DOI:
10.1039/C0MT00062K
(Communication)
Metallomics, 2011,
3, 42-48
Synthesis, structure and biological evaluation of bis salicylaldehyde-4(N)-ethylthiosemicarbazone ruthenium(III) triphenylphosphine†
Received
22nd October 2010
, Accepted 26th November 2010
First published on 6th December 2010
Abstract
Bis salicylaldehyde-4(N)-ethylthiosemicarbazone ruthenium(III) triphenylphosphine [Ru(Sal-etsc)(H-Sal-etsc)(PPh3)] was synthesized and structurally characterized by spectral and X-ray crystallographic studies and it showed 100% inhibition on the DPPH radical. It also exhibited a significant lymphocyte activity and inhibitory effect on the lung carcinoma A549 cell.
1. Introduction
The search for novel compounds that are able to prevent cancer cell proliferation is on going as much current chemotherapy remains inefficient against common and aggressive tumors e.g., brain cancer, melanoma, breast, prostate and lung cancer. Another major problem is drug resistance of certain cancer to traditional chemotherapeutics, many of which target DNA. This challenge calls for new strategies and drugs that take advantage of novel molecular targets that are key components of cellular metabolism and replication. A wide range of biological activities possessed by substituted thiosemicarbazones and their metal complexes include cytotoxic, antitumor and antileukemic properties.1–9 Thiosemicarbazones are of interest not only because of their biological relevancies but also due to their structural consequences. Usually, they bind to the metal ions either in the neutral thione form or in the negative thiolate form as a bidentate N,S donor by forming either an unusual four member NS chelate ring or an expected five member ring by utilizing either the N2 or N1 hydrazinic nitrogen atom.10–21 The incorporation of a third potential donor atom to the thiosemicarbazone ligands should increase their versatility and flexibility in the coordination behavior.10–13,19,22–34 From the literature, it has been found that there are two factors which are probably responsible for the formation of unusual four member NS chelation of thiosemicarbazone in ruthenium complexes.10–13,19 One is the intramolecular hydrogen bonding between the phenolic hydrogen and the imine nitrogen which reduces the availability of the lone pair on the imine nitrogen and thus prevents coordination from it. The second is the steric hindrance created by the bulky triphenylphosphine present in the starting complex. In addition, the size of group which is attached to the azomethine carbon atom of the thiosemicarbazone also plays an important role in determining its coordination behavior.10–14 Though few reasons have been offered for the unusual coordination behaviour of thiosemicarbazones, no one has explained clearly about their bonding behaviour. In order to find out the exact force which drives the coordination behaviour of thiosemicarbazones, we have carried out a number of reactions of various thiosemicarbazones with nickel, palladium and ruthenium complexes.25–28 In all these cases, we have obtained only ONS coordinated complexes irrespective of the size of the group which is attached to the thiosemicarbazones. To get more insight in this area, we reacted salicylaldehyde-4(N)-ethylthiosemicarbazone with [RuCl3(PPh3)3]. We obtained a complex with two units of thiosemicarbazones, one which formed a five and six member ring by behaving as an ONS tridentate bibasic ligand while the other formed a five membered NS chelate leaving the third potential donor site phenolic oxygen uncoordinated. From this, it is inferred that the intramolecular hydrogen bonding, the bulkiness of the co-ligands and the size of substituent on thiosemicarbazone are not the only responsible factors for the unusual coordination behaviour of thiosemicarbazones. Hence, it is anticipated that some other factors may be responsible for the observed behaviour of thiosemicarbazones. Though there are many reports proving the potential biological activities of ruthenium(II) complexes in various studies, the study on ruthenium(III) is limited. Hence, the d7Ru(III) complexes have been taken for studying the biological activity with the idea that they can undergo reduction to d6Ru(II) (like Cu(II) to Cu(I) reduction in a number of biological sites) which is the next common and stable oxidation state. This d6 complex is expected to behave like the d6Fe(II) analogue in biological systems. As a part of our systematic investigations, herein we report the synthesis, characterization, electrochemistry and biological activities of [Ru(Sal-etsc)(H-Sal-etsc)(PPh3)].
2. Results and discussion
Reaction of [RuCl3(PPh3)3] with [H2-Sal-etsc] in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio in benzene leads to a brown solution consisting of a stable dark brown [Ru(Sal-etsc)(H-Sal-etsc)(PPh3)] together with unreacted [RuCl3(PPh3)3] and triphenylphosphine oxide. It is to be noted that even when the reaction was carried out in 1
1 molar ratio in benzene leads to a brown solution consisting of a stable dark brown [Ru(Sal-etsc)(H-Sal-etsc)(PPh3)] together with unreacted [RuCl3(PPh3)3] and triphenylphosphine oxide. It is to be noted that even when the reaction was carried out in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio it afforded [Ru(Sal-etsc)(H-Sal-etsc)(PPh3)] in decent yield (Scheme 1).
2 molar ratio it afforded [Ru(Sal-etsc)(H-Sal-etsc)(PPh3)] in decent yield (Scheme 1).
![Preparation of [Ru(Sal-etsc)(H-Sal-etsc)(PPh3)].](/image/article/2011/MT/c0mt00062k/c0mt00062k-s1.gif) |
| | Scheme 1 Preparation of [Ru(Sal-etsc)(H-Sal-etsc)(PPh3)]. | |
Table 1
Crystal data and structure refinement for [Ru(Sal-etsc)(H-Sal-etsc)PPh3]·2DMF
| Empirical formula: |
C44H52N8O4PRuS2 |
| Moiety formula: |
C38H38N6O2PRuS2·2(C3H7NO) |
| Formula weight: |
953.10 |
| Temperature: |
100(2) K |
| Wavelength: |
0.71073 Å |
| Crystal system: |
Monoclinic |
| Space group: |
P21/c |
| Unit cell dimensions: |
|
| |
a = 25.792(3) Å, α = 90° |
| |
b = 8.6974(9) Å, β = 111.142(2)° |
| |
c = 21.213(2) Å, γ = 90° |
| Volume, Z: |
4438.1(8) Å3, 4 |
| Density (calculated): |
1.426 Mg m−3 |
| Absorption coefficient: |
0.535 mm−1 |
|
F(000): |
1980 |
| Crystal size: |
0.46 × 0.26 × 0.02 mm |
| Crystal shape, colour: |
platelet, red |
|
θ range for data collection: |
0.85 to 28.28° |
| Limiting indices: |
−34 ≤ h ≤ 33, −11 ≤ k ≤ 11, −27 ≤ l ≤ 28 |
| Reflections collected: |
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 654 654 |
| Independent reflections: |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (R(int) = 0.0666) 000 (R(int) = 0.0666) |
| Completeness to θ = |
28.28°: 99.7% |
| Absorption correction: |
multi-scan |
| Max. and min. transmission: |
1.0 and 0.525 |
| Refinement method: |
Full-matrix least-squares on F2 |
| Data/restraints/parameters: |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000/57/601 000/57/601 |
| Goodness-of-fit on F2: |
1.203 |
| Final R indices [I > 2σ(I)]: |
R
1 = 0.0908, wR2 = 0.1760 |
|
R indices (all data): |
R
1 = 0.1212, wR2 = 0.1875 |
| Largest diff. peak and hole: |
1.364 and −1.526 e × Å−3 |
Table 2 Selected bond lengths (Å) and angles (°) of [Ru(Sal-etsc)(H-Sal-etsc)PPh3]
| N(1)–Ru(1) |
2.0795(5) |
| N(4)–Ru(1) |
2.1075(5) |
| O(1)–Ru(1) |
2.0334(4) |
| P(1)–Ru(1) |
2.3270(14) |
| Ru(1)–S(2) |
2.2743(16) |
| Ru(1)–S(1) |
2.3581(14) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
| O(1)–Ru(1)–N(1) |
89.01(17) |
| O(1)–Ru(1)–N(4) |
83.70(16) |
| N(1)–Ru(1)–N(4) |
92.40(18) |
| O(1)–Ru(1)–S(2) |
98.48(13) |
| N(1)–Ru(1)–S(2) |
169.97(13) |
| N(4)–Ru(1)–S(2) |
81.88(13) |
| O(1)–Ru(1)–P(1) |
93.95(11) |
| N(1)–Ru(1)–P(1) |
91.22(13) |
| N(4)–Ru(1)–P(1) |
175.66(14) |
| S(2)–Ru(1)–P(1) |
94.89(6) |
| O(1)–Ru(1)–S(1) |
166.75(12) |
| N(1)–Ru(1)–S(1) |
80.27(13) |
| N(4)–Ru(1)–S(1) |
88.95(13) |
| S(2)–Ru(1)–S(1) |
91.35(6) |
| P(1)–Ru(1)–S(1) |
94.03(5) |
![Structure of [Ru(Sal-etsc)(H-Sal-etsc)(PPh3)].](/image/article/2011/MT/c0mt00062k/c0mt00062k-f2.gif) |
| | Fig. 2 Structure of [Ru(Sal-etsc)(H-Sal-etsc)(PPh3)]. | |
Table 3
Hydrogen bonds for [Ru(Sal-etsc)(H-Sal-etsc)PPh3] [Å and °]
| D–H⋯A |
d(D–H) |
d(H⋯A) |
d(D⋯A) |
∠(DHA)
|
| Symmetry transformations used to generate equivalent atoms: #1 x, y − 1, z; #2 x, y + 1, z. |
|
N(3A)–H(3A)⋯O(4A) |
0.88 |
2.06 |
2.923(19) |
167.9 |
|
N(3B)–H(3B)⋯O(4B) |
0.88 |
2.27 |
2.96(5) |
135.0 |
| N(6)–H(6)⋯N(2)#1 |
0.88 |
2.16 |
3.038(7) |
174.8 |
| O(2)–H(2A)⋯O(3A)#2 |
0.84 |
1.83 |
2.669(14) |
177.7 |
| O(2)–H(2A)⋯O(3B)#2 |
0.84 |
1.85 |
2.68(7) |
167.1 |
As suggested before, the non-participation of the phenolic oxygen from coordination in one of the thiosemicarbazone units may not be due to either intramolecular hydrogen bonding or due to the size of the substituent in the ligand or due to the bulkiness of the co-ligand. With the data available, it is believed that the non-participation of the phenolic oxygen from coordination and non-substitution of the last triphenylphosphine from the starting complex may be due to the back bonding effect: It is very difficult to replace the last triphenylphosphine ligand from the metal complex because of the very strong back bonding between the metal and phosphorus atom of triphenylphosphine.
Though the formation a stable five membered ring is stated as an impossible mode of coordination by Bhattacharya et al.,38 in our case, two units of thiosemicarbazone coordinated to the metal by forming not only a more stable five member ring but also a six member ring. Formation of a five member ring is very common and possible by the rotation of the C–N single bond of the thiosemicarbazone ligand followed by the tautomerisation to the thiol form and dissociation of the thiolate proton prior to coordination. It is to be noted that Bhattacharya et al. have also recently reported ONS coordination of thiosemicarbazones without mentioning their earlier arguments.39 Though many reasons can be stated as responsible for the versatile coordination behaviour of salicylaldehyde-4(N)-ethylthiosemicarbazone, no one undisputedly explains it. There may be some other factors which influenced the coordination behaviour of thiosemicarbazone ligand or it may be due to thiosemicarbazone itself. A systematic study is underway to find out the factors that drive the coordination mode of thiosemicarbazone ligands by using various thiosemicarbazone ligands having different substitutions with different transition metals with a different geometry.
Electrochemistry
An electrochemical investigation of the complex has been done with cyclic voltammetry in dichloromethane which showed a quasi- reversible oxidation at 0.602 V for Ru(III)–Ru(IV) and a reversible reduction at −0.690 V for Ru(III)–Ru(II) with the peak to peak separation of 545 mV and 45 mV.40 Moreover, the potential difference between the two successive oxidation processes is ≈1.5 V which agrees well with the average potential difference between the redox processes of the ruthenium center (RuII/III–RuIII/IV ≈ 1.0–1.5 V) observed for other mononuclear complexes.41 A quasi- reversible ligand oxidation at 0.98 V with a peak to peak separation of −520 mV and an irreversible reduction at −1.65 V were also exhibited by the coordinated thiosemicarbazone (Fig. 3).
DPPH (1,1-diphenyl-2-picryl-hydrazyl) radical scavenging assay
The free radical scavenging activity of [Ru(Sal-etsc)(H-Sal-etsc)(PPh3)] was tested by its ability to bleach the stable radical DPPH. This assay provided information on the reactivity of the compound with a stable free radical. Because of the odd electron, DPPH shows a strong absorption band at 517 nm in the visible spectrum.42 As this electron becomes paired off in the presence of a free radical scavenger, the absorption vanishes, and the resulting decolonization is stoichiometric with respect to the number of electrons taken up. The ruthenium complex exhibited an excellent DPPH radical-scavenging effect higher than that of well established vitamin E, a reference drug established elsewhere.43,44 The free radical scavenging activity of the complex increased with an increase in the concentration of the complex and 100% activity was shown at 350 μg ml−1 of the complex (Fig. 4) which is an encouraging sign for this complex to be a good anti-cancer drug. The observed antioxidant activity of the ruthenium complex may be due to the neutralization of the free radical character of DPPH either by transfer of an electron or a hydrogen atom.45 This can also be due to the inhibition of lipid peroxidation as has been indicated earlier.46
![DPPH scavenging activity of [Ru(Sal-etsc)(H-Sal-etsc)(PPh3)].](/image/article/2011/MT/c0mt00062k/c0mt00062k-f4.gif) |
| | Fig. 4
DPPH scavenging activity of [Ru(Sal-etsc)(H-Sal-etsc)(PPh3)]. | |
Antiproliferative activity studies on human lymphocyte
The encouraging results obtained from the free radical scavenging studies prompted us to carry out antiproliferative studies. The antiproliferative activity of [Ru(Sal-etsc)(H-Sal-etsc)(PPh3)] was determined by using the mitogen activated human lymphocytes. The mitotic metaphase stage cells were scored to calculate the Mitotic Index (Fig. 5). Table 4 summarizes the Mitotic Index observed in the control and experiments. No statistically significant reduction in Mitotic Index was observed in all concentrations of [H2-Sal-etsc]. But, [Ru(Sal-etsc)(H-Sal-etsc)(PPh3)] showed a decrease in Mitotic Index even in low concentrations (5 and 15 μM mL−1). A statistically significant (P < 0.05) decrease in Mitotic Index was observed at high concentrations (25, 50, 75 and 100 μM mL−1) of the ruthenium complex. Generally, cell proliferation or increase in the cell number is associated with cancer risk.47 The Mitotic Index represents the percentage of cells undergoing division to the total number of cells. Usually, an increase in Mitotic Index was identified in cells of colonic or rectal cancer and superficial bladder cancer patients. Most of the drugs used to treat cancer have antiproliferative activity.48,49 In the present study, the salicylaldehyde-4(N)-ethylthiosemicarbazone and its new ruthenium(III) complex were tested for their antiproliferative activity on mitogen induced proliferation on human lymphocytes.48 The ligand showed no reduction in mitotic cells compared with controls. Hence, the compound has no antiproliferative activity on mitogen activate human lymphocytes. But, the ruthenium complex showed significant reduction in the mitotic cell number. Statistically significant reduction in Mitotic Index was observed at 25, 50 and 75 μM mL−1 concentrations of the test compound. This shows that the ruthenium complex has potent antiproliferative activity indicating it to be a good candidate for therapeutic applications.
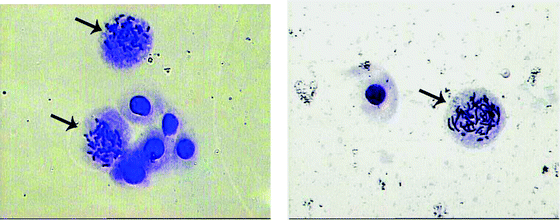 |
| | Fig. 5 Typical mitotic cells. The arrow indicates the cells with metaphase chromosomes. | |
Table 4 Mitotic Index in control and test compounds
| Concentration/μM mL−1 |
Mitotic Index (mean ± SD) |
| [H2-Sal-etsc] |
[Ru(Sal-etsc)(H-Sal-etsc)(PPh3)]
|
|
P < 0.05 compared with control.
|
| Control |
5.7 ± 0.2 |
5.6 ± 0.4 |
| 5 |
5.3 ± 0.2 |
5.0 ± 0.1 |
| 15 |
5.3 ± 0.4 |
3.1 ± 0.1 |
| 25 |
5.2 ± 0.4 |
2.1 ± 0.0a |
| 50 |
4.7 ± 0.2 |
1.8 ± 0.3a |
| 75 |
4.7 ± 0.2 |
1.6 ± 0.3a |
MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay50
Since the complex exhibited potent antiproliferative activity on human lymphocytes, a study of the complex on lung carcinoma A549 cells in a dose dependent manner was carried out to find out the activity. The complex showed a maximum inhibitory activity of 83.2% at the concentration of 20 μM. Further, the IC50 concentration of the compound was found to be at 12 μM where the proliferations of 50% of the cells were inhibited. There is so much literature available about the potential anti-cancer activity of cis-platin. Recently a report has been published with 50% growth (IC50) inhibition of cis-platin in human acute promyelocytic leukaemia cell line HL-60 cells at 15.61 μM concentration.51 Though we have not done any comparative studies of cis-platin and our compound, we are expecting that our compound also can be a potent anticancer agent because it exhibits IC50 at 12 μM on lung carcinoma A549 cells. This also indicates that the ruthenium complex can be considered as a potential anticancer agent.
3. Experimental section
All the regents used were chemically pure grade. RuCl3·3H2O was purchased from Himedia and was used without further purification. The starting complex [RuCl3(PPh3)3] and salicylaldehyde-4(N)-ethylthiosemicarbazone were prepared by the literature methods.52,53IR spectra of the ligand and complex were recorded from KBr pellets with Nicolet instruments in the 4000–400 cm−1 range. The electronic spectrum of the complex was measured in dichloromethane using a Systronics 119 spectrophotometer in the 800–200 nm range. Elemental analyses were performed by a Vario EL III CHNS analyzer. The EPR spectrum of the powder sample was recorded at room temperature with Jeol Tel −100 at X-band frequencies using 2,2′-diphenyl-1-picrylhydrazine hydrate as internal standard. The cyclic voltammetric study was done by using a CH Instrument Electrochemical Analyzer using a platinum working electrode at a scan rate of 100 mV s−1. The supporting electrolyte used was 0.1 M [NBu4]ClO4 in dichloromethane solution. The concentration of the complex was 0.001 M. All the potentials were referenced to Ag/AgCl electrode. Ferrocene is used as external standard. The melting point was recorded with a Lab India melting point apparatus.
Synthesis of [Ru(Sal-etsc)(H-Sal-etsc)(PPh3)]complex
A solution of [H2-Sal-etsc] (0.1 mmol; 0.023 g) in 20 cm3 of benzene was added dropwise to a boiling solution of [RuCl3(PPh3)3] (0.1 mmol; 0.0989 g) in 20 cm3 of benzene. The resulting mixture was refluxed for 6 h and it was subjected to thin layer chromatography wherein three spots were identified and they were isolated by silica gel column chromatography by using a methanol–benzene mixture. A light brown band was eluted with benzene and it was identified as unreacted [RuCl3(PPh3)3] and a dark brown band was eluted with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 methanol
9 methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) benzene mixture. On evaporation, it afforded a black compound and when it was crystallized in hot DMF gave shiny black needle shaped crystals of [Ru(Sal-etsc)(H-Sal-etsc)(PPh3)]. A brownish yellow band was eluted with 2
benzene mixture. On evaporation, it afforded a black compound and when it was crystallized in hot DMF gave shiny black needle shaped crystals of [Ru(Sal-etsc)(H-Sal-etsc)(PPh3)]. A brownish yellow band was eluted with 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 methanol
8 methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) benzene mixture and it was identified as triphenylphosphine oxide.
benzene mixture and it was identified as triphenylphosphine oxide.
[Ru(Sal-etsc)(H-Sal-etsc)(PPh3)]
Yield: 39% (30.89 mg). M.p. 196 °C. FT–IR (KBr): 1596 cm−1 (νC=N), 3445 cm−1 (νOH), 1332 cm−1 (νC−O), 748 cm−1 (νC−S), 3274 cm−1 (νN−H), 1448, 1076, 691 cm−1 (for PPh3). UV–Vis (CH2Cl2), νmax (267, 292, 373, 422 nm). Anal. Calc. for C38H38N6O2PS2Ru: C, 56.65; H, 4.74; N, 10.41; S, 7.95; Found: C, 56.48; H, 4.71; N, 10.26; S, 7.89.
Single crystals of [Ru(Sal-etsc)(H-Sal-etsc)(PPh3)] were obtained from hot DMF. Crystal data collections were performed at 287 K with a Bruker SMART 5.630 Diffractometer using mono chromated Mo-Kα (λ = 0.71073 Å) radiation.54 SAINT +6.45 Program(s) were used for cell refinement and data reduction and the structure was solved and refined by full matrix least squares on F2 using SHELXTL 6.14.55 The complex crystallized with two DMF molecules and they are disordered over two positions and the disorder of one of the DMF molecules is also associated with a disorder of one of the C–NH–CH2–CH3 moieties. The occupancy ratio for the first disordered DMF molecule is 0.824(8) to 0.196(8), that of the second and the C–NH–CH2–CH3 group is 0.697(7) to 0.303(7). In the disordered moieties all minor component atoms were set to have the same atomic displacement parameters (ADPs) as their major component counterparts. All equivalent bonds were restrained to be the same within a standard deviation of 0.02.
DPPH radical-scavenging assay
The potential antioxidant activity of ruthenium(III) complex was evaluated by DPPH radical-scavenging assay according to the procedure described previously with a slight modification.42DPPH free radicals are used for rapid analysis of antioxidants. While scavenging the free radicals, the antioxidants donate hydrogen and form a stable DPPH–H molecule. This reaction involves a color change from purple to yellow that can be measured spectroscopically.56 Briefly, the compound (25–350 μg ml−1) was added to 2 ml of DPPH (0.1 mM in methanol) and was mixed rapidly. Radical scavenging capacity was measured in 10 min intervals using spectrophotometer by monitoring the decrease in absorbance at 517 nm. The percent DPPH decolonization of the sample was calculated. L-Ascorbic acid was used as a positive control.
Human lymphocyte culture and Mitotic Index determination47,48
Blood samples were collected from a healthy volunteer. Cultures were set up with 0.5 ml of whole blood in 5 ml of RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) and 1% phytohemagglutinin (PHA). The culture was incubated at 37 °C for 48 h. At 24 h, the two test samples, [H2-Sal-etsc] and [Ru(Sal-etsc)(H-Sal-etsc)(PPh3)] dissolved in 0.4% DMSO were added to the individual culture in the concentration ranging from 5, 12, 25, 50 and 75 μM ml−1. Colchicine was added two hours prior to harvest. The cells were harvested, treated with hypotonic solution (0.075 M KCl) and fixed in methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetic acid (3
acetic acid (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The fixed cells were spread on a clean microscopic slide, air dried and stained with Giemsa stain. The cells were observed at 400× magnification in a Leica microscope, Germany. The Mitotic Index was determined by examination of 1000 cells per slide. The Mitotic Index (MI) was the percentage of mitotic cells (metaphase stage) in the total cells population.
1). The fixed cells were spread on a clean microscopic slide, air dried and stained with Giemsa stain. The cells were observed at 400× magnification in a Leica microscope, Germany. The Mitotic Index was determined by examination of 1000 cells per slide. The Mitotic Index (MI) was the percentage of mitotic cells (metaphase stage) in the total cells population.
Statistical analysis
A student's t-test was performed to determine the mean, standard deviation and significant values for the Mitotic Index.
Cell culture and treatment
Human lung carcinoma cells A549 were cultured in DMEM medium supplemented with 10% FBS and 1% penicillin-streptomycin in a 37 °C incubator with 5% CO2. Cells at the exponential growth stage were implanted on a 96 well plate.
MTT assay50
The MTT assay is used to analyze the viability of cells. The mitochondrial dehydrogenases of the living cells reduces the yellow MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) to purple formazan. The intensity of the purple color formed is measured using a spectrophotometer. The light absorbed by the formazan correlates with the live cells.50 The cells were treated with various concentrations of the compound and incubated for 24 h in 96 well microtitre plate. 20 μl of 5 mg ml−1 MTT in PBS was added to each well and incubated at 37 °C for further 4 h. The purple formazan crystals formed were dissolved in 150 μl of DMSO. The absorbency of each well was measured at 570 nm using Elisa reader (Biotek, USA) and the percentage viability was calculated.
4. Conclusion
In this communication, we have reported the exhibition of two different types of coordination behaviour of salicylaldehyde-4(N)-ethylthiosemicarbazone in a ruthenium(III) complex. From our studies, it is made clear that intramolecular hydrogen bonding and steric bulkiness of co-ligand are not the factors responsible in determining the coordination behaviour of thiosemicarbazone. The structure of the complex was confirmed by X-ray crystallography. The new complex has exhibited 100% scavenging activity over DPPH radical. Moreover, the complex exhibited significant antiproliferative activities on human lymphocyte culture and human lung cancer cells.
Acknowledgements
The authors gratefully acknowledge the Department of Science and Technology (DST), and the Council of Scientific and Industrial Research (CSIR), New Delhi, India for their financial assistance. The diffractometer was funded by a NSF grant 0087210 by the Ohio Board or Regents grants CAP-491 and by YSU.
References
- D. S. Kalinowski and D. R. Richardson, Pharmacol. Rev., 2005, 57, 547 CrossRef CAS.
- A. R. Cowley, J. R. Dilworth, P. S. Donnelly, A. D. Gee and J. M. Heslop, Dalton Trans., 2004, 2404 RSC.
- E. J. Blanz and F. A. French, Cancer Res., 1968, 28, 2419 CAS.
- S. B. Padhye and G. B. Kaufman, Coord. Chem. Rev., 1985, 63, 127 CrossRef CAS.
- D. L. Klayman, J. P. Scovill, J. F. Bartosevich and J. Bruce, J. Med. Chem., 1983, 26, 35 CrossRef CAS.
- M. C. Miller, C. N. Stineman, J. R. Vance, D. X. West and I. H. Hall, Anticancer Res., 1998, 18, 4131 CAS.
- R. W. Brockman, J. R. Thomson, M. J. Bell and H. E. Skipper, Cancer Res., 1956, 16, 167 CAS.
- N. C. Kusuya, K. Sekino, C. Koumo, N. Shimada, M. Ishikawa and K. Nomiya, J. Inorg. Biochem., 2001, 84, 55 CrossRef CAS.
- D. X. West, A. E. Liberta, S. B. Padhye, P. B. Jonawane, A. S. Kumbhar and R. G. Yerande, Coord. Chem. Rev., 1993, 123, 49 CrossRef CAS.
- F. Basuli, S. M. Peng and S. Bhattacharya, Inorg. Chem., 1997, 36, 5645 CrossRef CAS.
- F. Basuli, M. Ruf, C. G. Pierpontand and S. Bhattacharya, Inorg. Chem., 1998, 37, 6113 CrossRef CAS.
- F. Basuli, S. M. Peng and S. Bhattacharya, Inorg. Chem., 2000, 39, 1120 CrossRef CAS.
- I. Pal, F. Basuli, T. C. W. Makand and S. Bhattacharya, Angew. Chem. Int. Ed. Engl., 2001, 113, 3007 CrossRef.
- R. Archaryya, F. Basuli, S. M. Peng, G. H. Lee, L. R. Falvello and S. Bhattacharya, Inorg. Chem., 2006, 45, 1252 CrossRef CAS.
- E. I. Torres, M. A. Merdiola, C. J. Pastor and B. S. Perez, Inorg. Chem., 2004, 43, 5223.
- I. G. Sentos, U. Abram, R. Alberto, E. V. Lapez and A. Sanchez, Inorg. Chem., 2004, 43, 1834 CrossRef CAS.
- M. Marino, E. Gayoso, J. M. Antelo, L. A. Adrio, J. J. Fernandez and J. M. Vila, Polyhedron, 2006, 25, 1449 CrossRef CAS.
- J. Martinez, M. T. Pereira, I. Buceta, G. Alberdi, A. Amoeda, J. J. Fernandez, M. Lopez and J. M. Vila, Organometallics, 2003, 22, 5581 CrossRef CAS.
- F. Basuli, S. M. Peng and S. Bhattacharya, Inorg. Chem., 2001, 40, 1126 CrossRef CAS.
- J. P. Holland, F. I. Aigbirhio, H. M. Betts, P. D. Bonnitcha, P. Burke, M. Christlieb, G. C. Churchill, A. R. Cowley, J. R. Dilworth, P. S. Donnelly, J. C. Green, J. M. Peach, S. R. Vasudevan and J. E. Warren, Inorg. Chem., 2007, 46, 465 CrossRef CAS.
- D. Mishra, S. Naskar, M. G. B. Drew and S. K. Chattopadhyay, Inorg. Chim. Acta, 2006, 359, 585 CrossRef CAS.
- C. Paek, S. O. Kang and J. K. J. Carroll, Organometallics, 1997, 16, 2110 CrossRef CAS.
- C. Paek, S. O. Kang and J. K. J. Carroll, Organometallics, 1997, 16, 4755 CrossRef CAS.
- Y. Kang, N. Yang, S. O. Kang, J. C. H. Lee and J. K. J. Carroll, Organometallics, 1997, 16, 5522 CrossRef CAS.
- R. Prabhakaran, C. Jayabalakrishnan, V. Krishnan, K. Pasumpon, D. Sukanya, H. Bertagnolli and K. Natarajan, Appl. Organomet. Chem., 2006, 20, 203 CrossRef CAS.
- R. Prabhakaran, R. Huang, R. Karvembu, C. Jayabalakrishnan and K. Natarajan, Inorg. Chim. Acta, 2007, 360, 691 CrossRef CAS.
- R. Prabhakaran, R. Karvembu, T. Hashimoto, K. Shimizu and K. Natarajan, Inorg. Chim. Acta, 2005, 358, 2093 CrossRef CAS.
- R. Prabhakaran, S. V. Renukadevi, R. Karvembu, R. Huang, J. Mautz, G. Huttner, R. Subhaskumar and K. Natarajan, Eur. J. Med. Chem., 2008, 43, 268 CrossRef CAS.
- D. K. Demertzi, A. Galani, N. Kourkoumelis, J. R. Miller and M. A. Demertzis, Polyhedron, 2007, 26, 2871 CrossRef CAS.
- D. K. Demertzi, P. N. Yadav, J. Wiecek, S. Skoulika, T. Varadinova and M. A. Demertzis, J. Inorg. Biochem., 2006, 100, 1558 CrossRef CAS.
- M. A. Demertzis, P. N. Yadav and D. K. Demertzi, Helv. Chim. Acta, 2006, 89, 1959 CrossRef CAS.
- M. C. Aguirre, J. Borras, A. Castineiras, J. M. G. Monteagudo, I. G. Santos, J. Niclos and D. X. West, Eur. J. Inorg. Chem., 2006, 1231 CrossRef CAS.
- M. S. Shongwe, H. N. R. Al-Kharousi, H. Adams, M. J. Morris and E. Bill, Inorg. Chem., 2006, 45, 1103 CrossRef CAS.
- G. P. Gabian, E. M. Vazquez-Lopez, H. Braband and U. Abram, Inorg. Chem., 2005, 44, 834 CrossRef CAS.
- S. K. Chattopadhyay and S. Ghosh, Inorg. Chim. Acta, 1998, 163, 538.
- J. G. Tojal, J. L. Dizarro, A. G. Orad, A. R. P. Sanz, M. Ugalda, A. A. Diaz, J. L. Serra, M. I. Arriortua and T. Rojo, J. Inorg. Biochem., 2001, 86, 627 CrossRef.
- R. Prabhakaran, A. Geetha, M. Thilagavathi, R. Karvembu, V. Krishnan, H. Bertagnolli and K. Natarajan, J. Inorg. Biochem., 2004, 98, 2131 CrossRef CAS.
- I. Pal, F. Basuli and S. Bhattacharya, Proc.–Indian Acad. Sci., Chem. Sci., 2002, 114, 255 Search PubMed.
- S. Basu, R. Archaryya, W. S. Sheldrick, H. M. Figge and S. Bhattacharya, Struct. Chem., 2007, 18, 209 CrossRef CAS.
- J. Heinze, Angew. Chem., Int. Ed. Engl., 1984, 23, 831 CrossRef.
- S. Chakraborty, M. G. Walawalkar and G. K. Lahiri, J. Chem. Soc., Dalton Trans., 2000, 2875 RSC.
- M. S. Blios, Nature, 1958, 26, 1199 CrossRef.
- R. Singh, S. Singh, S. Kumar and S. Arora, Food Chem., 2003, 103, 1403.
- P. Mahakunakoran, M. Tohda, Y. Murakami, K. Matsumoto and H. Watanabe, Biol. Pharm. Bull., 2004, 27, 38 CrossRef.
- G. H. Naik, K. I. Priyadarsini, J. G. Satav, M. M. Banavalikar, P. P. Sohoni and M. K. Biyani, Phytochemistry, 2003, 63, 97 CrossRef CAS.
- E. Rekka and P. N. Kourounakis, J. Pharm. Pharmacol., 1991, 43, 486 CAS.
- P. Romagnoli, F. Filipponi, L. Bandettini and D. Brugnola, Dis. Colon Rectum, 1984, 27, 305 Search PubMed.
- T. J. O. Liukkonen, P. K. Lipponen, M. Helle, H. K. Haapasalo, S. Nordling and P. Rajala, Urol. Res., 1996, 24, 61 CrossRef CAS.
- J. Patole, S. Padhye, M. S. Moodbidri and N. Shirsat, Eur. J. Med. Chem., 2005, 40, 1052 CrossRef CAS.
- T. Mosmann, J. Immunol. Methods, 1983, 65, 55 CrossRef CAS.
- V. Moreno, J. Lorenzo, F. X. Aviles, M. Helena Garcia, J. P. Ribeiro, T. S. Morais, P. Florindo and M. P. Robalo, Bioinorg. Chem. Appl., 2010, 2010 DOI:10.1155/2010/936834 , Article ID 936834.
- S. Purohit, A. P. Kolay, L. S. Prasad, P. T. Manoharan and S. Ghosh, Inorg. Chem., 1989, 28, 3735 CrossRef CAS.
- D. L. Klayman, J. F. Brtosevich, T. S. Griffin, C. J. Mason and J. P. Scoveil, J. Med. Chem., 1997, 22, 855.
- Bruker 2002. SMART. Version 5.630. Bruker AXS Inc., Madison,Wisconsin, USA.
- Bruker 2003. SAINT-Plus (Version 6.45) and SHELXTL (Version 6.14). Bruker AXS Inc., Madison, Wisconsin, USA.
- D. Bandoniene and M. Murkovic, J. Biochem. Biophys. Methods, 2002, 53, 45 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2011 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio in benzene leads to a brown solution consisting of a stable dark brown [Ru(Sal-etsc)(H-Sal-etsc)(PPh3)] together with unreacted [RuCl3(PPh3)3] and triphenylphosphine oxide. It is to be noted that even when the reaction was carried out in 1
1 molar ratio in benzene leads to a brown solution consisting of a stable dark brown [Ru(Sal-etsc)(H-Sal-etsc)(PPh3)] together with unreacted [RuCl3(PPh3)3] and triphenylphosphine oxide. It is to be noted that even when the reaction was carried out in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio it afforded [Ru(Sal-etsc)(H-Sal-etsc)(PPh3)] in decent yield (Scheme 1).
2 molar ratio it afforded [Ru(Sal-etsc)(H-Sal-etsc)(PPh3)] in decent yield (Scheme 1).
![Preparation of [Ru(Sal-etsc)(H-Sal-etsc)(PPh3)].](/image/article/2011/MT/c0mt00062k/c0mt00062k-s1.gif)
![EPR spectrum of [Ru(Sal-etsc)(H-Sal-etsc)PPh3].](/image/article/2011/MT/c0mt00062k/c0mt00062k-f1.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 654
654![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (R(int) = 0.0666)
000 (R(int) = 0.0666)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000/57/601
000/57/601![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
![Structure of [Ru(Sal-etsc)(H-Sal-etsc)(PPh3)].](/image/article/2011/MT/c0mt00062k/c0mt00062k-f2.gif)
![Cyclic voltammogram of [Ru(Sal-etsc)(H-Sal-etsc)PPh3].](/image/article/2011/MT/c0mt00062k/c0mt00062k-f3.gif)
![DPPH scavenging activity of [Ru(Sal-etsc)(H-Sal-etsc)(PPh3)].](/image/article/2011/MT/c0mt00062k/c0mt00062k-f4.gif)

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 methanol
9 methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) benzene mixture. On evaporation, it afforded a black compound and when it was crystallized in hot DMF gave shiny black needle shaped crystals of [Ru(Sal-etsc)(H-Sal-etsc)(PPh3)]. A brownish yellow band was eluted with 2
benzene mixture. On evaporation, it afforded a black compound and when it was crystallized in hot DMF gave shiny black needle shaped crystals of [Ru(Sal-etsc)(H-Sal-etsc)(PPh3)]. A brownish yellow band was eluted with 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 methanol
8 methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) benzene mixture and it was identified as triphenylphosphine oxide.
benzene mixture and it was identified as triphenylphosphine oxide.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetic acid (3
acetic acid (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The fixed cells were spread on a clean microscopic slide, air dried and stained with Giemsa stain. The cells were observed at 400× magnification in a Leica microscope, Germany. The Mitotic Index was determined by examination of 1000 cells per slide. The Mitotic Index (MI) was the percentage of mitotic cells (metaphase stage) in the total cells population.
1). The fixed cells were spread on a clean microscopic slide, air dried and stained with Giemsa stain. The cells were observed at 400× magnification in a Leica microscope, Germany. The Mitotic Index was determined by examination of 1000 cells per slide. The Mitotic Index (MI) was the percentage of mitotic cells (metaphase stage) in the total cells population.
