Interplay between manganese and zinc homeostasis in the human pathogen Streptococcus pneumoniae†
Faith E.
Jacobsen
a,
Krystyna M.
Kazmierczak
b,
John P.
Lisher
a,
Malcolm E.
Winkler
b and
David P.
Giedroc
*a
aDepartment of Chemistry, Indiana University, Bloomington, IN 47405-7102, USA. E-mail: giedroc@indiana.edu
bDepartment of Biology, Indiana University, Bloomington, IN 47405-3700, USA
First published on 28th October 2010
Abstract
ICP-MS analysis of Streptococcus pneumoniae reveals a high cell-associated Mn(II) concentration that is comparable to that of Zn(II). Stressing these cells with 100–200 μM Zn(II) leads to a slow-growth phenotype and a total Mn(II) concentration that is reduced, with no decrease of other metal ions. Supplementation of the growth media with as little as 10 μM Mn(II) fully restores the growth defect and cell-associated Mn(II) to normal levels. DNA microarray analysis reveals that zinc stress induces the expected upregulation of czcD (encoding a zinc effluxer), but also a pleiotropic transcriptional response suggestive of mild cell wall stress. Genes encoding a nitric oxide (NO) detoxification system (nmlR) and the Mn(II) uptake system (psaBCA) are also induced. We conclude that Zn(II) toxicity results in a cytoplasmic Mn(II) deficiency, possibly caused by competition at the Mn(II) uptake transporter protein PsaA.
Zinc is an essential metal ion in all organisms and is used as an essential cofactor in metalloenzymes and for structural and regulatory roles.1,2Cellular homeostasis of zinc is maintained by the coordination of uptake, efflux and intracellular sequestration and allows an organism to respond to large fluctuations in extracellular zinc availability. This may be particularly important during the course of an infection of a mammalian host by the pathogen Streptococcus pneumoniae since zinc concentrations are reported to range from 5 μM in the nasopharynx to ≈300 μM in lung tissue.3
In S. pneumoniae and other streptococci, inactivation of the adc (adhesin competence) operon leads to both a reduction in virulence and adhesion.4 The adcoperon encodes an ABC (ATP-binding cassette) transporter AdcCBA that is proposed to uptake zinc in S. pneumoniae5 and is under the transcriptional control of AdcR.6Zinc efflux in S. pneumoniae requires the cation diffusion facilitator (CDF) protein CzcD,7 regulated by the TetR family regulator SczA (see below).8 Interestingly, a ΔadcCBA deletion strain is still able to acquire extracellular zinc, presumably through the PsaBCA ABC transporter,5 which has been shown to uptake manganese. The CDF protein MntE, a paralog of CzcD, has recently been reported to function as a manganese efflux protein and appears to be constitutively expressed.9
In this study we evaluate the effects of defined perturbations of zinc homeostasis in S. pneumoniae on a metabolic and genetic level and in so doing, characterize a clear connection between zinc and manganese homeostasis in S. pneumoniaeD39. ICP-MS analysis reveals that S. pneumoniae grown in limited aeration (static) liquid culture in brain heart infusion (BHI) broth is characterized by a cell-associated Mn concentration that is comparable to that of Zn and in excess of Fe (Fig. 1). The total [Fe] is 3–4 fold lower than previous estimates in S. pneumoniae R6 strain and in E. coli,10 for reasons that require further investigation. Other catalase-negative, hydrogen peroxide generating lactic acid bacteria are characterized by high intracellular Mn, and this could provide resistance to hydrogen peroxide toxicity.11–13S. pneumoniae also requires manganese for capsule production (tyrosine-protein phosphatase CpsB), metabolism (lactate dehydrogenase and pyruvate kinase) and for ROS detoxification (Mn superoxide dismutase).3 Remarkably, the total cellular concentrations of Mn and Zn are comparable in S. pneumoniae despite the fact that Mn is present at ≈50-fold lower concentration in this growth medium (Fig. S1, ESI†); this suggests that PsaBCA is a highly efficient Mn transporter even in the presence of a highly competitive metal like Zn, which sits near the top of the Irving–Williams series.14
 | ||
| Fig. 1 ICP-MS analysis of total WT S. pneumoniaecell-associated metal ion concentration (expressed as ng metal/mg protein) when grown in BHI media under microaerophilic conditions, 37 °C. | ||
Growth of the parent wild-type, ΔadcR and czcD::kan-rpsL strains in the presence of increasing concentrations of zinc reveal a growth deficiency in the ΔadcR strain characterized by a slightly longer lag phase and lower growth yield (Fig. S2, ESI†).6,15 In addition, a kinetic Zn67/Zn68 uptake experiment reveals the ΔadcR mutant shows an increased uptake of zinc from the media (Fig. S3, ESI†), thus suggesting that zinc import through AdcR-controlled expression of AdcBCA is at least partially repressed in the wild-type strain under these conditions (120 μM Zn). In contrast, the czcD::kan-rpsL strain does not grow in media containing ≥120 μM Zn (Fig. S2, ESI†).15 Metal contents as determined by ICP-MS show that the czcD::kan-rpsL and double mutant ΔadcR czcD::kan-rpsL strain in the presence of 100 μM added Zn contain ≈2-fold higher Zn than either the parent or ΔadcR strains under the same conditions (Fig. S4, ESI†). These data suggest that zinc efflux is primarily responsible for maintaining zinc homeostasis under these conditions of moderate zinc stress (100–200 μM).
Most significantly, all strains tested were characterized by a 40–50% decrease in cell-associated Mn under these conditions (Fig. 2). Addition of excess extracellular Mn rescued the growth phenotype of these strains (Fig. S2, ESI† and not shown) while restoring the intracellular Mn concentration to above normal (Fig. 2). In addition, Mn supplementation decreased the intracellular concentration of Zn (Fig. S4, ESI†). For the parent and ΔadcR strains as little as 10 μM manganese completely restores growth (other studies have found that as low as 3 μM manganese is sufficient).16 For the czcD::kan-rpsL strain, 300 μM manganese was required for a full recovery of growth. This effect is highly specific for Mn since the metal concentrations for copper, nickel and iron did not decrease in response to high external Zn concentrations (Fig. S5, ESI†); in addition, none of these metals could rescue the growth phenotype of any of these strains induced by Zn (Fig. S2, ESI†).
 | ||
| Fig. 2 Mn content of the parent wild-type , ΔadcR, ΔczcD::kan-rpsL and ΔadcR ΔczcD::kan-rpsL strains grown in BHI alone (green), in the presence of 100 μM zinc added (gray) and in the presence of 100 μM zinc and 300 μM manganese added (purple). | ||
Microarray analysis of the parent strain grown in BHI media (≈20 μM Zn) compared to BHI plus 200 μM zinc reveal a moderate number of transcriptomic changes (Table S3, ESI†), some of which were previously described.15 These changes are suggestive of mild cell envelope stress, given the up-regulation of members of the WalRK, CiaRH and TCS03 two-component system regulons.17 It is important to note that SczA-regulated genesczcD (encoding a Zn effluxer) and nmlR are highly upregulated at high Zn, while the expression of the adcRregulon6 is unchanged at 220 μM total Zn relative to 20 μM. This reveals that zinc efflux by CzcD is the primary homeostatic response to zinc stress under these conditions. This is fully consistent with microarray analysis of a strain harboring a nonfunctional adcRmissense allele (AdcR H108Q)6 in BHI with 200 μM zincvs. the parent strain with zinc (Table S4, ESI†). These data allow us to conclude that the expression of the adcRregulon is repressed in the wild-type strain in BHI (≈20 μM Zn),6 at 120 μM (above) and at 220 μM total Zn in the growth media, in striking contrast to the sczAregulon (see Fig. 3). Although more studies are required, these data suggest that Zn-regulated down-regulation of zinc import through AdcCBA and up-regulation of efflux through CzcD occurs are distinct cytoplasmic [Zn]free, in contrast to previous observations in E. coli.1 This in turn, suggests distinct sensitivities of AdcR-mediated repression and SczA-mediated depression of transcription in the cytoplasm.
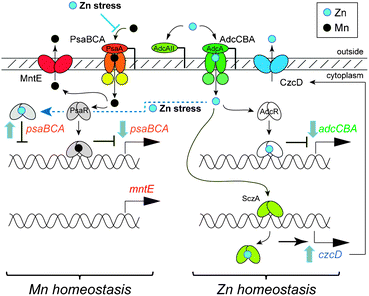 | ||
| Fig. 3 Schematic rendering of Zn and Mn homeostasis in S. pneumoniae highlighting the metal transporters and metal regulators discussed here and how Zn stress may interfere with Mn homeostasis (see text for details). | ||
A major finding is that the entire psaRregulon is derepessed at high Zn thus revealing that S. pneumoniae is sensing cytoplasmic Mn deficiency at 200 μM added Zn (Table S3, ESI†). As expected, a comparison of the parent strain grown in BHI with 200 μM zinc and 300 μM manganeseversus that grown in BHI shows very few transcriptional changes compared to when manganese is not added (Table S5, ESI†). Zinc stress is still sensed however, as czcD and nmlR are highly transcribed, and the psaRregulon is now repressed (Table S5, ESI†). Thus, the transcriptomic and ICP-MS analyses are internally consistent and reveal that a major consequence of external Zn stress is a cellular Mn deficiency. Interestingly, Mn deficiency induced by deletion of psaA reveals the same five transcriptional changes, including the psaRregulon (prtA, psaCBA) and czcD.16
We next tested if the Zn-dependent Mn deficiency was caused by efflux through the manganese CDF protein MntE.9 A deletion mutant of mntE was constructed and compared to the ΔadcR strain. A growth phenotype at 200 μM Zn was observed in the ΔmntE strain that was identical to the ΔadcR mutant (Fig. S6A, ESI†). ICP-MS reveals that Mn levels are similarly reduced by Zn stress in the ΔmntE mutant relative to ΔadcR or the wild-type strain (Fig. S6B, ESI†), indicating that the decrease in cell-associated Mn is not due to Mn efflux. As expected, addition of a moderate dose of Mn (50 μM) was capable of rescuing the effects of zinc toxicity of the ΔmntE strains (Fig. S6A, ESI†).
These data therefore suggest by process of elimination that cell-associated Mn levels are decreased by Zn toxicity as a result of competition with Zn for transport by PsaBCA (Fig. 3). Efforts to force Mn and Zn uptake through PsaBCA in a ΔadcA strain are complicated by the presence of an orphan Zn binding lipoprotein AdcAII18 that may well function to stimulate metal uptake through AdcBC; as a result, this strain was not investigated further. Although a ΔpsaA strain is sick due to manganese deficiency,5,16 addition of 200 μM zinc causes a clearly reduced growth phenotype which is rescued by addition of 300 μM Mn (Fig. S7A, ESI†), a finding in opposition to competition between Zn and Mn at PsaA alone. A Zn67/Zn68 isotope ratio experiment that compares the parent strain grown in 100 μM Zn67 to the ΔpsaA strain cultured in 100 μM Zn67 and 300 μM Mn (which have identical growth kinetics) reveals that less Zn67 is taken up from the media by the ΔpsaA strain. This finding is consistent with the observation that ΔpsaAcells contain about half the total concentration of Zn (when grown in excess Mn) relative to a wild-type counterpart (Fig. S7B, ESI†).16 This suggests competition between Zn and Mn for binding to AdcA lipoprotein and transport by AdcCBA. In contrast, Zn may be a competitive inhibitor of Mn transport by PsaCBA, further evidence for which is that the crystal structure of PsaA was solved with Zn bound,19 suggesting a significant affinity of the Mn transporter for its non-cognate metal (Fig. 3). We stress, however, that we have no direct evidence for zinc competition for Mn uptake by PsaCBA, although this seems likely.
In addition to possible competition at the transporter, a high extracellular Zn/Mn ratio could potentially alter the activity of the psaBCA regulator, PsaR.3,15DNA binding experiments show that PsaR binds psaBCA operator DNA tightly in the presence of 1 μM Mn(II) or Cd(II); however, in the presence of 1 μM Zn(II) the binding is weak (Fig. 4). Similar results have been reported for the Mn regulator from S. gordonii, ScaR.20 This lack of co-repression by the non-cognate metal Zn may help to ensure that expression of the high affinity Mn uptake system is expressed under conditions of both extracellular and intracellular Zn stress in an ongoing effort by S. pneumoniae to counteract the effects of cytoplasmic Mn deficiency (see Fig. 3). This may provide a physiological advantage to S. pneumoniae during colonization of the lung or bloodstream where Zn concentrations are reported to be high (15–300 μM) and Mn is deplete (20 nM).3,16 In fact, the extracellular zinc binding proteins Pht (pneumococcal histidine triad proteins) and AdcAII, which are regulated by AdcR, may favorably position S. pneumoniae to scavenge zinc in a switch from a Zn deplete to Zn replete environment during invasion, while reducing competition at PsaA when extracellular Zn concentrations are high as a result of innate immune responses.21 Detailed mechanistic and biochemical studies of the purified transporters will be required to obtain additional support for this model of metal competition.
![PsaR operator DNA binding curves for apo- Zn(ii)-, Mn(ii)- and Cd(ii)-substituted PsaR. The anisotropy (robs) of a fluorescein-labeled psaBCA operator DNA fragment was measured as a function of total [PsaR] and fit to a model that invokes the binding of a single dissociable PsaR dimer to the DNA (see supplementary methods, ESI).](/image/article/2011/MT/c0mt00050g/c0mt00050g-f4.gif) | ||
| Fig. 4 PsaR operator DNA binding curves for apo- Zn(II)-, Mn(II)- and Cd(II)-substituted PsaR. The anisotropy (robs) of a fluorescein-labeled psaBCA operator DNA fragment was measured as a function of total [PsaR] and fit to a model that invokes the binding of a single dissociable PsaR dimer to the DNA (see supplementary methods, ESI†). | ||
Supported by grants from the US National Institutes of Health (GM042569 to DPG, F32 AI084445 to FEJ and AI060744 to MEW).
Notes and references
- C. Outten and T. O'Halloran, Science, 2001, 292, 2488–2492 CrossRef CAS.
- J. Frausto da Silva and R. Williams, The Biological Chemistry of Elements: The Inorganic Chemistry of Life, Oxford University Press, Oxford, 2001 Search PubMed.
- J. W. Johnston, D. E. Briles, L. E. Myers and S. K. Hollingshead, Infect. Immun., 2006, 74, 1171–1180 CrossRef CAS.
- A. Riboldi-Tunnicliffe, N. W. Isaacs and T. J. Mitchell, FEBS Lett., 2005, 579, 5353–5360 CrossRef CAS.
- A. Dintilhac, G. Alloing, C. Granadel and J.-P. Claverys, Mol. Microbiol., 1997, 25, 727–739 CrossRef CAS.
- H. Reyes-Caballero, A. J. Guerra, F. E. Jacobsen, K. M. Kazmierczak, D. Cowart, U. M. K. Koppolu, M. E. Winkler and D. P. Giedroc, J. Mol. Biol., 2010, 403, 197–216 CrossRef CAS.
- A. Anton, C. Groe, J. Reimann, T. Pribyl and D. H. Nies, J. Bacteriol., 1999, 181, 6876–6881 CAS.
- T. G. Kloosterman, M. M. van der Kooi-Pol, J. J. E. Bijlsma and O. P. Kuipers, Mol. Microbiol., 2007, 65, 1049–1063 CrossRef CAS.
- J. W. Rosch, G. Gao, G. Ridout, Y.-D. Wang and E. I. Tuomanen, Mol. Microbiol., 2009, 72, 12–25 CrossRef CAS.
- C. D. Pericone, S. Park, J. A. Imlay and J. N. Weiser, J. Bacteriol., 2003, 185, 6815–6825 CrossRef CAS.
- F. S. Archibald and I. Fridovich, J. Bacteriol., 1981, 146, 928–936 CAS.
- J. A. Imlay, Annu. Rev. Biochem., 2008, 77, 755–776 CrossRef CAS.
- M. B. Yim, B. S. Berlett, P. B. Chock and E. R. Stadtman, Proc. Natl. Acad. Sci. U. S. A., 1990, 87, 394–398 CrossRef CAS.
- K. J. Waldron, J. C. Rutherford, D. Ford and N. J. Robinson, Nature (Lond.), 2009, 460, 823–830 Search PubMed.
- T. G. Kloosterman, R. M. Witwicki, M. M. van der Kooi-Pol, J. J. E. Bijlsma and O. P. Kuipers, J. Bacteriol., 2008, 190, 5382–5393 CrossRef CAS.
- A. D. Ogunniyi, L. K. Mahdi, M. P. Jennings, A. G. McEwan, C. A. McDevitt, M. B. Van der Hoek, C. J. Bagley, P. Hoffmann, K. A. Gould and J. C. Paton, J. Bacteriol., 2010, 192, 4489–4497 CrossRef CAS.
- S. Jordan, M. I. Hutchings and T. Mascher, FEMS Microbiol. Rev., 2008, 32, 107–146 CrossRef CAS.
- E. Loisel, L. Jacquamet, L. Serre, C. Bauvois, J. L. Ferrer, T. Vernet, A. M. Di Guilmi and C. Durmort, J. Mol. Biol., 2008, 381, 594–606 CrossRef CAS.
- M. Lawrence, P. Pilling, V. Epa, A. Berry, A. D. Ogunniyi and J. C. Paton, Structure, 1998, 6, 1553–1561 CrossRef CAS.
- K. E. Stoll, W. E. Draper, J. I. Kliegman, M. V. Golynskiy, R. A. T. Brew-Appiah, R. K. Phillips, H. K. Brown, W. A. Breyer, N. S. Jakubovics, H. F. Jenkinson, R. G. Brennan, S. M. Cohen and A. Glasfeld, Biochemistry, 2009, 48, 10308–10320 CrossRef CAS.
- A. S. Prasad, Mol. Cell. Biochem., 1998, 188, 63–69 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: These include supplementary methods, Tables S1–S5 and Fig. S1–S7. See DOI: 10.1039/c0mt00050g |
| This journal is © The Royal Society of Chemistry 2011 |
