Oxathiazole-2-one derivative of bortezomib: Synthesis, stability and proteasome inhibition activity†
Berkley E.
Gryder
a,
Will
Guerrant
a,
Chin Ho
Chen
b and
Adegboyega K.
Oyelere
*a
aSchool of Chemistry and Biochemistry, Parker H. Petit Institute for Bioengineering and Bioscience, Georgia Institute of Technology, Atlanta, GA 30332-0400, USA. E-mail: aoyelere@gatech.edu; Fax: +01-404-894-2291; Tel: +01-404-894-4047
bSurgical Science, Department of Surgery, Duke University Medical Center, Durham, NC 27710, USA
First published on 16th September 2011
Abstract
Oxathiazole-2-one is a new candidate for proteasome inhibition which has not been widely explored. We describe herein the synthesis and characterization of a new oxathiazole-2-one derived from the dipeptide backbone of Bortezomib. We found that this new oxathiazole-2-one compound 1 is modestly active against the human 20S proteasome, but surprisingly has no significant activity against the M. tuberculosis proteasome. Additionally, the compound has improved aqueous stability compared to previously reported oxathiazole-2-one compounds. Molecular docking analyses provided information on the structural basis of the observed disparity between the human and mycobacterium proteasomes inhibitory activity of compound 1.
The proteasome is the cell's recycling plant which functions to maintain intracellular protein homeostasis. The 20S core particle of the proteasome has multicatalytic cores, located on the β1, β2, and β5 sub-units, with caspase-like, trypsin-like, and chymotrypsin-like proteolytic activity, respectively.1,2 At the active site of each proteasome subunit is an N-terminus threonine which facilitates peptide bond hydrolysis using a rare mechanism that involves its β-hydroxyl group.3 Inhibition of proteasome proteolytic activity has been recognized as a powerful approach for therapeutic intervention against human diseases including cancer, neurodegenerative disorders and inflammation.4,5
The FDA approval of dipeptidyl boronic acid Bortezomib (also known as Velcade, Fig. 1), for the treatment of multiple myeloma, has significantly boosted the validity of proteasome inhibition as a viable therapeutic approach.6 In addition to Bortezomib, a high potency reversible inhibitor of the β5 site of the proteasome,1,7 several other proteasome inhibitors have been identified from natural sources and rational drug design approach.5,8 As with most chemotherapeutic agents, Bortezomib suffers from a wide spectrum of serious side effects, such as peripheral neuropathy, hypotension,9 acute development of congestive heart failure,10 acute respiratory distress syndrome,11 fatigue and nausea, that would preclude its use for the treatment of less serious diseases.12 Thus the search for safer, less toxic alternatives is ongoing.
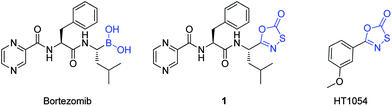 | ||
| Fig. 1 Structures of Bortezomib, Bortezomib-like oxathiazole-2-one 1 and mycobacterium proteasome inhibitor HT1054. | ||
Recently, Lin et al. disclosed a new class of small molecule proteasome inhibitor, derived from oxathiazole-2-one ring (see Fig. 1 for the structure of HT1054, a representative example), which are over 1000-fold more selective for the mycobacterium proteasome. These aryl oxathiazole-2-one compounds function through cyclocarboxylation of the active site threonine, with a concomitant constriction of the pocket to exclude the accommodation of the peptide substrate.13Oxathiazole-2-one compounds have shown antimycobacterial activity previously, but the mechanism of action was hitherto unknown.14 Although the catalytic active site threonine is conserved in both human and mycobacterium proteasomes, the greater accessibility of the inhibitor's carbonyl group to the mycobacterium proteasome active site threonine and protein landscape near the active site were suggested to be the major selectivity determinants.13 The particular contribution of either factor to inhibitor selectivity remains unresolved from this study. Intent on finding new generation of species-selective proteasome inhibitors, we sought to gain a further understanding of the role(s) of the oxathiazole-2-one moiety in dictating inhibitor's mycobacterium selectivity. We have investigated the effect of the replacement of the Bortezomib boronate group by oxathiazole-2-one ring on the proteasome inhibition activity. We report herein the synthesis, preliminary biological activity, aqueous stability and molecular docking analysis of the resulting Bortezomib-like oxathiazole-2-one.
The synthesis of the target Bortezomib-like oxathiazole-2-one 1, and its diastereomer 7, followed the route in Scheme 1. The coupling of pyrazine 2 with phenylalanine methyl ester 3, followed by saponification of the resulting intermediate ester with alkali, afforded carboxylic acid 4.15 The penultimate amide intermediates 6a and 6b were obtained when 4 was coupled to chirally pure leucine-amides 5a and 5b, respectively. The target oxathiazole-2-ones 1 and 7 were obtained by refluxing 6a and 6b with chlorocarbonyl sulfonyl chloride.14 The overall yield was 50%, with only the final cyclization step requiring chromatographic purification.
 | ||
Scheme 1 Synthesis of oxathiazole-2-one Bortezomib derivatives. Reagents and conditions: (a) TBTU, DIPEA, DMF:DCM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20), 0 °C, 12 h, 93%; (b) NaOH, acetone, 3 h, 25 °C, then HCl, 0 °C, 1 h, 96%; (c) TBTU, DIPEA, CH2Cl2, −5 to 25 °C, 1 h, 6a: 76%, 6b: 76%. (d) ClCOSCl, THF, reflux, 3 h, 1: 90%, 7: 85%. 20), 0 °C, 12 h, 93%; (b) NaOH, acetone, 3 h, 25 °C, then HCl, 0 °C, 1 h, 96%; (c) TBTU, DIPEA, CH2Cl2, −5 to 25 °C, 1 h, 6a: 76%, 6b: 76%. (d) ClCOSCl, THF, reflux, 3 h, 1: 90%, 7: 85%. | ||
The mycobacterium proteasome inhibition activities of compounds 1 and 7 were tested using an open-gate mutant M. tuberculosis proteasome (Mtb20SOG).16 We observed that both compounds were surprisingly inactive against the mycobacterium proteasome at concentrations in excess of 1000 μM (Table 1) with no significant loss in enzyme velocity up to 100 μM (Fig. 2A), tracked by cleavage of the fluorogenic peptide substrate Suc-LLVY-7-amido-4-methylcoumarin (AMC), used for measuring chymotrypsin-like peptidase activity.17 Because the primary target of Bortezomib, the starting template of compound 1, is the human proteasome, we probed to see if compounds 1 and 7 possessed any activity against it. The human proteasome inhibition activity was tested using the same cell free fluorescent assay, employing purified human erythrocyte 20S proteasome and Suc-LLVY-AMC. We found that these Bortezomib-like oxathiazole-2-ones modestly inhibit human proteasome activity, and the enzyme velocity was reduced in a dose dependent manner parallel to but more potently than with the Mtb20SOG (Fig. 2A). Using logit transformation to analyze the sigmoidal inhibition curves (Fig. 2B), the IC50 was derived from the x-intercept of the logit plot, determined by linear regression analysis.18 Significantly, compound 1, analog with the same chirality as Bortezomib, is twice as potent as compound 7 (Table 1). Bortezomib, the positive control compound, is active against both the human and mycobacterium proteasomes with IC50 similar to the values in the literature.19 These results suggest that substitution of the Bortezomib boronate group by oxathiazole-2-one ring is not compatible with the mycobacterium proteasome inhibition activity. Additionally, compounds 1 and 7 are preferential inhibitors human proteasome, although only modestly active.
 | ||
| Fig. 2 (A) Fraction of remaining enzyme velocity plotted as a function of inhibitor concentration for both human (H20S) and open-gate mutant M. tuberculosis 20SOG proteasome. (B) Logit analysis of the sigmoidal inhibition curves of human proteasome by compounds 1 and 7. (C) HeLa UbG76V-GFP cells, a proteasome inhibition detection system. | ||
To test if the modest activity of 1 and 7 translates into intracellular proteasome inhibition, we exposed these compounds at sub-IC50 levels (23 μM) to HeLa UbG76V-GFP for 16 h. Again, Bortezomib was used as a positive control. HeLa UbG76V-GFP cells accumulate UbG76V-GFP which emits green fluorescence in the presence of proteasome inhibitors.20,21 Under this condition, compound 1 resulted in a moderate accumulation of UbG76V-GFP while the level of green fluorescence in cells treated with 7 is almost indistinguishable from the control. As expected, Bortezomib (26 nM) resulted in a strong accumulation of UbG76V-GFP (Fig. 2C). This result paralleled the observed cell free proteasome inhibition activities of compounds 1 and 7 suggesting that the oxathiazole-2-one Bortezomib derivatives are able to penetrate the cell membrane.
The previously reported oxathiazole-2-one based proteasome inhibitors are hydrolytically labile with half-lives between a few minutes to a few hours.13 To investigate if the activity of the oxathiazole-2-one Bortezomib derivatives is compromised by their instability in aqueous media, we monitored the stability of 1 in 100 fold molar excess of deuterated water in DMSO by 1H-NMR at 37 °C. We found 1 to be very stable under this condition with a half-life of 6.5 days. Comparatively, HT1054 hydrolyzed with a half-life of 20 h under identical conditions (Fig. 3, see also the ESI†). This result strongly suggests that the weak proteasome inhibition activity of the oxathiazole-2-one Bortezomib derivatives is due to factors other than hydrolytic lability. Nevertheless, it gratifying to note that the oxathiazole-2-one Bortezomib derivatives have better aqueous stability than the previously reported oxathiazole-2-one based proteasome inhibitors.
 | ||
| Fig. 3 Hydrolytic stability of compound 1 and HT1054 monitored by 1H-NMR. | ||
We performed molecular docking analyses to obtain information on the structural basis of the observed disparity between the human and mycobacterium proteasomes inhibitory activity of compound 1 and HT1054 (a representative oxathiazole-2-one). Docking of the compounds against M. tuberculosis proteasome (PDB: 3KRD)22 and the human 20S proteasome (PDB: 2F16)7 was performed using Autodock Vina,23 with a 20 Å cube search area around the active site. Docked structures are ranked according to binding affinity scoring function,23 which gave highly repeatable and uniform output sets. In the human proteasome, the proximity of the 1,2-aminoalcohol moiety of the terminal catalytic threonine (Thr1) in the β5 subunit to the electrophilic carbon on the oxathiazole-2-one ring was within attack range for 1 (4.0 Å from the oxathiazole-2-one carbonyl group), but HT1054 preferentially docked in the cavity formed with the β6 subunit at about 11.0 Å away from Thr1 (Fig. 4A). In contrast, the oxathiazole-2-one ring of 1 was unable to come within reasonable distance of Thr1 in the M. tuberculosis proteasome, whereas the smaller HT1054 docked with its oxathiazole-2-one ring at 3.5 Å away from Thr1 (Fig. 4B), a distance sufficient to facilitate the Thr1 cyclocarboxylation observed by Lin et al.13 The differences in the binding orientations at the active sites of either proteasome could provide the molecular basis for the observed disparity in compound potency.
 | ||
| Fig. 4 In silico docking analysis of 1 (green) and HT1054 (yellow) with human 20S proteasome (A) and M. tuberculosis20S proteasome (B). Images were rendered using PyMol v1.2. | ||
We have described new oxathiazole-2-one derivative of Bortezomib compounds 1 and 7. Although these compounds showed moderate proteasome inhibition activity, they are selective for the human proteasome and have better aqueous stability relative to the previously reported oxathiazole-2-one based inhibitors. Efforts are currently underway in our laboratory to further probe the structure activity relationship of oxathiazole-2-one derived dipeptide proteasome inhibitors.
Acknowledgements
We are grateful to Dr Carl Nathan and Dr Gang Lin of Cornell University for a generous gift of the open-gate mutant M. tuberculosis 20SOG proteasome. This work was financially supported by Georgia Institute of Technology and by the Blanchard fellowship to AKO. B.G. and W.G. are recipients of the GAANN predoctoral fellowship from the Georgia Tech Center for Drug Design, Development, and Delivery.References
- J. Adams, The development of proteasome inhibitors as anticancer drugs, Cancer Cell, 2004, 5(5), 417–421 CrossRef CAS.
- A. L. Goldberg and A. C. Stjohn, Intracellular Protein Degradation in Mammalian and Bacterial-Cells. 2, Annu. Rev. Biochem., 1976, 45, 747–803 CrossRef CAS.
- O. Coux, K. Tanaka and A. L. Goldberg, Structure and functions of the 20S and 26S proteasomes, Annu. Rev. Biochem., 1996, 65, 801–847 CrossRef CAS.
- G. de Bettignies and O. Coux, Proteasome inhibitors: Dozens of molecules and still counting, Biochimie, 2010, 92(11), 1530–1545 CrossRef CAS.
- L. Huang and C. H. Chen, Proteasome Regulators: Activators and Inhibitors, Curr. Med. Chem., 2009, 16(8), 931–939 CrossRef CAS.
- B. A. Teicher, G. Ara, R. Herbst, V. J. Palombella and J. Adams, The proteasome inhibitor PS-341 in cancer therapy, Clin. Cancer Res., 1999, 5(9), 2638–2645 CAS.
- M. Groll, C. R. Berkers, H. L. Ploegh and H. Ovaa, Crystal structure of the boronic acid-based proteasome inhibitor bortezomib in complex with the yeast 20S proteasome, Structure, 2006, 14(3), 451–456 CrossRef CAS.
- A. Hershko, A. Ciechanover, H. Heller, A. L. Haas and I. A. Rose, Proposed Role of Atp in Protein Breakdown - Conjugation of Proteins with Multiple Chains of the Polypeptide Of Atp-Dependent Proteolysis, Proc. Natl. Acad. Sci. U. S. A., 1980, 77(4), 1783–1786 CrossRef CAS.
- J. Cortes, D. Thomas, C. Koller, F. Giles, E. Estey, S. Faderl, G. Garcia-Manero, D. McConkey, G. Patel, R. Guerciolini, J. Wright and H. Kantarjian, Phase I study of bortezomib in refractory or relapsed acute leukemias, Clin. Cancer Res., 2004, 10(10), 3371–3376 CrossRef CAS.
- J. Voortman and G. Giaccone, Severe reversible cardiac failure after bortezomib treatment combined with chemotherapy in a non-small cell lung cancer patient: a case report, BMC Cancer, 2006, 6 CrossRef.
- E. Chew, R. Filshie and A. Wei, Development of fatal bortezomib induced acute lung injury despite concurrent therapy with high-dose dexamethasone, Leuk. Lymphoma, 2007, 48(1), 212–213 CrossRef.
- P. G. Richardson, B. Barlogie, J. Berenson, S. Singhal, S. Jagannath, D. Irwin, S. V. Rajkumar, G. Srkalovic, M. Alsina, R. Alexanian, D. Siegel, R. Z. Orlowski, D. Kuter, S. A. Limentani, S. Lee, T. Hideshima, D.-L. Esseltine, M. Kauffman, J. Adams, D. P. Schenkein and K. C. Anderson, A Phase 2 Study of Bortezomib in Relapsed, Refractory Myeloma, N. Engl. J. Med., 2003, 348(26), 2609–2617 CrossRef CAS.
- G. Lin, D. Li, L. P. S. de Carvalho, H. Deng, H. Tao, G. Vogt, K. Wu, J. Schneider, T. Chidawanyika, J. D. Warren, H. Li and C. Nathan, Inhibitors selective for mycobacterial versus human proteasomes, Nature, 2009, 461(7264), 621–626 CrossRef CAS.
- M. H. Gezginci, A. R. Martin and S. G. Franzblau, Antimycobacterial activity of substituted isosteres of pyridine- and pyrazinecarboxylic acids. 2, J. Med. Chem., 2001, 44(10), 1560–1563 CrossRef CAS.
- M. Verdoes, B. I. Florea, W. A. van der Linden, D. Renou, A. van den Nieuwendijk, G. A. van der Marel and H. S. Overkleeft, Mixing of peptides and electrophilic traps gives rise to potent, broad-spectrum proteasome inhibitors, Org. Biomol. Chem., 2007, 5(9), 1416–1426 CAS.
- G. Lin, G. Q. Hu, C. Tsu, Y. Z. Kunes, H. L. Li, L. Dick, T. Parsons, P. Li, Z. Q. Chen, P. Zwickl, N. Weich and C. Nathan, Mycobacterium tuberculosis prcBA genes encode a gated proteasome with broad oligopeptide specificity, Mol. Microbiol., 2006, 59(5), 1405–1416 CrossRef CAS.
- R. L. Stein, F. Melandri and L. Dick, Kinetic characterization of the chymotryptic activity of the 20S proteasome, Biochemistry, 1996, 35(13), 3899–3908 CrossRef CAS.
- T. C. Chou and P. Talalay, Quantitative-Analysis Of Dose-Effect Relationships – The Combined Effects Of Multiple-Drugs Or Enzyme-Inhibitors, Adv. Enzyme Regul., 1984, 22, 27–55 CrossRef CAS.
- P. C. Trippier and C. McGuigan, Boronic acids in medicinal chemistry: anticancer, antibacterial and antiviral applications, Med. Chem. Commun., 2010, 1(3), 183–198 RSC.
- Z. Dang, A. Lin, P. Ho, D. Soroka, K. H. Lee, L. Huang and C. H. Chen, Synthesis and proteasome inhibition of lithocholic acid derivatives, Bioorg. Med. Chem. Lett., 2011, 21(7), 1926–1928 CrossRef CAS.
- N. P. Dantuma, K. Lindsten, R. Glas, M. Jellne and M. G. Masucci, Short-lived green fluorescent proteins for quantifying ubiquitin/proteasome-dependent proteolysis in living cells, Nat. Biotechnol., 2000, 18(5), 538–543 CrossRef CAS.
- G. Lin, D. Y. Li, T. Chidawanyika, C. Nathan and H. L. Li, Fellutamide B is a potent inhibitor of the Mycobacterium tuberculosis proteasome, Arch. Biochem. Biophys., 2010, 501(2), 214–220 CrossRef CAS.
- O. Trott and A. J. Olson, AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading, J. Comput. Chem., 2010, 31(2), 455–461 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c1md00208b |
| This journal is © The Royal Society of Chemistry 2011 |
