1 = 0.0966 | R 1 = 0.1804 | R 1 = 0.1515 | R 1 = 0.0959 |
| wR2 = 0.2036 | wR2 = 0.3330 | wR2 = 0.2953 | wR2 = 0.1591 | |
| Largest diff peak and hole/e Å−3 | 0.683 and −0.829 | 0.630 and −0.620 | 0.559 and −0.871 | 0.400 and −0.327 |
Conclusions
The molecular structures of nickel complexes revealed a four-coordination number around nickel with 2-phenyl substituted ligands, and five-coordination number around nickel with 2-chloro substituted ligands. All title nickel catalysts perform high activities in ethylene oligomerization with high selectivity of butenes and hexenes; and catalysts bearing 2-chloro substituted ligands generally show better activity than their analogues ligating 2-Ph substituted ligands.Acknowledgements
This work was supported by MOST 863 program No. 2009AA033601.References
- (a) W. Keim, F. H. Kowaldt, R. Goddard and C. Kruger, Angew. Chem., Int. Ed. Engl., 1978, 17, 466–467 CrossRef; (b) W. Keim, A. Behr, B. Limbäcker and C. Krüger, Angew. Chem., Int. Ed. Engl., 1983, 22, 503–503 CrossRef.
- L. K. Johnson, C. M. Killian and M. Brookhart, J. Am. Chem. Soc., 1995, 117, 6414–6415 CrossRef CAS.
- T. R. Younkin, E. F. Conner, J. I. Henderson, S. K. Friedrich, R. H. Grubbs and D. A. Bansleben, Science, 2000, 287, 460–462 CrossRef CAS.
- (a) C. M. Killian, L. K. Johnson and M. Brookhart, Organometallics, 1997, 16, 2005–2007 CrossRef CAS; (b) Z. L. Li, W. H. Sun, Z. Ma, Y. L. Hu and C. X. Shao, Chin. Chem. Lett., 2001, 12, 691–692 CAS; (c) E. Nelkenbaum, M. Kapon and M. S. Eisen, J. Organomet. Chem., 2005, 690, 2297–2305 CrossRef CAS; (d) C. Zhang, W.-H. Sun and Z.-X. Wang, Eur. J. Inorg. Chem., 2006, 4895–4902 CrossRef CAS; (e) P. Hao, S. Zhang, W.-H. Sun, Q. Shi, S. Adewuyi, X. Lu and P. Li, Organometallics, 2007, 26, 2439–2446 CrossRef CAS; (f) P. Yang, Y. Yang, C. Zhang, X.-J. Yang, H.-M. Hu, Y. Gao and B. Wu, Inorg. Chim. Acta, 2009, 362, 89–96 CrossRef CAS; (g) F.-Z. Yang, Y.-C. Chen, Y.-F. Lin, K.-H. Yu, Y.-H. Liu, Y. Wang, S.-T. Liu and J.-T. Chen, Dalton Trans., 2009, 1243–1250 RSC; (h) M. Zhang, T. Xiao and W.-H. Sun, Acta Polym. Sin., 2009, 9, 600–612 Search PubMed; (i) L. Wang, C. Zhang and Z.-X. Wang, Eur. J. Inorg. Chem., 2007, 2477–2487 CrossRef CAS.
- (a) T. V. Laine, K. Lappalainen, J. Liimatta, E. Aitola, B. Lofgren and M. Leskela, Macromol. Rapid Commun., 1999, 20, 487–491 CrossRef CAS; (b) T. V. Laine, U. Piironen, K. Lappalainen, M. Klinga, E. Aitola and M. Leskelä, J. Organomet. Chem., 2000, 606, 112–124 CrossRef CAS; (c) B. Lee, X. Bu and G. C. Bazan, Organometallics, 2001, 20, 5425–5431 CrossRef CAS; (d) S. Jie, D. Zhang, T. Zhang, W.-H. Sun, J. Chen, Q. Ren, D. Liu, G. Zheng and W. Chen, J. Organomet. Chem., 2005, 690, 1739–1749 CrossRef CAS; (e) X. Tang, W.-H. Sun, T. Gao, J. Hou, J. Chen and W. Chen, J. Organomet. Chem., 2005, 690, 1570–1580 CrossRef CAS; (f) F.-S. Liu, H.-Y. Gao, K.-M. Song, L. Zhang, F.-M. Zhu and Q. Wu, Polyhedron, 2009, 28, 1386–1392 CrossRef CAS.
- (a) J. Pietsch, P. Braunstein and Y. Chauvin, New J. Chem., 1998, 22, 467–472 RSC; (b) W.-H. Sun, Z. Li, H. Hu, B. Wu, H. Yang, N. Zhu, X. Leng and H. Wang, New J. Chem., 2002, 26, 1474–1478 RSC; (c) W. Keim, S. Killat, C. F. Nobile, G. P. Suranna, U. Englert, R. Wang, S. Mecking and D. L. Schröder, J. Organomet. Chem., 2002, 662, 150–171 CrossRef CAS; (d) Z. Weng, S. Teo and T. S. A. Hor, Organometallics, 2006, 25, 4878–4882 CrossRef CAS; (e) P. W. Dyer, J. Fawcett and M. J. Hanton, Organometallics, 2008, 27, 5082–5087 CrossRef CAS.
- (a) C. Wang, S. Friedrich, T. R. Younkin, R. T. Li, R. H. Grubbs, D. A. Bansleben and M. W. Day, Organometallics, 1998, 17, 3149–3151 CrossRef CAS; (b) L. Wang, W.-H. Sun, L. Han, Z. Li, Y. Hu, C. He and C. Yan, J. Organomet. Chem., 2002, 650, 59–64 CrossRef CAS; (c) T. Hu, L.-M. Tang, X.-F. Li, Y.-S. Li and N.-H. Hu, Organometallics, 2005, 24, 2628–2632 CrossRef CAS; (d) J. Hou, W.-H. Sun, D. Zhang, L. Chen, W. Li, D. Zhao and H. Song, J. Mol. Catal. A: Chem., 2005, 231, 221–233 CrossRef CAS; (e) Q. Shi, S. Zhang, F. Chang, P. Hao and W.-H. Sun, C. R. Chim., 2007, 10, 1200–1208 CrossRef CAS; (f) A. Kermagoret and P. Braunstein, Dalton Trans., 2008, 1564–1573 RSC.
- (a) W. Keim, Angew. Chem., Int. Ed. Engl., 1990, 29, 235–244 CrossRef; (b) J. Heinicke, M. He, A. Dal, H. Klein, O. Hetche, W. Keim, U. Flörke and H. Haupt, Eur. J. Inorg. Chem., 2000, 431–440 CrossRef CAS; (c) J. Heinicke, M. Köhler, N. Peulecke and W. Keim, J. Catal., 2004, 225, 16–23 CrossRef; (d) C.-Y. Guo, N. Peulecke, K. R. Basvani, M. K. Kinderman and J. Heinicke, Macromolecules, 2010, 43, 1416–1424 CrossRef CAS; (e) C.-Y. Guo, N. Peulecke, K. R. Basvani, M. K. Kinderman and J. Heinicke, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 258–266 CrossRef CAS.
- (a) L. Wang, W.-H. Sun, L. Han, H. Yang, Y. Hu and X. Jin, J. Organomet. Chem., 2002, 658, 62–70 CrossRef CAS; (b) F. A. Kunrath, R. F. de Souza, O. L. Casagrande, Jr., N. R. Brooks and V. G. Young, Jr., Organometallics, 2003, 22, 4739–4743 CrossRef CAS; (c) N. Ajellal, M. C. A. Kuhn, A. D. G. Boff, M. Hörner, C. M. Thomas, J. Carpentier and O. L. Casagrande, Jr., Organometallics, 2006, 25, 1213–1216 CrossRef CAS; (d) S. Adewuyi, G. Li, S. Zhang, W. Wang, P. Hao, W.-H. Sun, N. Tang and J. Yi, J. Organomet. Chem., 2007, 692, 3532–3541 CrossRef CAS.
- B. Su, J. Zhao, Q. Zhang and W. Qin, Polym. Int., 2009, 58, 1051–1057 CrossRef CAS.
- J. Hou, W.-H. Sun, S. Zhang, H. Ma, Y. Deng and X. Lu, Organometallics, 2006, 25, 236–244 CrossRef CAS.
- Y. Xie, H. Huang, W. Mo, X. Fan, Z. Shen, Z. Shen, N. Sun, B. Hu and X. Hu, Tetrahedron: Asymmetry, 2009, 20, 1425–1432 CrossRef CAS.
- F. Lions and K. Martin, J. Am. Chem. Soc., 1957, 79, 2733–2738 CrossRef CAS.
- (a) M. Gasperini, F. Ragaini and S. Cenini, Organometallics, 2002, 21, 2950–2957 CrossRef CAS; (b) M. A. Esteruelas, A. M. López, L. Méndez, M. Oliván and E. Oñate, Organometallics, 2003, 22, 395–406 CrossRef CAS; (c) C.-L. Song, L.-M. Tang, Y.-G. Li, X.-F. Li, J. Chen and Y.-D. Li, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 1964–1974 CrossRef CAS.
- (a) R. J. Butcher and E. Sinn, Inorg. Chem., 1977, 16, 2334–2343 CrossRef CAS; (b) G. J. P. Britovsek, S. P. D. Baugh, O. Hoarau, V. C. Gibson, D. F. Wass, A. J. P. White and D. J. Williams, Inorg. Chim. Acta, 2003, 345, 279–291 CrossRef CAS; (c) F. Speiser, P. Braunstein, L. Saussine and R. Welter, Inorg. Chem., 2004, 43, 1649–1658 CrossRef CAS; (d) W.-H. Sun, K. Wang, K. Wedeking, D. Zhang, S. Zhang, J. Cai and Y. Li, Organometallics, 2007, 26, 4781–4790 CrossRef CAS; (e) P. Chavez, I. Rios, A. Kermagoret, R. Pattacini, A. Meli, C. Bianchini, G. Giambastiani and P. Braunstein, Organometallics, 2009, 28, 1776–1784 CrossRef CAS.
- T. V. Laine, M. Klinga and M. Leskelä, Eur. J. Inorg. Chem., 1999, 959–964 CrossRef CAS.
- B. Bahuleyan, U. Lee, C. Ha and I. Kim, Appl. Catal., A, 2008, 351, 36–44 CrossRef CAS.
- (a) W.-H. Sun, S. Zhang, S. Jie, W. Zhang, Y. Li, H. Ma, J. Chen, K. Wedeking and R. Fröhlich, J. Organomet. Chem., 2006, 691, 4196–4203 CrossRef CAS; (b) R. Gao, M. Zhang, T. Liang, F. Wang and W.-H. Sun, Organometallics, 2008, 27, 5641–5648 CrossRef CAS.
- (a) G. J. P. Britovsek, S. Mastroianni, G. A. Solan, S. P. D. Baugh, C. Redshaw, V. C. Gibson, A. J. P. White, D. J. Williams and M. R. J. Elsegood, Chem.–Eur. J., 2000, 6, 2221–2231 CrossRef CAS; (b) W.-H. Sun, P. Hao, S. Zhang, Q. Shi, W. Zuo, X. Tang and X. Lu, Organometallics, 2007, 26, 2720–2734 CrossRef CAS.
- (a) W.-H. Sun, S. Jie, S. Zhang, W. Zhang, Y. Song, H. Ma, J. Chen and K. Wedeking. R. Fröhlich, Organometallics, 2006, 25, 666–677 CrossRef CAS; (b) S. Jie, S. Zhang and W.-H. Sun, Eur. J. Inorg. Chem., 2007, 5584–5598 CrossRef CAS; (c) M. Zhang, R. Gao, X. Hao and W.-H. Sun, J. Organomet. Chem., 2008, 693, 3867–3877 CrossRef CAS.
- G. M. Sheldrick, SHELXL-97, Program for the Refinement of Crystal Structures, University of Göttingen, Germany, 1997 Search PubMed.
Footnote |
| † CCDC reference numbers 779268–779271. For crystallographic data in CIF or other electronic format see DOI: 10.1039/c0nj00516a |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2011 |
