Synthesis and activity of a novel diether phosphonoglycerol in phospholipase-resistant synthetic lipid:peptide lung surfactants†
Adrian L.
Schwan
*a,
Suneel P.
Singh
a,
Jason A.
Davy
a,
Alan J.
Waring
*bc,
Larry M.
Gordon
b,
Frans J.
Walther
b,
Zhengdong
Wang
d and
Robert H.
Notter
*d
aDepartment of Chemistry, University of Guelph, Guelph, ON, Canada N1G 2W1
bDepartment of Pediatrics, LA Biomed Research Institute/Harbor-UCLA, Torrance, CA 90502, USA
cDepartment of Physiology and Biophysics, School of Medicine, UC, Irvine, 92697, USA
dDepartment of Pediatrics, University of Rochester, Rochester, NY 14642, USA
First published on 19th October 2011
Abstract
This paper reports the chemical synthesis and purification of a novel phospholipase-resistant C16:0, C16:1 diether phosphonoglycerol with structural analogy to ester-linked anionic phosphatidylglycerol (PG) in endogenous pulmonary surfactant. This diether phosphonoglycerol (PG 1) is studied for phospholipase A2 (PLA2) resistance and for surface activity in synthetic exogenous surfactants combined with Super Mini-B (S-MB) peptide and DEPN-8, a previously-reported diether phosphonolipid analog of dipalmitoyl phosphatidylcholine (DPPC, the major zwitterionic phospholipid in native lung surfactant). Activity experiments measured both adsorption and dynamic surface tension lowering due to the known importance of these surface behaviors in lung surfactant function in vivo. Synthetic surfactants containing 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DEPN-8:PG 1 + 3% S-MB were resistant to degradation by PLA2 in chromatographic studies, while calf lung surfactant extract (CLSE, the substance of the bovine clinical surfactant Infasurf®) was significantly degraded by PLA2. The 9
1 DEPN-8:PG 1 + 3% S-MB were resistant to degradation by PLA2 in chromatographic studies, while calf lung surfactant extract (CLSE, the substance of the bovine clinical surfactant Infasurf®) was significantly degraded by PLA2. The 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DEPN-8:PG 1 + 3% S-MB mixture also had small but consistent increases in both adsorption and dynamic surface tension lowering ability compared to DEPN-8 + 3% S-MB. Consistent with these surface activity increases, molecular dynamics simulations using Protein Modeller, GROMACS force-field, and PyMOL showed that bilayers containing DPPC and palmitoyl-oleoyl-PC (POPC) as surrogates of DEPN-8 and PG 1 were penetrated to a greater extent by S-MB peptide than bilayers of DPPC alone. These results suggest that PG 1 or related anionic phosphono-PG analogs may have functional utility in phospholipase-resistant synthetic surfactants targeting forms of acute pulmonary injury where endogenous surfactant becomes dysfunctional due to phospholipase activity in the innate inflammatory response.
1 DEPN-8:PG 1 + 3% S-MB mixture also had small but consistent increases in both adsorption and dynamic surface tension lowering ability compared to DEPN-8 + 3% S-MB. Consistent with these surface activity increases, molecular dynamics simulations using Protein Modeller, GROMACS force-field, and PyMOL showed that bilayers containing DPPC and palmitoyl-oleoyl-PC (POPC) as surrogates of DEPN-8 and PG 1 were penetrated to a greater extent by S-MB peptide than bilayers of DPPC alone. These results suggest that PG 1 or related anionic phosphono-PG analogs may have functional utility in phospholipase-resistant synthetic surfactants targeting forms of acute pulmonary injury where endogenous surfactant becomes dysfunctional due to phospholipase activity in the innate inflammatory response.
Introduction
Active lung surfactant is essential for respiration in air-breathing animals. Surfactant deficiency leads to the neonatal respiratory distress syndrome (NRDS) in preterm infants, and surfactant dysfunction (inhibition) is a major contributor to the pathophysiology of clinical acute lung injury (ALI) and the acute respiratory distress syndrome (ARDS) in patients of all ages.1,2 The inflammatory lung injury syndromes of ALI/ARDS have substantial mortality rates of 30–50 percent despite great advances in medical intensive care over the past several decades.3–5 Exogenous surfactant therapy with animal-derived drugs is currently used with great success to improve survival and minimize morbidity in premature infants with NRDS,1,6,7 and there is significant interest in extending this therapy to patients with ALI/ARDS.3,4,8 New synthetic lipid:peptide lung surfactant drugs now under development have the potential to increase the efficacy and cost-effectiveness of this therapy.9This paper reports the synthesis of PG 1 (Fig. 1), a novel analog of anionic phosphatidylglycerol (PG) compounds in native lung surfactant. This new PG 1lipid is studied for phospholipase resistance and activity in synthetic lung surfactants combined with Super Mini-B (S-MB) peptide and DEPN-8, a phospholipase-resistant diether phosphono analog of dipalmitoyl phosphatidylcholine (DPPC). Zwitterionic DPPC is the most prevalent phospholipid in native lung surfactant,1 and the active S-MB peptide is bioengineered to have functionally-crucial features from human surfactant protein (SP)-B including its N- and C-terminal amphipathic helices, Saposin fold character, and intramolecular disulfide connectivities.10 Synthetic surfactants containing a mixture of PG 1, DEPN-8, and S-MB as studied here thus encompass major zwitterionic/anionic lipid molecular interactions present in native lung surfactant and also mimic its most active protein component (SP-B).
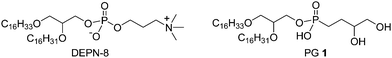 | ||
| Fig. 1 Chemical structures of phospholipase-resistant surface active lipids. DEPN-8 is well established for its effectiveness as a synthetic surface active agent, whereas C16:0, C16:1-diether based phosphonoglycerol 1 is introduced in this paper. | ||
Synthetic lipid/peptide lung surfactants have significant potential pharmaceutical advantages relative to animal-derived surfactant drugs. Compared to animal-derived surfactants, synthetic surfactants have greater purity, better compositional reproducibility, easier manufacturing quality-control, and significant scale-up economy for treating NRDS and ALI/ARDS. Synthetic surfactants are also free from the risk of prion transmission (i.e., bovine spongiform encephalitis, BSE), and are not subject to cultural/religious issues that can affect bovine or porcine surfactants. Synthetic surfactants can also be designed to contain novel constituents with beneficial molecular properties, such as phospholipase-resistance in the current work. Phospholipase-resistant lung surfactants may have particular utility in direct pulmonary ALI/ARDS, where these lytic enzymes are present during the innate pulmonary inflammatory response.11–17 Results here document the phospholipase resistance of PG 1 and 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DEPN-8: PG 1 + 3% (by wt.) S-MB, and show that this synthetic surfactant has high adsorption and dynamic surface activity equal to calf lung surfactant extract (CLSE) containing all of the hydrophobic constituents of endogenous surfactant. Molecular dynamics simulations are also performed to show that bilayers containing DPPC plus 10% palmitoyl-oleoyl PG (POPG) as surrogates of DEPN-8 and PG 1 are penetrated more rapidly by S-MB peptide compared to bilayers containing DPPC alone.
1 DEPN-8: PG 1 + 3% (by wt.) S-MB, and show that this synthetic surfactant has high adsorption and dynamic surface activity equal to calf lung surfactant extract (CLSE) containing all of the hydrophobic constituents of endogenous surfactant. Molecular dynamics simulations are also performed to show that bilayers containing DPPC plus 10% palmitoyl-oleoyl PG (POPG) as surrogates of DEPN-8 and PG 1 are penetrated more rapidly by S-MB peptide compared to bilayers containing DPPC alone.
Results
Synthesis of C16:0, C16:1 PG 1
The preparation of mono[2-[(9Z)-9-hexadecen-1-yloxy]-3-(hexadecyloxy)propyl] P-(3,4-dihydroxybutyl)phosphonate 1 is shown in Scheme 1 below based on our previously reported results for formation of the phosphonoglycerol headgroup.18Dimethyl 3,4-bis(benzoyloxy)butylphosphonate 2 was employed to introduce the phosphorus and carbon components of the polar head group of the lipid. In one pot, 2 was subjected to demethylative silylation of the methoxy groups using TMS-Br and the resulting bis(silylated) phosphonate 3 was directly converted to dichloride 4 using oxalyl chloride. Without purification, the dichloride was reacted with rac-2-[(9Z)-9-hexadecen-1-yloxy]-3-(hexadecyloxy)–propanol 519 to afford penultimate target compound 6. PG 1 was then obtained through careful methanolysis of only the carboxylic ester functionalities of 6, while leaving the phosphonate ester intact. PG 1 was purified by silica gel flash chromatography, and its assigned structure supported by high-resolution mass spectrometry and by 1H and 13C NMR spectroscopy (specific characterization data for PG 1 and lipid 6 are given in Materials and experimental procedures). NMR analysis indicated that no isomerization of the Z-alkene functionality occurred during the preparation of target PG 1.![Preparation of C16:0, C16:1 phosphonoglycerol 1. Preparation of C16:0, C16:1-diether phosphonoglycerol 1 from dimethyl 3,4-bis(benzoyloxy)butylphosphonate 2: a) 2.2 equiv. TMSBr, CH2Cl2, −40 °C-rt; b) (COOH)2, cat. DMF, CH2Cl2, rt; c) rac-2-([9Z]-9-hexadecen-1-yloxy)-3-(hexadecyloxy)-propanol (5), Et3N, CHCl3, 76% over 3 steps; d) K2CO3, CH2Cl2/MeOH, 48 h, rt, 48%.](/image/article/2011/MD/c1md00206f/c1md00206f-s1.gif) | ||
| Scheme 1 Preparation of C16:0, C16:1 phosphonoglycerol 1. Preparation of C16:0, C16:1-diether phosphonoglycerol 1 from dimethyl 3,4-bis(benzoyloxy)butylphosphonate 2: a) 2.2 equiv. TMSBr, CH2Cl2, −40 °C-rt; b) (COOH)2, cat. DMF, CH2Cl2, rt; c) rac-2-([9Z]-9-hexadecen-1-yloxy)-3-(hexadecyloxy)-propanol (5), Et3N, CHCl3, 76% over 3 steps; d) K2CO3, CH2Cl2/MeOH, 48 h, rt, 48%. | ||
Resistance of PG 1 and a synthetic surfactant containing 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) 1 DEPN-8:PG 1 + 3% S-MB to degradation by PLA2
1 DEPN-8:PG 1 + 3% S-MB to degradation by PLA2
The ability of PG 1 to resist degradation by phospholipase A2 (PLA2) was documented chromatographically. PG 1 and 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DEPN-8:PG 1 + 3% (by wt.) S-MB were incubated for 30 min with PLA2, and lyso-lipid degradation products were assessed by thin layer chromatography (Methods). Results showed no PLA2-induced degradation of PG 1 based on lyso-PG measurements, or of 9
1 DEPN-8:PG 1 + 3% (by wt.) S-MB were incubated for 30 min with PLA2, and lyso-lipid degradation products were assessed by thin layer chromatography (Methods). Results showed no PLA2-induced degradation of PG 1 based on lyso-PG measurements, or of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DEPN-8:PG 1 + 3% S-MB based on lyso-PC measurements (Table 1). In contrast, substantial PLA2-induced degradation was present in control experiments with POPG and CLSE (Table 1).
1 DEPN-8:PG 1 + 3% S-MB based on lyso-PC measurements (Table 1). In contrast, substantial PLA2-induced degradation was present in control experiments with POPG and CLSE (Table 1).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DEPN-8/PG 1 + 3% S-MB) to degradation by phospholipase A2a
1 DEPN-8/PG 1 + 3% S-MB) to degradation by phospholipase A2a
| Lipid Class | CLSE | CLSE + PLA2 |
DEPN-8 + PG 1 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) + 3% S-MB 1) + 3% S-MB |
DEPN-8 + PG 1 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) + 3% S-MB + PLA2 1) + 3% S-MB + PLA2 |
POPG | POPG + PLA2 | PG 1 | PG 1 + PLA2 |
|---|---|---|---|---|---|---|---|---|
a
Phospholipid classes are given in weight percent relative to total phospholipid based on phosphate analysis of thin-layer chromatographic bands following incubation with PLA2 (Methods). PLA2 did not degrade PG 1 based on lyso-PG measurements, or 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DEPN-8:PG 1 + 3% by weight S-MB based on lyso-PC measurements. In comparison, significant degradation by PLA2 was found for POPG based on lyso-PG production, and for CLSE based on lyso-PC production. See text for details. Abbreviations: Lyso-PC: lysophosphatidylcholine; SPH: sphingomyelin; PC: phosphatidylcholine; PI: phosphatidylinositol; PG: phosphatidylglycerol; Lyso-PG: lysophosphatidylglycerol. Data are Mean ± SEM (N = 3). 1 DEPN-8:PG 1 + 3% by weight S-MB based on lyso-PC measurements. In comparison, significant degradation by PLA2 was found for POPG based on lyso-PG production, and for CLSE based on lyso-PC production. See text for details. Abbreviations: Lyso-PC: lysophosphatidylcholine; SPH: sphingomyelin; PC: phosphatidylcholine; PI: phosphatidylinositol; PG: phosphatidylglycerol; Lyso-PG: lysophosphatidylglycerol. Data are Mean ± SEM (N = 3).
|
||||||||
| Lyso-PC | 0.9 ± 0.1 | 25.8 ± 1.9 | ||||||
| Sph | 1.2 ± 0.2 | 0.8 ± 0.6 | ||||||
| PC | 83.5 ± 0.7 | 61.2 ± 2.4 | 89.7 ± 0.9 | 90.2 ± 1.5 | ||||
| PI | 4.2 ± 0.3 | 3.4 ± 0.2 | ||||||
| PE | 3.6 ± 0.5 | 3.1 ± 0.5 | ||||||
| PG | 4.9 ± 0.7 | 3.6 ± 0.3 | 10.3 ± 0.5 | 9.8 ± 0.7 | 100 ± 0 | 91.3 ± 1.7 | 100 ± 0 | 100 ± 0 |
| Lyso-PG | 8.7 ± 1.2 | |||||||
| Residue | 1.5 ± 0.4 | 2.1 ± 0.7 |
Surface activity of synthetic lung surfactants containing PG 1, DEPN-8, and S-MB
Rapid adsorption and high dynamic surface activity are known to be crucial interfacial properties for the physiological efficacy of lung surfactants in respiratory function and mechanics.1 These two activity measures are shown in Tables 2 and 3 for synthetic surfactants containing DEPN-8 or 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DEPN-8: PG 1 combined with 3% S-MB. Mixtures of DEPN-8 + 3% S-MB had very high adsorption and overall dynamic surface tension lowering activity, but the addition of PG 1 led to small but consistent further improvements in both surface behaviors. Synthetic surfactants containing 9
1 DEPN-8: PG 1 combined with 3% S-MB. Mixtures of DEPN-8 + 3% S-MB had very high adsorption and overall dynamic surface tension lowering activity, but the addition of PG 1 led to small but consistent further improvements in both surface behaviors. Synthetic surfactants containing 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DEPN-8:PG 1 + 3% S-MB had adsorption (Table 2) and overall dynamic surface activity (Table 3) equal to the bovine surfactant extract CLSE. This bovine lung surfactant extract contains all of the hydrophobic components of endogenous surfactant, and is the substance of the exogenous surfactant drug Infasurf® that has defined clinical efficacy in preterm infants with NRDS1,6,7 and pediatric patients with direct pulmonary forms of ALI/ARDS.4,20–23
1 DEPN-8:PG 1 + 3% S-MB had adsorption (Table 2) and overall dynamic surface activity (Table 3) equal to the bovine surfactant extract CLSE. This bovine lung surfactant extract contains all of the hydrophobic components of endogenous surfactant, and is the substance of the exogenous surfactant drug Infasurf® that has defined clinical efficacy in preterm infants with NRDS1,6,7 and pediatric patients with direct pulmonary forms of ALI/ARDS.4,20–23
| Samples | Adsorption surface pressure (mN m−1) at time (minutes) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 5 | 10 | 15 | 20 | |
| a Adsorption was measured following injection of a surfactant bolus beneath the interface of a stirred subphase at time zero. Surface pressure is the amount of surface tension lowering below that of the pure subphase (normal saline adjusted to pH 7.0) at 37 ± 0.5 °C. Higher surface pressure equates to lower surface tension. Surfactant concentrations are in mg ml−1 based on phospholipid content for each mixture shown. Data are Means ± SEM for N = 4. CLSE: calf lung surfactant extract. See Methods for experimental procedures, and text for discussion. | |||||||
| 0.03125 mg ml−1 | |||||||
| DEPN-8 | 0 ± 0.0 | 7 7 ± 1.3 | 8.5 ± 1.4 | 9.0 ± 1.4 | 9.3 ± 1.3 | 9.5 ± 1.3 | 9.9 ± 1.3 |
| DEPN-8 + 3% SMB | 0 ± 0.0 | 42.3 ± 0.4 | 43.0 ± 0.3 | 45.3 ± 1.2 | 47.2 ± 0.6 | 47.5 ± 0.5 | 47.7 ± 0.3 |
DEPN-8 + PG 1 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) + 3% S-MB 1) + 3% S-MB |
0 ± 0.0 | 46.2 ± 0.6 | 47.5 ± 0.5 | 48.1 ± 0.2 | 48.3 ± 0.2 | 48.4 ± 0.2 | 48.5 ± 0.1 |
| 0.0625 mg ml−1 | |||||||
| DEPN-8 | 0 ± 0.0 | 12.9 ± 2.9 | 16.0 ± 1.6 | 17.8 ± 1.7 | 18.0 ± 1.7 | 18.2 ± 1.7 | 18.4 ± 1.8 |
| DEPN-8 + 3% SMB | 0 ± 0.0 | 44.4 ± 0.4 | 46.4 ± 0.3 | 47.1 ± 0.5 | 47.5 ± 0.6 | 47.6 ± 0.6 | 47.7 ± 0.6 |
DEPN-8 + PG 1 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) + 3% S-MB 1) + 3% S-MB |
0 ± 0.0 | 47.4 ± 0.3 | 47.8 ± 0.1 | 48.1 ± 0.1 | 48.2 ± 0.1 | 48.3 ± 0.1 | 48.6 ± 0.1 |
| CLSE | 0 ± 0.0 | 46.4 ± 0.1 | 47.4 ± 0.2 | 48.0 ± 0.1 | 48.1 ± 0.1 | 48.1 ± 0.1 | 48.1 ± 0.1 |
| Samples | Minimum surface tension (mN m−1) at time (minutes) of bubble pulsation | ||||||
|---|---|---|---|---|---|---|---|
| 0.25 | 0.5 | 1 | 2 | 5 | 10 | 15 | |
| a Surface tension at minimum bubble radius (minimum surface tension) is shown as a function of time of pulsation on a bubble surfactometer at physical conditions relevant for respiration in vivo (37 °C, 20 cycles/min, and 50% area compression). Under rapid dynamic compression on this apparatus, lung surfactant films reach significantly lower surface tensions than in adsorption studies, which are limited by the equilibrium spreading pressure. Surfactant mixture concentrations in bubble studies were at 1 and 2.5 mg ml−1phospholipid as shown. CLSE: Calf lung surfactant extract. Data are means ± SEM for N = 4. See Methods for experimental details and text for discussion. | |||||||
| 1.0 mg ml−1 | |||||||
| DEPN-8 | 36.6 ± 2.2 | 30.1 ± 2.0 | 24.0 ± 2.0 | 17.7 ± 2.3 | 10.7 ± 1.3 | 4.8 ± 1.3 | <1 |
| DEPN-8 + 3% S-MB | 26.0 ± 1.3 | 24.2 ± 1.3 | 20.8 ± 1.3 | 15.0 ± 2.3 | 7.2 ± 2.7 | <1 | |
DEPN-8 + PG 1 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) + 3% S-MB 1) + 3% S-MB |
21.5 ± 1.0 | 20.4 ± 0.9 | 16.7 ± 2.0 | 11.3 ± 1.7 | 3.6 ± 0.8 | <1 | |
| 2.5 mg ml−1 | |||||||
| DEPN-8 | 31.2 ± 2.0 | 27.1 ± 1.8 | 21.2 ± 1.5 | 14.6 ± 1.2 | 7.8 ± 1.7 | 1.5 ± 1.0 | <1 |
| DEPN-8 + 3% S-MB | 11.6 ± 2.0 | 5.8 ± 1.7 | 3.0 ± 1.5 | 2.3 ± 1.4 | <1 | ||
DEPN-8 + PG 1 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) + 3% SMB 1) + 3% SMB |
6.8 ± 1.7 | 3.5 ± 1.1 | 2.2 ± 1.0 | 1.2 ± 0.5 | <1 | ||
| CLSE | 6.8 ± 0.7 | 4.2 ± 0.5 | 2.4 ± 0.6 | <1 | |||
Molecular dynamics simulations showing the penetration of S-MB into bilayers of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) 1 DPPC:POPG compared to DPPC alone
1 DPPC:POPG compared to DPPC alone
To test the interaction affinity of S-MB with different lipids, we performed molecular dynamics simulations defining the penetration of the peptide into bilayers containing zwitterionic DPPC or 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DPPC:POPG. These glycerophospholipids (DPPC, POPG) were used as surrogates of DEPN-8 and PG 1 in computer simulations because of their well-known molecular coordinates and parameters from prior spectroscopic and X-ray analyses. Simulations were initiated with S-MB peptide positioned so that its N-terminal phenylalanine made contact with the lipid polar head group region of the bilayer, and the insertion of the peptide was then allowed to evolve over 100 ns of molecular dynamics analysis. S-MB peptide was found to insert more deeply into the bilayer when POPG was present (Fig. 2A, 2B). After 100 ns of molecular dynamics, the N-terminal phenylalanine of S-MB penetrated to within 12.8 angstroms of the cross-section center of a 9
1 DPPC:POPG. These glycerophospholipids (DPPC, POPG) were used as surrogates of DEPN-8 and PG 1 in computer simulations because of their well-known molecular coordinates and parameters from prior spectroscopic and X-ray analyses. Simulations were initiated with S-MB peptide positioned so that its N-terminal phenylalanine made contact with the lipid polar head group region of the bilayer, and the insertion of the peptide was then allowed to evolve over 100 ns of molecular dynamics analysis. S-MB peptide was found to insert more deeply into the bilayer when POPG was present (Fig. 2A, 2B). After 100 ns of molecular dynamics, the N-terminal phenylalanine of S-MB penetrated to within 12.8 angstroms of the cross-section center of a 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DPPC:POPG bilayer compared to 20.9 angstroms for a bilayer of DPPC alone. Similarly, helical domains of S-MB penetrated to within 15.2 angstroms of the bilayer cross-section center at 100 ns compared to a distance of 24 angstroms in bilayers of DPPC alone.
1 DPPC:POPG bilayer compared to 20.9 angstroms for a bilayer of DPPC alone. Similarly, helical domains of S-MB penetrated to within 15.2 angstroms of the bilayer cross-section center at 100 ns compared to a distance of 24 angstroms in bilayers of DPPC alone.
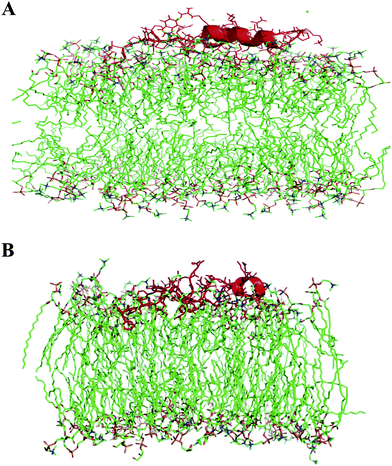 | ||
Fig. 2 Cross-sectional view of the insertion of Super Mini-B peptide into bilayers of (a) DPPC and (b) 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (mole:mole) DPPC:POPG after 100 ns of molecular dynamics. The S-MB backbone is shown in red highlight, lipid acyl chains are in green stick format, and lipid polar head groups are in yellow, orange and blue. Water molecules have been removed for clarity. Molecular dynamics simulations used Protein Modeller, Gromacs force-field, and PyMOL (Methods). 1 (mole:mole) DPPC:POPG after 100 ns of molecular dynamics. The S-MB backbone is shown in red highlight, lipid acyl chains are in green stick format, and lipid polar head groups are in yellow, orange and blue. Water molecules have been removed for clarity. Molecular dynamics simulations used Protein Modeller, Gromacs force-field, and PyMOL (Methods). | ||
Molecular dynamics analyses also examined the positioning of S-MB peptide in a surface view of the 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DPPC:POPG bilayer to examine potential lateral phase separation effects (Fig. 3). After 100 ns, the environment of S-MB was shown to be enriched in POPG molecules, presumptively reflecting preferential pairing between cationic Arg/Lys residues in the peptide and the anionic head groups of POPG molecules.
1 DPPC:POPG bilayer to examine potential lateral phase separation effects (Fig. 3). After 100 ns, the environment of S-MB was shown to be enriched in POPG molecules, presumptively reflecting preferential pairing between cationic Arg/Lys residues in the peptide and the anionic head groups of POPG molecules.
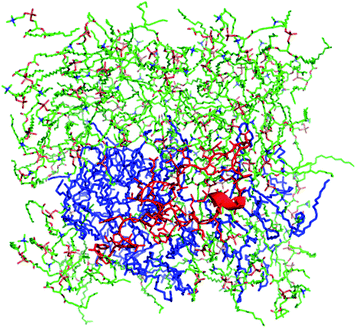 | ||
Fig. 3 Molecular dynamics surface view of S-MB peptide in a bilayer of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (mole:mole) DPPC:POPG. Zwitterionic DPPC lipids are in a green stick format, POPG anionic lipids are shown in blue highlight, and S-MB peptide is in red. Simulation results are after 100 ns of molecular dynamics using Protein Modeller, Gromacs force-field, and PyMOL (Methods). 1 (mole:mole) DPPC:POPG. Zwitterionic DPPC lipids are in a green stick format, POPG anionic lipids are shown in blue highlight, and S-MB peptide is in red. Simulation results are after 100 ns of molecular dynamics using Protein Modeller, Gromacs force-field, and PyMOL (Methods). | ||
Discussion
This paper has reported the synthesis of an anionic phospholipase-resistant C16:0, C16:1 diether phosphono PG compound (PG 1), and studied its effects on surface activity in a synthetic lipid/peptide exogenous lung surfactant. The anionic charge of PG 1 at neutral pH gives it the capability to interact directly with positively-charged Lys/Arg residues on S-MB or related SP-B peptides. PG 1 was shown to enhance both adsorption and overall dynamic surface activity when combined in a synthetic surfactant with zwitterionic DEPN-8 and S-MB peptide. Previous studies from our laboratory have documented the high surface activity of synthetic surfactants containing DEPN-8 and S-MB alone.24 The addition of PG 1 (10% by mole relative to DEPN-8) gave small but consistent further improvements in adsorption and dynamic surface tension lowering (Tables 2, 3). Mixtures of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DEPN-8:PG 1 + 3% S-MB had adsorption and overall dynamic surface activity equal to CLSE, the substance of the bovine-derived clinical surfactant drug Infasurf®. As a chloroform-methanol extract of lavage from calf lungs, CLSE contains the complete mix of lipids in alveolar surfactant plus the active hydrophobic surfactant proteins (SP-B and SP-C) in the endogenous ratio.1,25 However, during acute pulmonary injury, CLSE can be degraded by inflammation-induced phospholipases, while 9
1 DEPN-8:PG 1 + 3% S-MB had adsorption and overall dynamic surface activity equal to CLSE, the substance of the bovine-derived clinical surfactant drug Infasurf®. As a chloroform-methanol extract of lavage from calf lungs, CLSE contains the complete mix of lipids in alveolar surfactant plus the active hydrophobic surfactant proteins (SP-B and SP-C) in the endogenous ratio.1,25 However, during acute pulmonary injury, CLSE can be degraded by inflammation-induced phospholipases, while 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DEPN-8:PG 1 + 3% S-MB is resistant to this effect (Table 1).
1 DEPN-8:PG 1 + 3% S-MB is resistant to this effect (Table 1).
Our prior work26,27 has shown that exogenous surfactants containing DEPN-8 combined with column-isolated bovine hydrophobic surfactant proteins (SP-B/SP-C) maintain high surface and physiological activity in the presence of PLA2, while CLSE activity is inhibited by exposure to this enzyme. In addition to PLA2, ether chain linkages in DEPN-8 and PG 1 are also structurally resistant to phospholipase A1, and their phosphonate groups are resistant to cleavage by phospholipase D. DEPN-8 has also been found to be partially resistant to phospholipase C due to steric hindrance.28 Chromatographic experiments here on the phospholipase resistance of PG 1 and 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DEPN-8:PG 1 + 3% S-MB compared to CLSE and POPG in Table 1 used PLA2 as a representative enzyme because it has been shown to be elaborated in the lungs of patients with ALI/ARDS (e.g.,16,17).
1 DEPN-8:PG 1 + 3% S-MB compared to CLSE and POPG in Table 1 used PLA2 as a representative enzyme because it has been shown to be elaborated in the lungs of patients with ALI/ARDS (e.g.,16,17).
Although detailed molecular mechanisms of lipid:peptide activity in synthetic surfactants were not examined in this study, proteomic molecular dynamics simulations were used to assess interactions between S-MB and DPPC bilayers with and without an added anionic POPG component. These simulations used DPPC and POPG as surrogates for DEPN-8 and PG 1 because of their known molecular parameters. Proteomic modeling indicated that the ability of S-MB peptide to penetrate DPPC bilayers was increased by the presence of POPG (Fig. 2). Molecular dynamics analyses also indicated that the cationic S-MB peptide had preferential molecular interactions with anionic POPG molecules (Fig. 3). Both these simulation findings are consistent with anionic PG 1 having specific molecular interactions with S-MB that could enhance surface activity.
Current animal-derived clinical exogenous surfactants (Infasurf®, Curosurf®, and Survanta®) all contain DPPC plus other phosphatidylcholines and anionic lipids including PG that are sensitive to phospholipase degradation (1,2 for review). The synthetic surfactants Venticute® and Surfaxin® (KL4) also include phospholipase-sensitive DPPC and PG components in their compositions.1,2 The present study is promising in showing that the addition of phospholipase-resistant PG 1 increases the already high adsorption and dynamic surface activity of a synthetic exogenous surfactant containing DEPN-8 + 3% S-MB. Phospholipase-resistant synthetic exogenous surfactants may have particular utility in therapies targeting surfactant dysfunction in direct forms of ALI/ARDS, where phospholipases are known to be induced in the lungs during the innate inflammatory response11–17 and have the potential to degrade endogenous surfactant and non-resistant exogenous surfactants.
Materials and experimental procedures
Synthesis details for phosphonolipid 6 (Scheme 1)
Dibenzoyl-protected phosphonolipid 6 for use in preparing PG 1 (Scheme 1) was synthesized as follows. Neat TMSBr (0.704 g, 0.610 mL, 4.60 mmol) was added dropwise to a solution of phosphonate 2 (0.747 g, 1.84 mmol) dissolved in CH2Cl2 (8 mL) stirring at −40 °C. The reaction mix was slowly warm to rt and stirred for 3 h. Removal of the solvent under anhydrous conditions using an aspirator equipped with CaCl2 drying tube afforded a corresponding silyl ester 3 as thick yellow oil. Crude silyl ester 3 was placed under high vacuum for 1.5 h and then redissolved in freshly dried CH2Cl2 (8 mL). While stirring at rt, DMF (two drops) was added followed by oxalyl chloride (0.700 g, 0.481 mL, 5.51 mmol). The solution was stirred at rt for 30 min. After the reaction had completed, solvents and other volatiles were stripped off using an aspirator and drying tube. Crude dichloride 4 was placed under high vacuum for 1.5 h at 50 °C and then used without further purification. Acid chloride 4 was redissolved in dry, ethanol free CHCl3 (8 mL) at 50 °C and the solution was cooled to 0 °C and a solution of alcohol 5 (0.500 g, 0.919 mmol) and triethylamine (0.558 g, 0.770 mL, 5.51 mmol) in dry, ethanol free CHCl3 (8 mL) was slowly added. The reaction warmed to rt and stirred for 48 h at which time water (2 mL), was added and the solution was stirred for an additional 1 h. After concentrating under vacuum, the residue was dissolved in CHCl3/MeOH/H2O (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10 mL) and stirred with Amberlite® ion exchange resin (15 mL) for 1.5 h. The resin was filtered and rinsed with the same solvent system. The filtrate was concentrated by rotary evaporation and the resulting residue was dissolved in CH2Cl2, gently washed with water and brine, and then dried over MgSO4. After filtration and concentration, the crude material was purified by silica gel flash chromatography (10% MeOH/90% CHCl3) to provide 0.642 g (76%) of the dibenzoyl-protected phosphonolipid 6. Characterization data 6: 1H NMR (400 MHz, CDCl3) δ: 7.96 (m, 4H), 7.43 (m, 2H), 7.31 (m, 4H), 5.31 (m, 2H) 4.55 (m, 1H), 4.44 (m, 1H), 4.07 (m, 2H), 3.58–3.29 (m, 6H), 2.16 (m, 2H), 2.00–1.73 (m, 6H), 1.42 (m, 4H), 1.23 (m, 46H), 0.86 (t, J = 6.7 Hz, 6H) ppm; 31P NMR (162 MHz, CDCl3): 22.8 ppm; HRMS, ESI (+ve), m/z: calcd for C53H88O9P [M + H]+: 899.6166; found: 899.6151.
1, 10 mL) and stirred with Amberlite® ion exchange resin (15 mL) for 1.5 h. The resin was filtered and rinsed with the same solvent system. The filtrate was concentrated by rotary evaporation and the resulting residue was dissolved in CH2Cl2, gently washed with water and brine, and then dried over MgSO4. After filtration and concentration, the crude material was purified by silica gel flash chromatography (10% MeOH/90% CHCl3) to provide 0.642 g (76%) of the dibenzoyl-protected phosphonolipid 6. Characterization data 6: 1H NMR (400 MHz, CDCl3) δ: 7.96 (m, 4H), 7.43 (m, 2H), 7.31 (m, 4H), 5.31 (m, 2H) 4.55 (m, 1H), 4.44 (m, 1H), 4.07 (m, 2H), 3.58–3.29 (m, 6H), 2.16 (m, 2H), 2.00–1.73 (m, 6H), 1.42 (m, 4H), 1.23 (m, 46H), 0.86 (t, J = 6.7 Hz, 6H) ppm; 31P NMR (162 MHz, CDCl3): 22.8 ppm; HRMS, ESI (+ve), m/z: calcd for C53H88O9P [M + H]+: 899.6166; found: 899.6151.
Conversion of dibenzoate protected lipid 6 to target PG 1
Anhydrous K2CO3 (0.377 g, 2.27 mmol) was suspended in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mixture of dry MeOH (15 mL) and dry CH2Cl2 (30 mL). Dibenzoate 6 (0.490 g, 0.545 mmol) was added and reaction stirred for 48 h at room temperature, after which time water (5 mL) was added. The pH was carefully lowered to between 2 and 3 using dilute HCl. The aqueous phase was then extracted with MeOH/CH2Cl2 (1
2 mixture of dry MeOH (15 mL) and dry CH2Cl2 (30 mL). Dibenzoate 6 (0.490 g, 0.545 mmol) was added and reaction stirred for 48 h at room temperature, after which time water (5 mL) was added. The pH was carefully lowered to between 2 and 3 using dilute HCl. The aqueous phase was then extracted with MeOH/CH2Cl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) (3X) and the organic extracts were gently washed with water (2X) and brine (1X). The organic phase was dried over MgSO4, filtered, and concentrated. Purification by silica gel flash chromatography (10% MeOH/90% CHCl3 to 45% MeOH/55% CHCl3) afforded 0.180 g (48%) of PG 1. Characterization data for 1: 1H NMR (400 MHz, CDCl3/CD3OD); 5.34 (m, 2H), 3.89 (br, 2H), 3.59–3.43 (m, 10H), 2.01 (m, 4H), 1.70 (m, 4H), 1.56 (m, 4H), 1.26 (s, 44H), 0.88 (t, J = 6.5 Hz, 6H) ppm; 13C NMR (100 MHz, CDCl3/CD3OD), δ: 129.4, 129.3, 77.2, 71.4, 71.1, 70.3, 69.5, 65.4, 63.7, 31.5, 31.3, 29.5, 29.24, 29.20, 29.14, 29.09, 29.05, 28.9, 28.5, 26.7, 25.61, 25.56, 22.2, 13.5 ppm; 31P NMR (162 MHz, CDCl3/CD3OD), δ: 27.1 ppm; IR (neat): 3500–3100 (br), 1465, 1120, 1070 cm−1; HRMS, ESI (+ve), m/z: calcd for C39H80O7P [M + H]+: 691.5642; found: 691.5616.
2) (3X) and the organic extracts were gently washed with water (2X) and brine (1X). The organic phase was dried over MgSO4, filtered, and concentrated. Purification by silica gel flash chromatography (10% MeOH/90% CHCl3 to 45% MeOH/55% CHCl3) afforded 0.180 g (48%) of PG 1. Characterization data for 1: 1H NMR (400 MHz, CDCl3/CD3OD); 5.34 (m, 2H), 3.89 (br, 2H), 3.59–3.43 (m, 10H), 2.01 (m, 4H), 1.70 (m, 4H), 1.56 (m, 4H), 1.26 (s, 44H), 0.88 (t, J = 6.5 Hz, 6H) ppm; 13C NMR (100 MHz, CDCl3/CD3OD), δ: 129.4, 129.3, 77.2, 71.4, 71.1, 70.3, 69.5, 65.4, 63.7, 31.5, 31.3, 29.5, 29.24, 29.20, 29.14, 29.09, 29.05, 28.9, 28.5, 26.7, 25.61, 25.56, 22.2, 13.5 ppm; 31P NMR (162 MHz, CDCl3/CD3OD), δ: 27.1 ppm; IR (neat): 3500–3100 (br), 1465, 1120, 1070 cm−1; HRMS, ESI (+ve), m/z: calcd for C39H80O7P [M + H]+: 691.5642; found: 691.5616.
Other surfactant materials (DEPN-8, S-MB peptide, CLSE)
DEPN-8 [(±)-trimethyl(3 phosphonopropyl)ammonium, mono(2,3-bis(hexadecyloxy)propyl) ester] was synthesized and purified using methods detailed previously by Notter, Schwan and co-workers.19,26,27,29DEPN-8 has molecular structural resistance to phospholipases A1, A2, and D, and is also partially resistant to phospholipase C due to steric effects.28 S-MB peptide (41 residues, linear sequence NH2-FPIPLPYCWLCRALIKRIQAMIPKGGRMLPQLVCRLVLRCS-COOH) was prepared using a Symphony Multiple Peptide Synthesizer (Protein Technologies, Tucson, AZ) with standard FastMoc™ chemistry, as detailed by Walther et al.10 A low substitution (0.3 mmole/gm) pre-derivatized Fmoc-serine (tBu) Wang resin (NovaBiochem, San Diego, CA) minimized the formation of truncated sequences, and all residues were double-coupled during synthesis to optimize yield.30 The linear peptide was cleaved from the resin with the assistance of vacuum-assisted filtration, and deprotection was done over a period of 2 h to insure complete reaction.10,30 Filtered crude S-MB was precipitated in tertiary butyl ether, and separated by centrifugation at 2000 × g for 10 min (2–3 cycles of precipitation/centrifugation were done to minimize cleavage-deprotection byproducts). The mass of the crude reduced peptide was verified by MALDI-TOF spectroscopy, followed by solution in trifluoroethanol (TFE):10 mM HCl (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v:v), freeze-drying purification by preparative HPLC, air-oxidation for ≥24 h at 25 °C in TFE and 10 mM ammonium bicarbonate buffer (4
1, v:v), freeze-drying purification by preparative HPLC, air-oxidation for ≥24 h at 25 °C in TFE and 10 mM ammonium bicarbonate buffer (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, v:v) at pH 8.0, and re-purification by reverse phase HPLC. Final pure oxidized S-MB was then verified for mass via MALDI-TOF, and disulfide connectivity confirmed by mass spectroscopy of enzyme-digested fragments (trypsin and chymotrypsin digestion).10 Calf lung surfactant extract (CLSE) for activity comparisons with synthetic surfactants was a gift from ONY, INC, Amherst, NY, and was prepared by chloroform:methanol extraction of large aggregate surfactant lavaged from calf lungs, as described previously by Notter and co-workers.31–33
6, v:v) at pH 8.0, and re-purification by reverse phase HPLC. Final pure oxidized S-MB was then verified for mass via MALDI-TOF, and disulfide connectivity confirmed by mass spectroscopy of enzyme-digested fragments (trypsin and chymotrypsin digestion).10 Calf lung surfactant extract (CLSE) for activity comparisons with synthetic surfactants was a gift from ONY, INC, Amherst, NY, and was prepared by chloroform:methanol extraction of large aggregate surfactant lavaged from calf lungs, as described previously by Notter and co-workers.31–33
Phospholipase A2 degradation methods
The ability of PG 1 and 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DEPN-8:PG 1 + 3% S-MB to resist degradation by PLA2 was studied in comparison with CLSE and POPG as non-resistant controls. Surfactant substances in chloroform were dried under nitrogen and resuspended by vortexing in 5 mM Tris (hydroxymethylaminomethane) buffer containing 5 mM CaCl2 (pH 7.4). Lyophilized PLA2 (0.1 units/ml) suspended in the same buffer was added, and the mixture was incubated for 30 min at 37 °C. Phospholipid class compositions in the presence and absence of PLA2 were analyzed by thin layer chromatography (TLC) using one of two solvent systems: (1) chloroform-methanol-2-propanol-water-triethylamine (30
1 DEPN-8:PG 1 + 3% S-MB to resist degradation by PLA2 was studied in comparison with CLSE and POPG as non-resistant controls. Surfactant substances in chloroform were dried under nitrogen and resuspended by vortexing in 5 mM Tris (hydroxymethylaminomethane) buffer containing 5 mM CaCl2 (pH 7.4). Lyophilized PLA2 (0.1 units/ml) suspended in the same buffer was added, and the mixture was incubated for 30 min at 37 °C. Phospholipid class compositions in the presence and absence of PLA2 were analyzed by thin layer chromatography (TLC) using one of two solvent systems: (1) chloroform-methanol-2-propanol-water-triethylamine (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7
7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 by volume),34 and (2) chloroform-methanol-water (65
25 by volume),34 and (2) chloroform-methanol-water (65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 by volume).35 The first of these solvent systems was used to resolve lysophosphatidylcholine (lyso-PC) as a measure of PLA2 degradation for CLSE and DEPN-8 + PG 1 + 3% S-MB, and the second solvent system was used to resolve lysophosphatidylglycerol (lyso-PG) as a measure of PLA2 degradation for POPG and PG 1. Percentages of each phospholipid class were quantitated by the phosphate assay of Ames36 applied to specific identifiable spots scraped from the TLC plate relative to total phosphate content.
4 by volume).35 The first of these solvent systems was used to resolve lysophosphatidylcholine (lyso-PC) as a measure of PLA2 degradation for CLSE and DEPN-8 + PG 1 + 3% S-MB, and the second solvent system was used to resolve lysophosphatidylglycerol (lyso-PG) as a measure of PLA2 degradation for POPG and PG 1. Percentages of each phospholipid class were quantitated by the phosphate assay of Ames36 applied to specific identifiable spots scraped from the TLC plate relative to total phosphate content.
Surfactant activity methods
The surface activity of synthetic surfactants was measured by two methods relevant for surfactant function in respiration: (1) adsorption to the air–water interface;32,37 and (2) overall surface tension lowering during dynamic cycling on a pulsating bubble surfactometer (General Transco, Largo, FL).38,39 For both bubble and adsorption studies, surfactant components were combined in organic solvent (chloroform for PG 1, DEPN-8 or CLSE; and TFE for S-MB), dried under nitrogen, exposed to house vacuum to remove residual solvent, resuspended by hand vortexing in 0.15 M NaCl (normal saline) adjusted to pH 7.0 with 0.1 N sodium bicarbonate, heated to 65 °C intermittently for 30 min, and refrigerated for 12 h prior to use. Adsorption experiments were done at 37 ± 0.5 °C in a Teflon® dish containing a 35 ml subphase of pH 7 normal saline stirred with a Teflon-coated magnetic bar to minimize diffusion resistance. Dispersed surfactants were injected at time zero into the stirred subphase to final concentrations of 0.03125 or 0.0625 mg ml−1phospholipid (i.e., 1.25 or 2.5 mg of surfactant phospholipid in 5 ml of pH 7 normal saline was injected into the stirred 35 ml subphase). Adsorption surface pressure (surface tension lowering below that of the pure subphase) was measured over time from the force on a partially submerged, sandblasted platinum Wilhelmy slide. In bubble surfactometer studies, an air bubble communicating with ambient air was formed in a 40-μl sample of surfactant (1 and 2.5 mg ml−1phospholipid concentration). The bubble was then pulsated at 37 ± 0.5 °C between maximum and minimum radii of 0.55 and 0.4 mm (50% area compression for a truncated sphere) at a rate of 20 cycles/min. Surface tension at minimum bubble radius (minimum surface tension) was calculated as a function of time from the Laplace equation38,39 based on the measured pressure drop across the bubble interface.Methods for molecular dynamics computer modeling
For molecular dynamics simulations, S-MB structure was taken from the study of Walther et al.10 based on the lowest energy conformers of overlapping SP-B(1–25) (PDB: 1DFW)40 and Mini-B peptide (PDB: 1SSZ)30 structures as templates followed by homology-modeling with Protein Modeller version 9v4 (http://www.salilab.org/modeller/). The homology structure for S-MB was then manually docked onto the surface of a pre-equilibrated bilayer of either DPPC or 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (mole:mole) DPPC:POPG in a periodic 65 × 65 × 90 Å box with chloride counter ions added for electro-neutrality.41 The solvated lipid/peptide system then minimized by the steepest descent method as implemented in the GROMACS version 4.5.4 environment42 (http://www.gromacs.org). The ensemble was then subjected to 100 ps of molecular dynamics at 310°K using the ffG53a6 force field option to allow the solvent and lipid to equilibrate while restraining the peptide, followed by 100 ns of molecular dynamics without any constraints utilizing Berendsen temperature and pressure coupling and the Particle Mesh Ewald method for evaluating long-range electrostatic interactions. Molecular model structure was rendered using PyMOL v0.99 (http://www.pymol.org).
1 (mole:mole) DPPC:POPG in a periodic 65 × 65 × 90 Å box with chloride counter ions added for electro-neutrality.41 The solvated lipid/peptide system then minimized by the steepest descent method as implemented in the GROMACS version 4.5.4 environment42 (http://www.gromacs.org). The ensemble was then subjected to 100 ps of molecular dynamics at 310°K using the ffG53a6 force field option to allow the solvent and lipid to equilibrate while restraining the peptide, followed by 100 ns of molecular dynamics without any constraints utilizing Berendsen temperature and pressure coupling and the Particle Mesh Ewald method for evaluating long-range electrostatic interactions. Molecular model structure was rendered using PyMOL v0.99 (http://www.pymol.org).
Acknowledgements
The authors gratefully acknowledge the financial support of the National Institutes of Health through Bioengineering Research Partnership (BRP) grant HL094641 and grants HL080775, HL092158, and ES015330. We would like to thank the Office of Information Technology and Broadcom Corp. at UC Irvine for providing the Broadcom Distributed/Unified Cluster (BDUC) computer facilities used for the molecular dynamics simulations.References
- R. H. Notter, Lung surfactants: Basic science and clinical applications, Marcel Dekker, Inc, New York, 2000 Search PubMed.
- R. H. Notter, J. N. Finkelstein and B. A. Holm, ed., Lung injury: Mechanisms, pathophysiology and therapy, Taylor Francis Group, Inc, Boca Raton, 2005 Search PubMed.
- K. Raghavendran, G. S. Pryhuber, P. R. Chess, B. A. Davidson, P. R. Knight and R. H. Notter, Curr. Med. Chem., 2008, 15, 1911–1924 CrossRef CAS.
- D. F. Willson, P. R. Chess and R. H. Notter, Pediatr. Clin. North Am., 2008, 55, 545–575 CrossRef.
- G. D. Rubenfeld, E. Caldwell, E. Peabody, J. Weaver, D. P. Martin, M. Neff, E. J. Stern and L. J. Hudson, N. Engl. J. Med., 2005, 353, 1685–1693 CrossRef CAS.
- A. H. Jobe, N. Engl. J. Med., 1993, 328, 861–868 CrossRef CAS.
- R. F. Soll, Biol. Neonate, 1997, 71, 1–7 Search PubMed.
- K. R. Raghavendran, D. Willson and R. H. Notter, Crit. Care Clin., 2011, 27, 525–559 CrossRef CAS.
- R. H. Notter, A. L. Schwan, Z. Wang and A. J. Waring, Mini-Rev. Med. Chem., 2007, 7, 932–944 CrossRef CAS.
- F. J. Walther, A. J. Waring, J. M. Hernandez-Juviel, L. M. Gordon, Z. Wang, C.-L. Jung, P. Ruchala, A. P. Clark, W. M. Smith, S. Sharma and R. H. Notter, PLoS One, 2010, 5, e8672, DOI:10.1371/journal.pone.0008672.
- D. K. Kim, T. Fukuda, B. T. Thompson, B. Cockrill, C. Hales and J. V. Bonventre, Am. J. Physiol., 1995, 269, L109–L118 CAS.
- L. Touqui and L. Arbibe, Mol. Med. Today, 1999, 5, 244–249 CrossRef CAS.
- P. Vadas, J. Lab. Clin. Med., 1984, 104, 873–881 CAS.
- P. Vadas and W. Pruzanski, Lab. Invest., 1986, 55, 391–404 CAS.
- S. J. Ackerman, M. A. Kwatia, C. B. Doyle and G. Enhorning, Chest, 2003, 123, 255S CrossRef.
- H. L. Attalah, Y. Wu, M. Alaoui-El-Azher, F. Thouron, K. Koumanov, C. Wolf, L. Brochardz, A. Harf, C. Delclaux and L. Touqui, Eur. Respir. J., 2003, 21, 1040–1045 CrossRef CAS.
- G. Nakos, E. Kitsiouli, E. Hatzidaki, V. Koulouras, L. Touqui and M. E. Lekka, Crit. Care Med., 2005, 33, 772–779 CrossRef CAS.
- R. H. Notter, Z. Wang, Z. Wang, J. Davy and A. L. Schwan, Bioorg. Med. Chem. Lett., 2007, 17, 113–117 CrossRef CAS.
- Y. Chang, Z. Wang, A. L. Schwan, Z. Wang, B. A. Holm, J. E. Baatz and R. H. Notter, Chem. Phys. Lipids, 2005, 137, 77–93 CrossRef CAS.
- D. F. Willson, N. J. Thomas, B. P. Markovitz, J. V. DiCarlo, S. Pon, B. R. Jacobs, L. S. Jefferson, M. R. Conaway and E. A. Egan, JAMA, J. Am. Med. Assoc., 2005, 293, 470–476 CrossRef CAS.
- D. F. Willson, L. A. Bauman, A. Zaritsky, K. Dockery, R. L. James, M. Stat, D. Conrad, H. Craft, W. E. Novotny, E. A. Egan and H. Dalton, Crit. Care Med., 1999, 27, 188–195 CrossRef CAS.
- D. F. Willson, J. H. Jiao, L. A. Bauman, A. Zaritsky, H. Craft, K. Dockery, D. Conrad and H. Dalton, Crit. Care Med., 1996, 24, 1316–1322 CrossRef CAS.
- R. L. Auten, R. H. Notter, J. W. Kendig, J. M. Davis and D. L. Shapiro, Pediatrics, 1991, 87, 101–107 CAS.
- F. J. Walther, A. J. Waring, J. M. Hernandez-Juviel, L. M. Gordon, A. L. Schwan, C.-L. Jung, Y. Chang, Z. Wang and R. H. Notter, PLoS One, 2007, 2, e1039, DOI:10.1371/journal.pone.0001039.
- R. H. Notter, Z. Wang, E. A. Egan and B. A. Holm, Chem. Phys. Lipids, 2002, 114, 21–34 CrossRef CAS.
- Z. Wang, Y. Chang, A. L. Schwan and R. H. Notter, Am. J. Respir. Cell Mol. Biol., 2007, 37, 387–394 CrossRef CAS.
- Z. Wang, A. L. Schwan, L. L. Lairson, J. S. O'Donnell, G. F. Byrne, A. Foye, B. A. Holm and R. H. Notter, Am. J. Physiol., 2003, 285, L550–L559 CAS.
- W. H. Lin, S. G. Cramer, J. G. Turcotte and R. S. Thrall, Respiration, 1997, 64, 96–101 CrossRef CAS.
- J. G. Turcotte, W. H. Lin, P. E. Pivarnik, A. M. Sacco, M. S. Bermel, Z. Lu and R. H. Notter, Biochim. Biophys. Acta, Lipids Lipid Metab., 1991, 1084, 1–12 CrossRef CAS.
- A. J. Waring, F. J. Walther, L. M. Gordon, J. M. Hernandez-Juviel, T. Hong, M. A. Sherman, C. Alonso, T. Alig, A. Braun, D. Bacon and J. A. Zasadzinski, J. Pept. Res., 2008, 66, 364–374 CrossRef.
- Z. Wang, S. B. Hall and R. H. Notter, J. Lipid Res., 1995, 36, 1283–1293 CAS.
- S. B. Hall, A. R. Venkitaraman, J. A. Whitsett, B. A. Holm and R. H. Notter, Am. J. Respir. Crit. Care Med., 1992, 145, 24–30 CrossRef CAS.
- S. B. Hall, Z. Wang and R. H. Notter, J. Lipid Res., 1994, 35, 1386–1394 CAS.
- J. C. Touchstone, J. C. Chen and K. M. Beaver, Lipids, 1980, 15, 61–62 CrossRef CAS.
- R. E. Stafford, T. Fanni and E. A. Dennis, Biochemistry, 1989, 28, 5113–5120 CrossRef CAS.
- B. N. Ames, Methods Enzymol., 8, 115–118 CAS.
- R. H. Notter, D. P. Penney, J. N. Finkelstein and D. L. Shapiro, Pediatr. Res., 1986, 20, 97–101 CrossRef CAS.
- G. Enhorning, J. Appl. Physiol., 1977, 43, 198–203 CAS.
- S. B. Hall, M. S. Bermel, Y. T. Ko, H. J. Palmer, G. A. Enhorning and R. H. Notter, J. Appl. Physiol., 1993, 75, 468–477 CAS.
- L. M. Gordon, K. Y. C. Lee, M. M. Lipp, J. M. Zasadzinski, F. J. Walther, M. A. Sherman and A. J. Waring, J. Pept. Res., 2000, 55, 330–347 CrossRef CAS.
- A. Kukol, J. Chem. Theory Comput., 2009, 5, 615–626 CrossRef CAS.
- B. Hess, C. Kutzner, D. van der Spoel and E. Lindahl, J. Chem. Theory Comput., 2008, 4, 435–447 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c1md00206f |
| This journal is © The Royal Society of Chemistry 2011 |
