DOI:
10.1039/C1MD00130B
(Concise Article)
Med. Chem. Commun., 2011,
2, 1087-1092
Solution-phase synthesis of chiral O-acyl isodipeptides†
Received
19th May 2011
, Accepted 16th August 2011
First published on 14th September 2011
Abstract
O-Acylation of N-Boc-protected-serine and -threonine with N-Pg-(α-aminoacyl)benzotriazoles afforded the chiral O-acylated isodipeptides at 23 °C in yields of 74–91%.
The synthesis of peptides and proteins is of great importance to the understanding of biological functions. The solid-phase synthesis of peptides with “difficult sequences” remains problematic due to low yields and purity.1 In addition, intermolecular hydrophobic interactions in “difficult sequences” can promote aggregation in solution and hydrogen bond networks in resin-bound peptides can form extended structures such as β-sheets.1b–c,2
Kiso et al.1d demonstrated that the introduction of an O-acyl in place of an N-acyl residue within a peptide backbone significantly altered the secondary structure of native peptides. Furthermore, these “O-acyl isopeptides” or “click peptides”‡ are more hydrophilic, and easier to purify by HPLC.1d He found that a subsequent O–N intramolecular acyl migration, triggered by change in pH, could rapidly generate a target natural peptide under physiological conditions (pH 7.4) (Fig. 1).1c,3a This “O-acyl isopeptide method”3 has been used to develop new water-soluble taxoid prodrugs,4 HIV-1 protease inhibitors,5 the anti-tumor agent, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundpaclitaxel,6 difficult sequence-containing peptides including COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundAc-Val-Val-Ser-Val-Val-NH2,1b,4,7 Alzeheimer's disease-related amyloid βpeptide (Aβ) 1–42,4,7–13 and cyclic peptides.14
 |
| | Fig. 1
O-Acyl isopeptide methodology. | |
However, epimerization during the esterification step in the solid-phase synthesis of O-acyl isopeptides has remained a major problem.1c,e,15 Based on the hypothesis that epimerization during esterification should be suppressed in solution due to the faster coupling rate as compared to that on a solid support, Kiso1c,e synthesized O-acyl isodipeptides in three steps (Scheme 1): (i) protection of the carboxylic acid group in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundserine or COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundthreonine by benzyl esterification, (ii) O-acylation and (iii) deprotection using Pd/C. Treatment of Cbz-protected isodipeptides containing COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCys and Met with Pd/C–H2 failed, although catalytic hydrogen transfer (CTH) to Cys- and Met-containing protected isodipeptides gave 45% of the desired product.1c
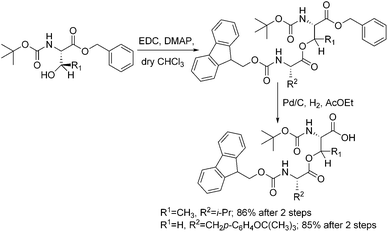 |
| | Scheme 1 Synthetic scheme of “O-acyl isodipeptide unit”. | |
We now report an efficient single-step preparation of chiral O-acyl isodipeptides from COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundserine and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundthreonine. The use of N-acylbenzotriazoles are advantageous for N-, O-, C-, S- acylation,16–18 especially where the corresponding acid chlorides are unstable or difficult to prepare. N-(Protected-α-aminoacyl)benzotriazoles have enabled fast preparations of biologically relevant peptides and peptide conjugates in high yields and purity, under mild reaction conditions, with full retention of the original chirality.18
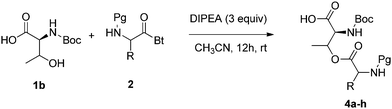
O-Acyl isoserinedipeptides 3a–h were prepared by O-acylation of Boc-protected serine 1a with various N-Pg-(α-aminoacyl)benzotriazoles 2 in the presence of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compounddiisopropylethylamine in CH3CN at 23 °C for 12 h in yields of 74–90%. This proved to be the optimum condition under which neither epimerization of 3 nor hydrolysis of 2 occurred. The presence of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundwater (MeCN/COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH2O, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in the reaction caused minimal hydrolysis of 2 (4%) but no epimerization was detected according to HPLC-MS analysis on 3b and (3b + 3b′). The O-acylated serines were characterized by NMR and elemental analysis (Table 1). HPLC analysis [chirobiotic T column (250 mm × 4.6 mm), detection at 254 nm, flow rate 2.5 mL min−1, COMPOUND LINKS
1) in the reaction caused minimal hydrolysis of 2 (4%) but no epimerization was detected according to HPLC-MS analysis on 3b and (3b + 3b′). The O-acylated serines were characterized by NMR and elemental analysis (Table 1). HPLC analysis [chirobiotic T column (250 mm × 4.6 mm), detection at 254 nm, flow rate 2.5 mL min−1, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundMeOH] on 3b (single peak, retention time 1.3 min) and (3b + 3b′) (two peaks, retention times, 1.3 min and 1.5 min) as well as HPLC-MS and (-)ESI-MS on 3b (Fig. 2) confirmed the absence of racemization in the targeted isodipeptides.
Table 1 The preparation of serine-based O- acyl-isodipeptides 3a–h
|

|
| Entry |
O-acyl isodipeptides 3a–h |
Yield (%) |
mp/°C |
|
1
|
Boc-L-Ser(Cbz-L-Ala)OH
3a |
84 |
oil |
|
2
|
Boc-L-Ser(Cbz-L-Phe)OH
3b |
81 |
oil |
|
3
|
Boc-L-Ser(Cbz-DL-Phe)OH(3b + 3b′) |
81 |
60–62 |
|
4
|
Boc-L-Ser(Cbz-L-Trp)OH
3c |
87 |
67–69 |
|
5
|
Boc-L-Ser(Cbz-L-Met)OH
3d |
74 |
oil |
|
6
|
Boc-L-Ser(Cbz-L-Val)OH
3e |
90 |
oil |
|
7
|
Boc-L-Ser(Cbz-Gly)OH
3f |
87 |
57–58 |
|
8
|
Boc-L-Ser(Cbz-Cys(Bzl))OH
3g |
85 |
oil |
|
9
|
Boc-L-Ser(Boc-Gly)OH
3h |
82 |
58–59 |
 |
| | Fig. 2
HPLC-MS and (-)ESI-MS analysis of 3b. | |
O-Acylated threonine based isodipeptides 4a–h were also prepared by O-acylation of Boc-protected threonine 1b with various N-Pg-(α-aminoacyl)benzotriazoles 2 in the presence of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compounddiisopropylethylamine in CH3CN at room temperature in yields of 86–91% (Table 2). The synthesized compounds were characterized by NMR and elemental analysis.
Table 2 The preparation of O-isodipeptides 4a–h
|
|
| Entry |
O-acyl isodipeptides 4a–h |
Yield (%) |
mp/°C |
|
1
|
Boc-L-Thr(Cbz-L-Ala)-OH
4a |
91 |
oil |
|
2
|
Boc-L-Thr(Cbz-L-Phe)-OH
4b |
87 |
oil |
|
3
|
Boc-L-Thr(Cbz-D,L-Phe)-OH (4b + 4b′) |
87 |
60–62 |
|
4
|
Boc-L-Thr(Cbz-L-Trp)-OH
4c |
91 |
89–90 |
|
5
|
Boc-L-Thr(Cbz-L-Met)-OH
4d |
90 |
oil |
|
6
|
Boc-L-Thr(Cbz-Gly)-OH
4e |
86 |
54–58 |
|
7
|
Boc-L-Thr(Cbz-Cys(Bzl))-OH
4f |
86 |
oil |
|
8
|
Boc-L-Thr(Boc-Gly)-OH
4g |
85 |
64–65 |
|
9
|
Boc-L-Thr(Boc-L-Phe)-OH
4h |
90 |
47–50 |
HPLC analysis [chirobiotic T column (250 mm × 4.6 mm), detection at 254 nm, flow rate 0.5 mL min−1, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundMeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) COMPOUND LINKS
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH2O, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1] on 4b (single peak, retention time 7.23 min) and (4b + 4b′) (two peaks, retention times, 6.54 min and 7.20 min) confirmed the absence of racemization in the desired isodipeptides.
1] on 4b (single peak, retention time 7.23 min) and (4b + 4b′) (two peaks, retention times, 6.54 min and 7.20 min) confirmed the absence of racemization in the desired isodipeptides.
Conclusions
In conclusion, the mild protocol reported herein enables the efficient preparation of optically pure O-acyl isopeptides from COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundserine and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundthreonine without protection of their carboxyl groups.
Materials and methods
General
Melting points were determined on a capillary point apparatus equipped with a digital thermometer and are uncorrected. The NMR spectra were recorded in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3 with TMS for 1H (300 MHz) and 13C (75 MHz) as an internal reference.
General procedure for the preparation of N-(Pg-α-aminoacyl)benzotriazoles 2
(S)-Benzyl (1-(1H-benzo[d][1,2,3]triazol-1-yl)-1-oxopropan-2-yl)carbamate (2a).
white solid (90%); mp 115 °C (lit.19c mp 113–115 °C); 1H NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 8.16 (d, J = 8.1 Hz, 1H), 8.04 (d, J = 8.4 Hz, 1H), 7.57 (t, J = 7.8 Hz, 1H), 7.43 (t, J = 7.7 Hz, 1H), 7.40–7.03 (m, 6H), 5.80–5.60 (m, 2H), 5.10–4.99 (m, 1H), 1.59 (d, J = 6.3 Hz, 3H); 13C NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 172.2, 155.6, 145.9, 136.0, 131.0, 130.6, 128.4, 128.1, 126.4, 120.2, 114.3, 67.1, 50.5, 19.0.
(S)-Benzyl (1-(1H-benzo[d][1,2,3]triazol-1-yl)-1-oxo-3-phenylpropan-2-yl)carbamate (2b).
white solid (90%); mp 150–152 °C (lit.20 mp 149.0–150.0 °C); 1H NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 8.23 (d, J = 7.8 Hz, 1H), 8.15 (d, J = 7.8 Hz, 1H), 7.68 (t, J = 7.4 Hz, 1H), 7.54 (t, J = 7.5 Hz, 1H), 7.32–7.23 (m, 7H), 7.14 (br s, 3H), 6.09 (d, J = 4.2 Hz, 1H), 5.57 (d, J = 6.6 Hz, 1H), 5.08 (s, 2H), 3.48 (d, J = 9.6 Hz, 1H), 3.24 (d, J = 7.8 Hz, 1H); 13C NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 170.8, 155.7, 146.0, 135.9, 134.9, 131.0, 130.8, 129.2, 128.7, 128.5, 128.1, 127.4, 126.5, 120.4, 114.3, 67.2, 55.6, 38.8.
Benzyl (1-(1H-benzo[d][1,2,3]triazol-1-yl)-1-oxo-3-phenylpropan-2-yl)carbamate (2b + 2b′).
white solid (90%); mp 141–142 °C (lit.21 mp 141–142 °C); 1H NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 8.23 (d, J = 7.8 Hz, 1H), 8.15 (d, J = 7.8 Hz, 1H), 7.68 (t, J = 7.4 Hz, 1H), 7.54 (t, J = 7.5 Hz, 1H), 7.32–7.23 (m, 7H), 7.14 (br s, 3H), 6.09 (d, J = 4.2 Hz, 1H), 5.57 (d, J = 6.6 Hz, 1H), 5.08 (s, 2H), 3.48 (d, J = 9.6 Hz, 1H), 3.24 (d, J = 7.8 Hz, 1H); 13C NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 170.8, 155.7, 146.0, 135.9, 134.9, 131.0, 130.8, 129.2, 128.7, 128.5, 128.1, 127.4, 126.5, 120.4, 114.3, 67.2, 55.6, 38.8.
(S)-Benzyl (1-(1H-benzo[d][1,2,3]triazol-1-yl)-3-(1H-indol-3-yl)-1-oxopropan-2-yl)carbamate (2c).
yellow solid (80%); mp 101 °C (lit.19 mp 98–100 °C); 1H NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 8.23 (br s, 1H), 8.14–8.06 (m, 2H), 7.57 (t, J = 7.5 Hz, 1H), 7.46 (t, J = 7.5 Hz, 1H), 7.38 (d, J = 7.8 Hz, 1H), 7.31–7.24 (m, 4H), 7.20 (s, 1H), 7.10–7.04 (m, 1H), 6.95–6.90 (m, 2H), 6.87–6.85 (m, 1H), 6.14–6.10 (m, 1H), 5.70 (d, J = 7.5 Hz, 1H), 5.03 (s, 2H), 3.58 (dd, J = 15, 4.5 Hz, 1H), 3.40 (dd, J = 14.9, 7.7 Hz, 1H); 13C NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 171.1, 155.9, 145.8, 136.1, 131.0, 130.6, 128.4, 128.1, 127.0, 126.4, 123.2, 122.2, 120.2, 119.7, 118.3, 114.3, 111.2, 108.9, 67.2, 55.1, 28.7.
(S)-Benzyl (1-(1H-benzo[d][1,2,3]triazol-1-yl)-4-(methylthio)-1-oxobutan-2-yl)carbamate (2d).
beige solid (85%); mp 108–109 °C (lit.19 mp 108–109 °C); 1H NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 8.15 (d, J = 8.1 Hz, 1H), 8.05 (d, J = 8.1 Hz, 1H), 7.59 (t, J = 7.8 Hz, 1H), 7.44 (t, J = 7.7 Hz, 1H), 7.30–7.24 (m, 5H), 5.83–5.79 (m, 1H), 5.67–5.65 (m, 1H), 5.03 (s, 2H), 2.59 (t, J = 7.1 Hz, 2H), 2.35 (br s, 1H), 2.10–2.03 (m, 1H), 1.97 (s, 3H);13C NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 171.2, 155.7, 130.9, 146.0, 130.8, 128.4, 128.5, 128.3, 126.6, 120.4, 114.3, 67.4, 54.2, 32.4, 30.0, 15.4; Anal. Calcd for C19H20N4O3S: C, 59.36; H, 5.24; N, 14.57. Found: C, 59.69; H, 5.23; N, 14.54.
(S)-Benzyl (1-(1H-benzo[d][1,2,3]triazol-1-yl)-3-methyl-1-oxobutan-2-yl)carbamate (2e).
white powder (87%); mp 107 °C (lit.20 mp 73–74 °C); 1H NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 8.28 (d, J = 8.0 Hz, 1H), 8.15 (d, J = 8.2 Hz, 1H), 7.68 (dd, J = 8.0, 7.4 Hz, 1H), 7.54 (dd, J = 8.1, 7.3 Hz, 1H), 7.37 (br s, 5H), 5.78–5.74 (m, 1H), 5.57 (d, J = 9.2 Hz, 1H), 5.14 (s, 2H), 2.61–2.43 (m, 1H), 1.13 (d, J = 6.6 Hz, 3H), 0.97 (d, J = 6.6 Hz, 3H); 13C NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 171.5, 156.2, 146.0, 136.0, 131.0, 130.7, 128.5, 128.2, 126.5, 120.3, 114.3, 67.3, 59.4, 31.6, 19.7, 17.0.
(S)-Benzyl (2-(1H-benzo[d][1,2,3]triazol-1-yl)-2-oxoethyl)carbamate (2f).
white solid (90%); mp 110–111 °C (lit.22 mp 106–108 °C); 1H NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 8.24 (d, J = 8.4 Hz, 1H), 8.14 (d, J = 8.1 Hz, 1H), 7.68 (t, J = 7.5 Hz, 1H), 7.53 (t, J = 7.5 Hz, 1H), 7.39–7.34 (m, 5H), 5.61 (br s, 1H), 5.19 (s, 2H), 5.09 (d, J = 5.7 Hz, 2H); 13C NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 168.3, 156.4, 145.9, 136.0, 130.8, 128.5, 128.2, 128.1, 126.5, 120.3, 114.0, 67.4, 44.8.
(R)-Benzyl (1-(1H-benzo[d][1,2,3]triazol-1-yl)-3-(benzylthio)-1-oxopropan-2-yl)carbamate (2g).
yellow oil (87%); 1H NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 8.31 (d, J = 8.4 Hz, 1H), 8.20 (d, J = 8.1 Hz, 1H), 7.74 (t, J = 8.1 Hz, 1H), 7.60 (t, J = 8.1 Hz, 1H), 7.47– 7.17 (m, 10H), 6.03 (br s, 2H), 5.22 (br s, 2H), 3.79 (br s, 2H), 3.28–2.89 (m, 2H); 13C NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 169.8, 155.7, 145.9, 136.0, 135.9, 130.8, 128.8, 128.4, 128.2, 128.1, 127.1, 126.6, 125.8, 120.3, 114.3, 67.4, 53.7, 36.1, 33.6; Anal. Calcd for C24H22N4O3S: C, 64.56; H, 4.51; N 12.55. Found: C, 64,26; H, 4.93; N 12.90.
General procedure for the preparation of O-acyl isodipeptides 3 and 4
DIPEA (0.44 mL, 3 equiv) was added to a solution of Boc-L-SerOH or Boc-L-ThrOH (0.49 mmol, 1 equiv) in MeCN (15 mL). The appropriate N-(Pg-α-aminoacyl)benzotriazole 2 (0.49 mmol, 1 equiv) dissolved in MeCN (5 mL) was added to the clear solution and the mixture was stirred for 12 h at room temperature. Complete reaction was judged by the disappearance of starting material. The solution was acidified with 1N HCl, evaporated; the residue was dissolved in EtOAc and washed with 1N HCl. The organic portion was dried over anhyd Na2SO4, filtered and dried to give the corresponding O-acyl isodipeptide. All samples were then freeze-dried and fully characterized.
(R)-3-(((S)-2-(((Benzyloxy)carbonyl)amino)propanoyl)oxy)-2-((tert-butoxycarbonyl)amino)propanoic acid (3a).
prepared from 2a. colorless oil (84%); 1H NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 10.2 (br s, 1H), 7.27–7.15 (m, 5H), 5.62–5.49 (m, 1H), 5.25–4.96 (m, 2H), 4.63–4.29 (m, 4H), 4.10–3.98 (m, 1H), 1. 36 (s, 9H); 13C NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 172.8, 172.4, 156.1, 155.6, 136.0, 128.4, 128.1, 80.6, 67.1, 64.9, 52.7, 49.7, 28.2, 18.1; Anal. Calcd for C19H26N2O8: C, 55.60; H, 6.39; N, 6.83. Found: C, 55.31; H, 6.41; N, 6.70.
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound(R)-3-(((S)-2-(((Benzyloxy)carbonyl)amino)-3-phenylpropanoyl)oxy)-2-((tert-butoxycarbonyl)amino)propanoic acid (3b).
prepared from 2b. yellow transparent oil (81%); 1H NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 10.8 (bs, 1H), 7.31–7.18 (m, 10H), 7.11–7.05 (m, 2H), 5.53–5.44 (m, 1H), 5.10–4.97 (m, 2H), 4.64–4.55 (m, 2H), 4.41–4.32 (m, 2H), 3.04–2.98 (m, 1H), 1.42 (br s, 9H); 13C NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 172.6, 171.2, 156.0, 135.5, 129.3, 129.1, 128.6, 128.5, 128.4, 128.1, 128.0, 127.2, 80.6, 67.2, 65.2, 54.9, 52.5, 38.0, 28.2; Anal. Calcd for C25H30N2O8: C, 61.72; H, 6.22; N, 5.76. Found: C, 61.79; H, 6.34; N, 5.42.
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound3-(((S)-2-(((Benzyloxy)carbonyl)amino)-3-phenylpropanoyl)oxy)-2-((tert-butoxycarbonyl)amino)propanoic acid (3b + 3b′).
prepared from (2b + 2b′). colorless solid (81%); mp 60–62 °C; 1H NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 8.85 (br s, 1H), 7.33–7.10 (m, 10H), 7.05–6.95 (m, 2H), 5.59–5.41 (m, 1H), 5.03 (bs, 2H), 4.64–4.17 (m, 3H), 3.03 (bs, 2H), 1.41 (bs, 9H), 1.23–1.18 (m, 3H) 13C NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 175.2, 172.5, 171.1, 156.0, 155.5, 135.9, 135.5, 129.1, 128.5, 128.4, 128.0, 127.1, 80.5, 67.1, 65.2, 60.5, 54.9, 53.6, 52.5, 38.8, 37.9, 37.7, 29.6, 28.2, Anal. Calcd for C25H30N2O8: C, 61.72; H, 6.22; N, 5.76. Found: C, 61.79; H, 6.35; N, 5.42.
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound(S)-3-(((S)-2-(((Benzyloxy)carbonyl)amino)-3-(1H-indol-3-yl)propanoyl)oxy)-2-((tert-butoxycarbonyl)amino)propanoic acid (3c).
prepared from 2c. yellow gel (85%); 1H NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 8.37–8.31 (m, 1H), 7.92 (br s, 1H), 7.55–7.42 (m, 1H), 7.25–7.04 (m, 7H), 6.90–6.81 (m, 1H), 5.50–5.44 (m, 1H), 5.12–4.98 (m, 2H), 4.70–4.65 (m, 1H), 4.54 (br s, 1H), 4.38–4.28 (m, 1H), 3.31–3.21 (m, 2H), 1.44 (br s, 9H); 13C NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 175.9, 172.8, 171.6, 156.1, 155.7, 136.1, 128.5, 128.1, 127.3, 123.0, 122.0, 120.0, 118.3, 111.4, 109.3, 80.7, 67.2, 65.0, 54.7, 52.7, 28.3; Anal. Calcd for C27H31N3O8: C, 61.71; H, 5.95; N, 8.00. Found: C, 61.96; H, 6.00; N, 7.61.
(R)-3-(((S)-2-(((Benzyloxy)carbonyl)amino)-4-(methylthio)butanoyl)oxy)-2-((tert-butoxycarbonyl)amino)propanoic acid (3d).
prepared from 2d. yellow oil (74%); 1H NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 9.40 (bs, 1H), 7.27–7.12 (m, 5H), 5.72–5.62 (m, 1H), 5.08–4.97 (m, 2H), 4.57–4.42 (m, 3H), 2.49–2.41 (m, 2H), 2.22–1.84 (m, 5H), 1.36 (br s, 9H); 13C NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 175.7, 172.7, 171.4, 156.2, 155.5, 135.8, 128.4, 128.1, 80.6, 67.2, 65.1, 53.2, 52.7, 31.5, 29.7, 28.2, 15.2; Anal. Calcd for C21H30N2O8S: C, 53.60; H, 6.43; N, 5.95. Found: C, 53.94; H, 6.56; N, 5.52.
(S)-3-(((S)-2-(((Benzyloxy)carbonyl)amino)-3-methylbutanoyl)oxy)-2-((tert-butoxycarbonyl)amino)propanoic acid (3e).
prepared from 2e. yellow oil (90%); 1H NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 9.54 (br s, 1 H), 7.34–7.22 (m, 5H), 5.80–5.51 (m, 1H), 5.12–5.00 (m, 2H), 4.62–4.02 (m, 3H), 2.13–2.00 (m, 1H), 1.39 (br s, 9H), 0.96–0.83 (m, 6H); 13C NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 172.5, 171.4, 156.5, 155.5, 135.9, 128.4, 128.1, 80.5, 67.2, 64.7, 59.1, 52.7, 31.0, 28.2, 18.9, 17.4; Anal. Calcd for C21H30N2O8: C 57.52; H 6.90; N 6.39. Found: C: 57.79, H 6.69, N 6.45.
(S)-3-(2-(((Benzyloxy)carbonyl)amino)acetoxy)-2-((tert-butoxycarbonyl)amino)propanoic acid (3f).
prepared from 2f. colorless solid (87%); mp 57–58 °C; 1H NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 10.18 (br s, 1H), 7.13–7.11 (m, 5H), 5.62–5.47 (m, 1H), 4.90 (br s, 2H), 4.41–4.17 (m, 2H), 3.73 (br s, 2H), 1.23 (br s, 9H); 13C NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 172.5, 169.7, 156.7, 155.5, 135.9, 128.4, 128.0, 80.5, 67.2, 65.0, 52.5, 42.5, 28.2; Anal. Calcd for C18H24N2O8: C, 54.54; H, 6.10; N, 7.07. Found: C, 54.72; H, 6.04; N, 6.73.
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound(S)-3-(((R)-2-(((Benzyloxy)carbonyl)amino)-3-(benzylthio)propanoyl)oxy)-2-((tert-butoxycarbonyl)amino)propanoic acid (3g).
prepared from 2g. yellow gel (85%); 1H NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 10.9 (br s, 1H), 7.49–7.16 (m, 11H), 5.81–5.71 (m, 1H), 5.09–4.98 (m, 2H), 4.61–4.32 (m, 3H), 3.66 (s, 2H), 2.79–2.73 (m, 2H), 1.36 (br s, 9H); 13C NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 172.3, 170.1, 156.0, 155.4, 137.2, 135.7, 128.8, 128.4, 128.0, 127.1, 80.4, 67.2, 65.2, 53.3, 52.5, 36.1, 33.1, 28.1; Anal. Calcd for C26H32N2O8S: C, 58.63; H, 6.06; N, 5.26. Found: C, 58.95; H, 6.08; N, 5.12.
(S)-2-((Tert-butoxycarbonyl)amino)-3-(2-((tert-butoxycarbonyl)amino)acetoxy)propanoic acid (3h).
prepared from 2h. white microcrystals (82%); mp 58–59 °C; 1H NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 7.38–7.28 (s, 2H), 5.59–5.20 (m, 1H), 4.58–4.45 (m, 3H), 3.86 (d, J = 4.2 Hz, 2H), 1.38 (s,18H); 13C NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 172.3, 170.1, 156.1, 155.6, 126.1, 114.9, 82.1, 80.5, 65.1, 52.7, 43.9, 42.3, 28.3; Anal. Calcd for C15H26N2O8: C, 49.72; H, 7.23; N, 7.73. Found: C, 50.41; H, 7.29; N, 8.16.
(2S)-3-(((S)-2-(((Benzyloxy)carbonyl)amino)propanoyl)oxy)-2-((tert-butoxycarbonyl)amino)butanoic acid (4a).
prepared from 2a. colorless oil (91%); 1H NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 9.73 (bs, 1H), 7.13–7.10 (m, 6H), 5.56–5.53 (m, 1H), 5.28 (br s, 1H), 4.89 (br s, 2H), 4.31–3.82 (m,2H); 1.24 (s, 9H), 1.11–1.05 (m, 6H); 13C NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 173.8, 173.2, 171.8, 156.1, 136.0, 128.4, 128.1, 80.3, 71.9, 67.0, 58.2, 57.0, 49.6, 28.2, 18.0, 16.6; Anal. Calcd for C20H28N2O8: C, 56.60; H, 6.65; N, 6.60. Found: C, 56.21; H, 6.55; N, 6.40.
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound(2S)-3-(((S)-2-(((Benzyloxy)carbonyl)amino)-3-phenylpropanoyl)oxy)-2-((tert-butoxycarbonyl)amino)butanoic acid (4b).
prepared from 2b. colorless oil (87%); 1H NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 9.86 (bs, 1H), 7.31–7.13 (m, 10 H), 7.13 (br s, 2H), 5.42 (br s, 1H), 5.10–4.99 (m, 2H), 4.79–4.40 (m, 2H), 3.03 (d, J = 6.0 Hz, 2H), 1.46 (s, 9H), 1.28–1.18 (m, 3H); 13C NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 173.4, 170.6, 156.1, 135.7, 129.2, 128.5, 128.0, 127.0, 80.3, 72.2, 67.1, 56.8, 54.8, 38.1, 28.2, 16.6; Anal. Calcd for C26H32N2O8: C, 62.39; H, 6.44; N, 5.60. Found: C, 61.99; H, 6.44; N, 5.53.
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound(2S)-3-((2-(((Benzyloxy)carbonyl)amino)-3-phenylpropanoyl)oxy)-2-((tert-butoxycarbonyl)amino)butanoic acid (4b + 4b′).
prepared from (2b + 2b′). yellow solid (87%); mp 60–62 °C; 1H NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 9.56 (br s, 1H), 7.38–7.19 (m, 10H), 7.08 (br s, 2H), 5.36–5.30 (m, 2H), 5.01 (br s, 2H), 4.64–4.44 (m, 2H), 3.13–2.99 (m, 3H), 1.42 (br s, 9H), 1.19 (d, J = 22.8 Hz, 3H); 13C NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 175.7, 173.5, 170.8, 156.0, 136.0, 135.6, 129.3, 128.5, 128.4, 128.1, 127.0, 80.4, 72.3, 67.0, 56.8, 54.6, 38.1, 37.7, 28.2, 16.7; Anal. Calcd for C26H32N2O8: C, 62.39; H, 6.44; N, 5.60. Found: C, 62.46; H, 6.50; N, 5.59.
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound(2S)-3-(((S)-2-(((Benzyloxy)carbonyl)amino)-3-(1H-indol-3-yl)propanoyl)oxy)-2-((tert-butoxycarbonyl)amino)butanoic acid (4c).
prepared from 2c. yellow oil (90%); 1H NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 8.38–8.15 (m, 1H), 7.54 (d, J = 5.4 Hz, 1H), 7.42–6.90 (m, 12H), 5.52–5.39 (m, 2H), 5.10–4.96 (m, 3H), 3.22 (s, 2H), 1.45 (s, 9H), 1.25–1.14 (m, 3H); 13C NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 172.7, 170.9, 156.1, 136.0, 128.5, 128.1, 127.5, 122.9, 122.3, 119.7, 118.6, 111.2, 109.6, 80.5, 72.0, 67.2, 56.8, 54.6, 28.3, 27.7, 16.5; Anal. Calcd for C28H33N3O8: C, 62.33; H, 6.16; N, 7.79. Found: C, 62.34; H, 6.30; N, 7.60.
(2R)-3-(((S)-2-(((Benzyloxy)carbonyl)amino)-4-(methylthio)butanoyl)oxy)-2-((tert-butoxycarbonyl)amino)butanoic acid (4d).
prepared from 2d. yellow oil (90%); 1H NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 8.84 (br s, 1H), 8.58 (br s, 1H), 7.34–7.27 (m, 5H), 5.86–5.83 (m, 1H), 5.71 (t, J = 8.9 Hz, 1H), 5.52 (br s, 1H), 5.10 (br s 2H), 4.63–4.38 (m, 1H), 2.54–2.47 (m, 2H), 2.05–1.91 (m, 4H), 1.38 (s, 9H), 1.28–1.27 (m, 3H); 13C NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 173.6, 170.7, 156.3, 156.1, 135.9, 128.4, 128.1, 80.4, 72.1, 67.2, 56.8, 53.2, 31.5, 29.8, 28.2, 16.7, 15.4; Anal. Calcd for C22H32N2O8S: C, 54.53; H, 6.66; N, 5.78. Found: C, 54.87; H, 6.77; N, 5.76.
(2S)-3-(2-(((Benzyloxy)carbonyl)amino)acetoxy)-2-((tert-butoxycarbonyl)amino)butanoic acid (4e).
prepared from 2f. colorless solid (86%); mp 54–57 °C; 1H NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 7.33–7.31 (m, 5H), 7.01 (bs, 1H), 5.62–5.40 (m, 2H), 5.10 (s, 2H), 3.90 (d, J = 5.7 Hz, 2H), 1.45 (br s, 9H), 1.29 (d, J = 6.9 Hz, 3H); 13C NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 173.3, 169.2, 156.5, 156.0, 136.0, 128.5, 128.1, 80.5, 72.0, 67.2, 56.8, 42.6, 28.2, 16.8; HRMSm/z for for C19H26N2O8Na [M + Na]+ calcd 433.1581, found 433.1597
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound(2S)-3-(((R)-2-(((Benzyloxy)carbonyl)amino)-3-(benzylthio)propanoyl)oxy)-2-((tert-butoxycarbonyl)amino)butanoic acid (4f).
prepared from 2g. yellow gel (86%); 1H NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 9.05 (br s, 1H), 7.50–7.09 (m, 11H), 5.83–5.78 (m, 1H), 5.44–5.39 (m, 1H), 5.08–4.96 (m, 2H), 4.56–4.44 (m, 1H), 3.62 (s, 2H), 2.82–2.50 (m, 2H), 1.39 (br s, 9H), 1.20 (d, J = 6.3 Hz, 3H); 13C NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 173.2, 169.5, 156.0, 137.4, 135.8, 128.8, 128.4, 128.0, 127.1, 80.3, 72.4, 67.1, 56.7, 53.3, 36.2, 33.0, 28.2, 16.6; HRMSm/z for for C27H34N2O8SNa [M + Na]+ calcd. 569.1928, found 569.1939.
(2S,3S)-2-((Tert-butoxycarbonyl)amino)-3-(2-((tert-butoxycarbonyl)amino)acetoxy)butanoic acid (4g).
prepared from 2h. white microcrystals (85%); mp 64–65 °C; 1H NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 7.89 (s, 2H), 5.58–5.38 (m, 3H), 4.47–4.45 (m, 1H), 3.84 (s, 2H), 1.43 and 1.42 (overlapped s, 18H), 1.31 (d, J = 5.7 Hz, 3H);13C NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 173.2, 169.7, 156.6, 156.1, 81.8, 80.4, 72.0, 56.9, 43.6, 28.3, 16.8; Anal. Calcd for C16H28N2O8: C, 51.05; H, 7.50; N, 7.44. Found: C, 50.72; H, 7.64; N, 7.41.
(2S,3S)-2-((Tert-butoxycarbonyl)amino)-3-(((S)-2-((tert-butoxycarbonyl)amino)-3-phenylpropanoyl)oxy)butanoic acid (4h).
prepared from 2i. white microcrystals (90%); mp 47–50 °C; 1H NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 7.07–7.01 (m, 5H), 6.97–6.95 (m, 2H), 5.21 (s, 1H), 4.92–4.80 (m, 3H), 4.31–4.25 (m, 2H), 2.83 (s, 1H), 1.28 (s, 9H), 1.21 (s, 9H), 1.06–1.05 (m, 3H); 13C NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 171.0, 156.1, 155.5, 129.2, 128.6, 127.1, 80.4, 72.1, 56.8, 54.4, 38.1, 28.3, 16.6; Anal. Calcd for C23H34N2O8: C, 59.21; H, 7.35; N, 6.00. Found: C, 59.62; H, 7.92; N, 6.54.
Acknowledgements
We thank the University of Florida, The Kenan Foundation and King Abdulaziz University, Jeddah, Saudi Arabia for financial support. We thank Dr C. D. Hall for helpful discussions.
Notes and references
-
(a) B. Bacsa, S. Bősze and C. O. Kappe, J. Org. Chem., 2010, 75, 2103 CrossRef CAS;
(b) Y. Sohma, M. Sasaki, Y. Hayashi, T. Kimura and Y. Kiso, Chem. Commun., 2004, 124 RSC;
(c) Y. Sohma, A. Taniguchi, M. Skwarczynski, T. Yoshiya, F. Fukao, T. Kimura, Y. Hayashi and Y. Kiso, Tetrahedron Lett., 2006, 47, 3013 CrossRef CAS;
(d) Y. Sohma, T. Yoshiya, A. Taniguchi, T. Kimura, Y. Hayashi and Y. Kiso, Biopolymers, 2007, 88, 253 CrossRef;
(e) T. Yoshiya, A. Taniguchi, Y. Sohma, F. Fukao, S. Nakamura, N. Abe, N. Ito, M. Skwarczynski, T. Kimura, Y. Hayashi and Y. Kiso, Org. Biomol. Chem., 2007, 5, 1720 RSC;
(f) I. Coin, R. Dölling, E. Krause, M. Bienert, M. Beyermann, C. D. Sferdean and L. A. Carpino, J. Org. Chem., 2006, 71, 6171 CrossRef;
(g) L. A. Carpino, E. Krause, C. D. Sferdean, M. Schümann, H. Fabian, M. Bienert and M. Beyermann, Tetrahedron Lett., 2004, 45, 7519 CrossRef CAS;
(h) R. B. Merrifield, J. Am. Chem. Soc., 1963, 85, 2149 CrossRef CAS;
(i) J. A. Moore, J. R. Dice, E. D. Nicolaides, R. D. Westland and E. L. Wittle, J. Am. Chem. Soc., 1954, 76, 2884 CrossRef CAS;
(j) T. Wöhr, F. Wahl, A. Nefzi, B. Rohwedder, T. Sato, X. Sun and M. Mutter, J. Am. Chem. Soc., 1996, 118, 9218 CrossRef;
(k) G. L. Thomas and R. J. Payne, Chem. Commun., 2009, 4260 RSC.
- J. P. Tam and Y.-A. Lu, J. Am. Chem. Soc., 1995, 117, 12058 CrossRef CAS.
-
(a) M. Skwarczynski and Y. Kiso, Curr. Med. Chem., 2007, 14, 2813 CrossRef CAS;
(b) M. Mutter, A. Chandravarkar, C. Boyat, J. Lopez, S. D. Santos, B. Mandal, R. Mimna, K. Murat, L. Patiny, L. Saucède and G. Tuchscherer, Angew. Chem., Int. Ed., 2004, 43, 4172 CrossRef;
(c) S. D. Santos, A. Chandravarkar, B. Mandal, R. Mimna, K. Murat, L. Saucède, P. Tella, G. Tuchscherer and M. Mutter, J. Am. Chem. Soc., 2005, 127, 11888 CrossRef CAS.
-
(a) Y. Sohma, Y. Hayashi, M. Skwarczynski, Y. Hamada, M. Sasaki, T. Kimura and Y. Kiso, Biopolymers, 2004, 76, 344 CrossRef CAS;
(b) M. Skwarczynski, Y. Sohma, M. Kimura, Y. Hayashi, T. Kimura and Y. Kiso, Bioorg. Med. Chem. Lett., 2003, 13, 4441 CrossRef CAS.
- Y. Hamada, H. Matsumoto, T. Kimura, Y. Hayashi and Y. Kiso, Bioorg. Med. Chem. Lett., 2003, 13, 2727 CrossRef CAS.
- Y. Hayashi, M. Skwarczynski, Y. Hamada, Y. Sohma, T. Kimura and Y. Kiso, J. Med. Chem., 2003, 46, 3782 CrossRef CAS.
- Y. Sohma, Y. Hayashi, M. Kimura, Y. Chiyomori, A. Taniguchi, M. Sasaki, T. Kimura and Y. Kiso, J. Pept. Sci., 2005, 11, 441 CrossRef CAS.
- Y. Sohma, M. Sasaki, Y. Hayashi, T. Kimura and Y. Kiso, Tetrahedron Lett., 2004, 45, 5965 CrossRef CAS.
- Y. Sohma, Y. Chiyomori, M. Kimura, F. Fukao, A. Taniguchi, Y. Hayashi, T. Kimura and Y. Kiso, Bioorg. Med. Chem., 2005, 13, 6167 CrossRef CAS.
- A. Taniguchi, Y. Sohma, M. Kimura, T. Okada, K. Ikeda, Y. Hayashi, T. Kimura, S. Hirota, K. Matsuzaki and Y. Kiso, J. Am. Chem. Soc., 2006, 128, 696 CrossRef CAS.
- Y. Sohma, A. Taniguchi, T. Yoshiya, Y. Chiyomori, F. Fukao, S. Nakamura, M. Skwarczynski, T. Okada, K. Ikeda, Y. Hayashi, T. Kimura, S. Hirota, K. Matsuzaki and Y. Kiso, J. Pept. Sci., 2006, 12, 823 CrossRef CAS.
- A. Taniguchi, T. Yoshiya, N. Abe, F. Fukao, Y. Sohma, T. Kimura, Y. Hayashi and Y. Kiso, J. Pept. Sci., 2007, 13, 868 CrossRef CAS.
- A. Taniguchi, M. Skwarczynski, Y. Sohma, T. Okada, K. Ikeda, H. Prakash, H. Mukai, Y. Hayashi, T. Kimura, S. Hirota, K. Matsuzaki and Y. Kiso, ChemBioChem, 2008, 9, 3055 CrossRef CAS.
- T. Yoshiya, H. Kawashima, Y. Hasegawa, K. Okamoto, T. Kimura, Y. Sohma and Y. Kiso, J. Pept. Sci., 2010, 16, 437 CAS.
- T. Yoshiya, Y. Sohma, T. Kimura, Y. Hayashi and Y. Kiso, Tetrahedron Lett., 2006, 47, 7905 CrossRef CAS.
- A. R. Katritzky, M. El Khatib, O. Bol'shakov, L. Khelashvili and P. J. Steel, J. Org. Chem., 2010, 75, 6532 CrossRef CAS.
- A. R. Katritzky, S. Suzuki and S. K. Singh, Synthesis, 2004, 2645 CrossRef CAS.
- A. R. Katritzky, L. Khelashvili and M. A. Munawar, J. Org. Chem., 2008, 73, 9171 CrossRef.
-
(a) E. Masiukiewicz, B. Rzeszotarska, I. Wawrzycka-Gorczyca and E. Kolodziejczyk, Synth. Commun., 2007, 37, 1917 CrossRef CAS;
(b) A. R. Katritzky, A. A. Shestopalov and K. Suzuki, Synthesis, 2004, 1806 CrossRef CAS;
(c) A. R. Katritzky, S. R. Tala, N. E. Abo-Dya, T. S. Ibrahim, S. A. El-Feky, K. Gyanda and K. M. Pandya, J. Org. Chem., 2011, 76, 85 CrossRef CAS.
- A. R. Katritzky, A. A. Shestopalov and K. Suzuki, Arkivoc, 2005, vii, 36.
- A. R. Katritzky and P. Angrish, Steroids, 2006, 71, 660 CrossRef CAS.
- A. R. Katritzky, A. S. Vincek and K. Suzuki, Arkivoc, 2005, v, 116.
Footnotes |
| † Electronic Supplementary Information (ESI) available: 1H and 13 C NMR for compounds 2a–g, 3a–h, 4a–h. See DOI: 10.1039/c1md00130b/ |
| ‡ Kiso denotes O-acyl peptides as “click peptides” because of their easy conversion to target native peptides under physiological conditions.1d |
|
| This journal is © The Royal Society of Chemistry 2011 |
Click here to see how this site uses Cookies. View our privacy policy here. 

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in the reaction caused minimal hydrolysis of 2 (4%) but no epimerization was detected according to HPLC-MS analysis on 3b and (3b + 3b′). The O-acylated serines were characterized by NMR and elemental analysis (Table 1). HPLC analysis [chirobiotic T column (250 mm × 4.6 mm), detection at 254 nm, flow rate 2.5 mL min−1, MeOH] on 3b (single peak, retention time 1.3 min) and (3b + 3b′) (two peaks, retention times, 1.3 min and 1.5 min) as well as HPLC-MS and (-)ESI-MS on 3b (Fig. 2) confirmed the absence of racemization in the targeted isodipeptides.
1) in the reaction caused minimal hydrolysis of 2 (4%) but no epimerization was detected according to HPLC-MS analysis on 3b and (3b + 3b′). The O-acylated serines were characterized by NMR and elemental analysis (Table 1). HPLC analysis [chirobiotic T column (250 mm × 4.6 mm), detection at 254 nm, flow rate 2.5 mL min−1, MeOH] on 3b (single peak, retention time 1.3 min) and (3b + 3b′) (two peaks, retention times, 1.3 min and 1.5 min) as well as HPLC-MS and (-)ESI-MS on 3b (Fig. 2) confirmed the absence of racemization in the targeted isodipeptides.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O, 4
H2O, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1] on 4b (single peak, retention time 7.23 min) and (4b + 4b′) (two peaks, retention times, 6.54 min and 7.20 min) confirmed the absence of racemization in the desired isodipeptides.
1] on 4b (single peak, retention time 7.23 min) and (4b + 4b′) (two peaks, retention times, 6.54 min and 7.20 min) confirmed the absence of racemization in the desired isodipeptides.

