Hierarchical mesoporous bio-polymer/silica composites co-templated by trimethyl chitosan and a surfactant for controlled drug delivery†
Vijay Kumar
Rana
acd,
Sung Soo
Park
a,
Surendran
Parambadath
a,
Mi Ju
Kim
b,
Sun-Hee
Kim
b,
Satyendra
Mishra
c,
Raj Pal
Singh
d and
Chang-Sik
Ha
*a
aDepartment of Polymer Science and Engineering, Pusan National University, Busan, 609-735, South Korea. E-mail: csha@pnu.edu; Fax: +82-51-514-4331; Tel: +82-51-510-2407
bDepartment of Biochemistry, School of Medicine, Pusan National University, Yangsan, 626-870, South Korea
cDepartment of Chemical Technology, North Maharashtra University, Jalgaon, India
dDivision of Polymer Science and Engineering, National Chemical Laboratory, Dr Homi Bhabha Road, Pune, 411008, India
First published on 23rd August 2011
Abstract
In this communication, we report the facile synthesis of hierarchical mesoporous bio-polymer/silica composite materials with bimodal mesopores using a dual-template of the cationic N,N,N-trimethyl chitosan (TMCs) and the anionic sodium dodecyl sulfate (SDS) via one-step synthetic strategy. Tetraethoxysilane (TEOS) was used as a silica source. The nitrogen adsorption/desorption measurements and transmission electron microscopy analysis showed the hierarchical structure of the mesoporous bio-polymer/silica composites with bimodal mesopores having an average pore size of 5–7 nm with the visible voids between the silica nanoparticles, which allow the mesoporous bio-polymer/silica composites to encapsulate a large number of guest drug molecules, Ibuprofen (IBU) or 5-fluorouracil (5-FU), due to their high surface area and pore volume. In addition, the mesoporous chitosan–silica composites also had a long term biocompatibility for the target release of the drug molecules to the CEM cells, MCF cells, etc. as well as a pH sensitive controlled release behavior of the drug molecules.
Mesoporous silica has attracted much interest for various applications in the field of biotechnology because of its large surface area, tunable pore size and morphology with its pore volume, bioinertness and notable biocompatibility.1–6 On the other hand, mesoporous polymer/silica composites are very attractive materials with a high surface area and pore volume because they can encapsulate large quantities of guest molecules and subsequently release them at later stages in an optimal manner.7,8 Various research groups have produced hollow polymer spheres and mesoporous polymers8 with submicrometre and micrometre sizes using pH-induced micellization of grafted copolymers9 through emulsion polymerization or interfacial polymerization strategies10 and the assembly of positively charged polyelectrolytes with negatively charged nanoparticles.11
Moreover, many of these techniques suffer from lower drug loading capacity and poor biocompatibility. From a biomedical point of view, chitosan and its derivatives are very attractive polymers because of their biocompatibility, antibacterial and antifungal action in the form of gels, films, emulsions, microspheres,12 and so on, for the prolonged release of drugs,13 including insulin14 and anticancer agents such as 5-flurouracil15 among others. Recently, Yan et al. produced chitosan–poly(acrylic acid) hollow nanospheres for drug release. However, these hollow spheres have a relatively small surface area.16
Well defined interconnected mesoporous polymer/silica composites with a large specific surface area and pore volume are vital for the ideal delivery system and must be highly biocompatible in order to be promising materials for biomedical applications.17
Herein, a facile, dual-template method was reported for the synthesis of hierarchical mesoporous bio-polymer/silica composite materials with bimodal mesopores. This route involved a one-step synthetic strategy. Prominently, these mesoporous bio-polymer/silica composites exhibited a high surface area and pore volume with a desirable drug loading capacity. First, a sodium dodecyl sulfate (SDS) micelle was formed in an aqueous solution, and then the cationic polymer N,N,N-trimethyl chitosan (TMCs) surrounded the anionic SDS micelle to form the comicelle through an electrostatic force. In this way, the surface of the comicelle exhibited a positive charge that was derived from TMCs. Under an alkaline condition, tetraethoxysilane (TEOS) was hydrolyzed and concentrated around the surface of the comicelle to form the silica frameworks. The cationic polymer (TMCs) acted as a cotemplate that connected the anionic silicates to the anionic SDS micelle (see Scheme 1 and also the ESI†). To the best of our knowledge, this study was the first to use the anionic surfactant/cationic bio-polymer template system for the synthesis of bio-polymer/silica composite materials. The mesoporous bio-polymer/silica composites were obtained after the anionic surfactant was removed. This work could potentially provide a synthesis technique for hierarchical mesoporous silica immobilized bio-polymers with bimodal mesopores that can be used as a stable matrix for drug loading and release.
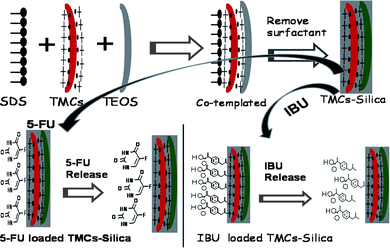 | ||
| Scheme 1 Schematic illustration of the synthesis process of TMCs–silica and drug-loaded TMCs–silica. | ||
The textural properties of the TMCs–silica composites were analyzed using the N2 sorption isotherm in Fig. 1(a) and Table S1 (ESI†). The TMCs–silica composites exhibited the characteristic type IV isotherms of the mesoporous materials with an H2 hysteresis at higher pressures (P/Po of 0.8–1.0) along with an H4 hysteresis in the range of relative pressures, P/Po, from 0.3 to 0.8. A similar isotherm behaviour was obtained for mesoporous silica (red). Notably, the two types of hysteresis loops probably suggested the presence of two types of pores and hierarchically mesoporous silica. The inset of Fig. 1(a) shows the pore size distribution of both the TMCs–silica composites and pure mesoporous silica. The size of the pores, i.e. 5–7 nm, was probably caused by the comicelle template of TMCs–silica, which was also identified using the TEM measurements. The large pores with a size of ca. 35 nm were ascribed to the inner voids between the TMCs–silica nanoparticles.
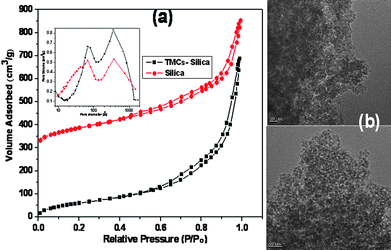 | ||
| Fig. 1 N2-Sorption isotherms (a) TMCs–silica and pure silica, inset: corresponding pore size distribution curves. (b) TEM images of TMCs–silica. | ||
The BET surface area and total pore volume of TMCs–silica composites were 351 m2 g−1 and 0.78 m3 g−1, respectively. The relatively high surface area and volume are desirable for future biomedical applications in order to carry the guest bio-organic or organic molecules into the interconnecting mesochannels of the silica nanoparticles for drug loading and to control drug and gene release behaviour, which could easily penetrate into the hierarchical mesoporous TMCs–silica nanoparticles.
In Fig. 1(b), the TEM image proved the hierarchical structure of the mesoporous bio-polymer/silica composites with bimodal mesopores having an average pore size of 5–7 nm with the visible voids between the silica nanoparticles (see also Fig. S1†). In Fig. S2†, the 29Si NMR spectrum of TMCs–silica exhibited three Qn peaks. In practice, these three signals were centered at ca., −114.3, −105.8 and −97.3 ppm and were assigned to Q4 (Si(OSi)4), Q3 (SiOH(OSi)3), and Q2 (Si(OH)2(OSi)2), respectively. The relative intensity of the Q4 site was higher than the Q3 and Q2 sites, indicating the formation of a highly condensed stable silica framework. A similar trend was observed for the pure silica materials. The SEM image of the TMCs–silica nanoparticles is shown in Fig. S3†. All of the particles exhibited apparently aggregation having spherical morphology. From the SEM image, the average particle size of the TMCs–silica particles was 40–50 nm.
The presence of the hierarchical mesoporosity with bimodal mesopores in the TMCs–silica sample was attributed to the use of the molecular and aggregated cationic polymer TMCs. The existence of the repulsive force among the adjacent polymer chains made the cationic polymer incompactly surround the SDS micelle. When TEOS was hydrolyzed and interacted with the comicelle, the silica species penetrated into the intervals between the polymer chains. The cationic polymers effectively interacted with the negatively charged inorganic silica species in the alkaline media. Moreover, the electrostatic force between the surfactant micelle and the cation polymer comicelle possibly unmatched, and the random coating of the polymers caused the nonuniformity in the size of the comicelles, resulting in the hierarchical mesoporosity with bimodal mesopores. In the FT-IR spectrum of the TMCs–silica nanoparticles and silica (Fig. S4a†), all known characteristic absorption bands for standard silica materials are available. The peak at 1380 cm−1 for the N–CH3 group (arrow) of TMCs–silica confirmed the existence of standard silica materials.
The peak at 1380 cm−1 for the N–CH3 group (arrow) of TMCs–silica confirmed the existence of a glucopyranose ring in the framework of TMCs–silica. Additionally, the absorption band at 1486 cm−1 was ascribed to the bending vibration of C–H. The vibrations of CHx were also observed at 2935, 2983, and 2840 cm−1. A comparison of the FT-IR spectra for TMCs–silica and pure silica confirmed that TMCs–silica contained a cationic polymer, which maintained its dual-template behaviour. This conclusion was further proved by the TGA and EDX analyses as well as 13C-NMR spectrum (Fig. S4b, S4c, S5, and S6†, respectively). The drug loading weight percentages of Ibuprofen (IBU, pKa = 4.43) in the TMCs–silica and pure silica nanoparticles were 41.86 and 44.18 wt%, respectively. The amounts of drug in the host materials were estimated by thermogravimetric analysis (TGA) (Fig. S7†). Although TMCs–silica contained TMCs that strongly interacted with the drug molecule, the presence of TMCs also reduced the surface area of the mesoporous TMCs–silica. However, the drug loading weight percentages of 5-fluorouracil (5-FU, pKa = 8.2) in the TMCs–silica and silica nanoparticles were 33.43 and 29.85 wt%, respectively. The differences in the drug loading wt% occurred because IBU is a hydrophobic drug. The hydrophobic interaction between the pure silica nanoparticles and TMCs–silica was almost the same, but the pure silica nanoparticles had a higher surface area than TMCs–silica. Apparently, 5-FU is a hydrophilic drug molecule. Consequently, TMCs–silica had a higher drug loading capacity than the pure silica nanoparticles because of the stronger interaction between TMCs and 5-FU, which was further supported by the higher pore volume. Moreover, TMCs in TMCs–silica is a biopolymer and creates more interaction with the guest drug molecules. The BET surface area, the pore size and the volume were reduced after the introduction of the drug molecules into the host materials (Table S1†). These results indicated that most of the pores were filled after the drugs were loaded into the host materials. It is interesting to note here that the loading amount of 5-FU was smaller than that of IBU in the mesoporous TMCs–silica and pure silica in spite of the hydrophilic nature of 5-FU. When IBU and 5-FU were loaded in the mesoporous TMCs–silica and mesoporous pure silica, hexane or water was used as a solvent, respectively, for IBU with hydrophobic nature and for 5-FU with hydrophilic nature. The surface of the mesoporous TMCs–silica adsorbent with hydrophilic nature may have stronger affinity with water molecules than with 5-FU.18 Consequently, the adsorption and loading amount of 5-FU are assumed to be smaller than those of IBU due to the disturbance of water molecules in the mesoporous TMCs–silica, although 5-FU has a hydrophilic nature.
Fig. 2(a) shows the IBU release profiles from the IBU loaded TMCs–silica and pure silica nanoparticles at 37 °C into both simulated intestinal fluid (SIF, pH = 7.5) and simulated stomach fluid (SSF, pH = 1.4). Apparently, obvious differences were observed between the drug-release rates of the different delivery systems with different releasing media. In the SIF, the pure silica nanoparticles exhibited a burst release of 45 wt% within 24 h, and the total release was achieved after about 120 h. However, the burst release of IBU into TMCs–silica was controlled to 15 wt% within 24 h and a release of 30 wt% took place at 60 h. The delivery process was optimally controlled until a time of 120 h when 50 wt% was released because of the strong interaction between the IBU and the cationic TMCs in TMCs–silica. Furthermore, in the SSF, the IBU was released from TMCs–silica (13 wt%), which was slower than the silica nanoparticles (30 wt%) during the same observation time. In contrast, the IBU release from the IBU-loaded silica nanoparticles in the media with pH 7.5 and pH 1.4 was much more rapid than the TMCs–silica nanoparticles. Therefore TMCs played a key role in the release of the IBU molecules with the pH stimulation, and an enhanced pH-controlled release.
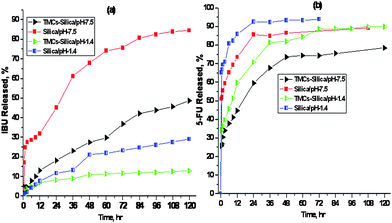 | ||
| Fig. 2 In vitro release profiles of (a) IBU and (b) 5-FU at different pH for the TMCs–silica and pure silica nanoparticles at 37 °C. | ||
The significantly different IBU release behaviour between the TMCs–silica and pure silica nanoparticles under the pH stimulation was mainly attributed to the swelling behaviour of TMCs and the ionization of IBU at different pH values. At pH = 7.5, TMCs in TMCs–silica (pKa = 6.5) and pure silica (pKa = 4.8) have some negative charge due to the deprotonation. IBU (pKa = 4.43) has also negative charge due to the ionization of carboxyl groups which causes the repulsion between host materials and drug molecules at the pH value. When the silica materials were immersed at pH = 7.5, the pores of the TMCs–silica and the pure silica nanoparticles would not be affected, but the TMCs chains in the TMCs–silica are readily swelled due to their hydrophilic nature. The swollen TMCs chains may close the TMCs–silica pores because of the hierarchical structure of the TMCs–silica nanoparticles with dual mesopores. This is why IBU was not freely released in TMCs–silica than in pure silica.
At pH = 1.4, the amino groups of TMCs and silanol groups in pure silica have positive charges due to protonation (pKa = 6.5). Hence, the nature of hierarchical mesoporous bio-polymer/silica composites with dual mesopores changes dramatically, where the dense packed pore structure is formed therein (though swelling also takes place). Even though IBU has also positive charge due to the protonation of carboxyl groups which causes the repulsion between host materials and drug molecules, nevertheless, it is hard to release out IBU from the pores. Thus, IBU was not freely released for TMCs–silica, and only 13 wt% was released after 120 h. Moreover, the drug release capability of a material usually relies on the affinity between the carrier and the drug molecules. When a drug molecule is absorbed into pure silica mesopores exposing silanol groups, only weak interactions including hydrogen bonding exist. Under acidic conditions (pH 1.4) the silanol groups at the pore surface are protonated. At the pH = 4, the IBU molecules feature with protonated carboxyl groups and hydrogen bonding to the pore walls. However, this weak interaction does not prevent the diffusion of the drug molecules out of the mesopores. Meanwhile, TMCs–silica would create higher interatomic interactions with the IBU molecules than pure silica, which would be helpful for controlled release. We assume that the IBU release behaviour for TMCs–silica is affected simultaneously by several different factors such as the swelling, interatomic interactions, the protonation of TMCs, the ionization of IBU at different pH values, etc.
Fig. 2(b) shows the 5-FU release behaviour from the 5-FU loaded TMCs–silica and pure silica nanoparticles that were incubated in the SIF and SSF solutions, respectively, at 37 °C.
The 5-FU release kinetic models were classified into two stages. The first stage presented the burst-drug release behaviour and the second was the controlled-drug release process. For the first stage, the large pores, i.e. the pores that were caused by the voids, facilitated the burst release of 5-FU in both release media, which were further controlled by the mesopores of the TMCs–silica nanoparticles in the second stage controlled release of 5-FU. In the SIF (pH = 7.5), TMCs in TMCs–silica (pKa = 6.5) and pure silica (pKa = 4.8) are ionized, whereas at pH 7.5, approximately one-fourth of 5-FU is charged, electrorepulsive and electroosmotic. Therefore, only weak attraction exists between 5-FU molecules and TMCs–silica. Consequently, the release rate was further controlled by the swelling nature and hierarchical structure of the respective silica composite with dual mesopores, as mentioned above for IBU. In this way, the release of 5-FU was controlled up to 70% in the case of the TMCs–silica composite, while 90% release was observed for pure silica carrier within 120 h. In the SSF, the protonated drug molecules were easily released because 5-FU, TMCs of the TMCs–silica (ζ potential 18.5 mV) and pure silica (ζ potential 3.7 mV) nanoparticles were protonated at pH = 1.4 that might cause increased repulsion. In the SSF, however, the burst release of 5-FU was reduced from 90 wt% for the pure silica nanoparticles to 58.5 wt% for the TMCs–silica nanoparticles within 12 h. After 24 h, 5-FU was almost completely released from the pure silica nanoparticles, while 90 wt% was released from TMCs–silica within 120 h.
The MTT assay was used to determine the cytotoxicity of the neat TMCs–silica nanoparticles and the drug loaded TMCs–silica nanoparticles. Herein, the viabilities of two types of in vitro cancer cell lines with different concentrations, i.e. CEM-human lymphoblastic leukemia and MCF7-human breast cancer cell lines were studied to examine the target potent cancer cell killing effects. The 5-FU loaded TMCs–silica afforded high potent cell viability from 97.4% to 38.5% at concentrations of 10 to 100 μg ml−1 for the CEM cell line after incubation for 5 days. The consistency of the cell line viability was almost the same for the IBU loaded TMCs–silica samples. Importantly, the toxicity of the CEM cell line did not change for various concentrations of TMCs–silica without drug loading, suggesting that the TMCs–silica nanoparticles had a very good biocompatibility, as shown in Fig. 3(a).
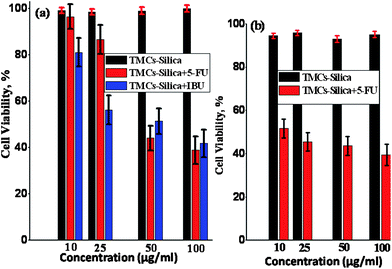 | ||
| Fig. 3 (a) CEM cell line viability of TMCs–silica, IBU and 5-FU loaded TMCs–silica and (b) MCF-7 cell line viability tests for TMCs–silica and 5-FU loaded TMCs–silica. | ||
5-Fluorouracil is a widely used chemotherapy drug for targeting various cancers. The 5-FU loaded polymer silica (TMCs–5-FU) again induced significant death in the MCF-7 cancer cells, as shown in Fig. 3(b). The 5-FU loaded polymer/silica composites (TMCs–silica) were believed to transport 5-FU inside the cellsviaendocytosis. One of the potential advantages of using TMCs–silica as a drug carrier compared to the drug alone is the ability to target the delivery for the selective destruction of certain types of cells, reducing the toxicity to the non-targeted cells. The 5-FU loaded TMCs–silica exhibited a higher concentration-dependent toxicity (10 to 100 μg ml−1) to the CEM cells than the MCF cells, suggesting that the chitosan conjugation to the TMCs–silica composites enhanced in the 5-FU delivery to the MCF cells. These results suggested that the toxicity of the drugs to certain types of cells could potentially be enhanced using TMCs–silica that was loaded with a targeting moiety as the drug carriers. Notably, the targeting, cell killing anti-cancer drug loaded TMCs–silica had a high, long term biocompatibility (5 days) with different types of cancer cell lines (CEM and MCF-7).
In summary, hierarchical mesoporous bio-polymer/silica composites with bimodal mesopores were synthesized using a one step facile route with a co-template of the TMCs bio-polymer. The mesoporous chitosan–silica composites with a high surface area and pore volume encapsulated a large number of guest drug molecules and exhibited a pH sensitive controlled release behaviour of drug molecules. Furthermore, the mesoporous chitosan/silica composites had a long term biocompatibility for the target release of the drug molecules to the CEM cells, MCF cells, etc. These mesoporous bio-polymer/silica composite materials with bimodal mesopores could possibly be useful in future biomedical applications.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) through the Acceleration Research Program (no. 2011-0000385) and the Pioneer Research Center Program (no. 2011-0001667/2011-0001668) and the Brain Korea 21 Project funded by the Ministry of Education, Science and Technology, Korea.Notes and references
- W. Zhao, J. Gu, L. Zhang, H. Chen and J. Shi, J. Am. Chem. Soc., 2005, 127, 8916–8917 CrossRef CAS.
- F. Y. Qu, G. S. Zhu, S. Y. Huang, S. G. Li, J. Y. Sun, D. L. Zhang and S. L. Qiu, Microporous Mesoporous Mater., 2006, 92, 1–9 CrossRef CAS.
- S. Moudgil and J. Y. Ying, Adv. Mater., 2007, 19, 3130–3135 CrossRef CAS.
- D. R. Radu, Y. L. Cheng, K. Jeftinija, E. W. Rowe, S. Jeftinija and V. S. Y. Lin, J. Am. Chem. Soc., 2004, 126, 13216–13217 CrossRef CAS.
- C. Y. Lai, B. G. Trewyn, D. M. Jeftinija, K. Jeftinija, S. Xu, S. Jeftinija and V. S. Y. Lin, J. Am. Chem. Soc., 2003, 125, 4451–4459 CrossRef CAS.
- S. Giri, B. G. Trewyn, M. P. Stellmaker and V. Lin, Angew. Chem., Int. Ed., 2005, 44, 5038–5044 CrossRef CAS.
- W. Meier, Chem. Soc. Rev., 2000, 29, 295–303 RSC.
- H. Lin, G. Zhu, J. Xing, B. Gao and S. Qiu, Langmuir, 2009, 25, 10159–10164 CrossRef CAS.
- H. Dou, M. Jiang, H. Peng, D. Chen and Y. Hong, Angew. Chem., Int. Ed., 2003, 42, 1516–1519 CrossRef CAS.
- (a) M. Okubo, Y. Konishi and H. Minami, Colloid Polym. Sci., 1998, 276, 638–642 CrossRef CAS; (b) K. B. Thurmond, T. Kowalewski and K. L. Wooley, J. Am. Chem. Soc., 1997, 119, 6656–6665 CrossRef CAS.
- J. N. Cha, H. Birkedal, L. E. Euliss, M. H. Bartl, M. S. Wong, T. J. Deming and G. D. Stucky, J. Am. Chem. Soc., 2003, 125, 8285–8289 CrossRef CAS.
- C. Peniche, M. Fernandez, A. Gallardo, A. López-Bravo and J. S. Román, Macromol. Biosci., 2003, 3, 540–545 CrossRef CAS.
- V. K. Rana, O. S. Kushwaha, R. P. Singh, S. Mishra and C. S. Ha, Macromol. Res., 2010, 18, 845–852 CrossRef CAS.
- J. P. Nam, C. Choi, M. K. Jang, Y. I. Jeong, J. W. Nah and Y. Park, Macromol. Res., 2010, 18, 630–635 CrossRef CAS.
- Y. Ohya, T. Takei, H. Kobayashi and T. Ouchi, J. Microencapsulation, 1993, 10, 1–9 CrossRef CAS.
- E. Yan, Y. Ding, C. Chen, R. Li, Y. Hu and X. Jiang, Chem. Commun., 2009, 2718–2720 RSC.
- M. Vallet-regí, F. Balas and D. Arcos, Angew. Chem., Int. Ed., 2007, 46, 7548–7558 CrossRef.
- J. H. Shin, S. S. Park and C.-S. Ha, Colloids Surf., B, 2011, 84, 579–584 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c0md00222d |
| This journal is © The Royal Society of Chemistry 2011 |
