Identification of multifunctional small molecule-based reversible monoamine oxidase inhibitors
Werner J.
Geldenhuys
a,
Kristin S.
Ko
b,
Hilary
Stinnett
a,
Cornelis J.
Van der Schyf
*a and
Mi Hee
Lim
*bc
aDepartment of Pharmaceutical Sciences, Northeast Ohio Medical Univ., Rootstown, OH 44272, USA. E-mail: cvanders@neomed.edu
bDepartment of Chemistry, University of Michigan, Ann Arbor, MI 48109-1055, USA. E-mail: mhlim@umich.edu
cLife Sciences Institute, University of Michigan, Ann Arbor, MI 48109-2216, USA
First published on 15th September 2011
Abstract
The design of multifunctional, neuroprotective compounds has received increasing attention due to their perceived utility in targeting not only the diverse comorbidity of neurological disorders, but also the typically complex pathoetiological pathways leading to these disorders, including insoluble proteins associated with many human neurodegenerative diseases of aging and radical-generating enzyme actions in the brain. For this purpose, herein, we present inhibitory studies on monoamine oxidase (MAO) of a chemical library composed of stilbene-like derivatives that have potential applications in Alzheimer's disease (AD) and dementia with Lewy bodies (DLB), with the ability to modulate metal-induced amyloid-β (Aβ) aggregation and neurotoxicity in vitro and in living cells. Two isoforms of MAO, MAOA and MAOB, are well-known drug targets for depression and Parkinson's disease (PD), respectively. Interestingly, inhibition and binding affinity studies of MAO with our chemical series indicated that three compounds, L1-b, L2-b, and L3-b, exhibited potent and relatively selective inhibitory effects of MAOB. In particular, L2-b was observed to be the most effective MAOB inhibitor in our chemical library showing a reversible and competitive inhibition. Generally, compounds having a dimethylamino moiety in our chemical family showed greater MAO inhibition suggesting a structure–activity relationship. Overall, our findings demonstrate that the stilbene-like scaffolds could be utilized for developing promising multifunctional, neuroprotective agents for several neurodegenerative diseases.
Introduction
Monoamine oxidase (MAO), found in the outer mitochondrial membrane, is a neurotransmitter-degrading flavoenzyme that catalyzes deamination of monoamines such as serotonin and dopamine.1–4 Two isoforms of MAO (MAOA and MAOB) have been identified in most mammalian tissues and distinguished by their substrate specificity. Typically, MAOA catalyzes the oxidation of 5-hydroxyltrptamine (5-HT or serotonin) while MAOB is most active with benzylamine and 2-phenylethylamine (e.g., dopamine). MAOA and MAOB share more than 70% homology in their amino acid sequences and their catalytic sites are very similar. MAO is associated with depression, anxiety, and multiple severe neurological disorders such as Parkinson's and Alzheimer's diseases (PD and AD), making it a desirable drug target.3–7A wide range of selective and non-selective small molecule-based inhibitors for MAOA and MAOB have been developed and used clinically to treat depression (MAOA inhibitors; moclobemide and toloxatone) and PD (MAOB inhibitors; e.g., selegiline and rasagiline).5,7–10 These MAOB inhibitors increase the levels of dopamine in the striatum, thereby improving movement in PD. In addition to relieving PD symptoms, the therapeutic value of MAOB inhibitors in a variety of neurodegenerative diseases has received attention due to their potential neuroprotective effects.5–14 With aging, the activity of MAOB increases, thereby promoting the increased generation of reactive oxygen species (ROS; hydrogen peroxide, a product of enzyme catalysis) leading to oxidative stress, an underlying cause of many neurodegenerative diseases. Considering this aspect, recent drug discovery efforts have focused on the development of multi-purpose, multifunctional molecules that can benefit several illnesses and target an array of pathoetiologies.5–7,12,15,16 For example, the drug ladostigil was constructed by combining the propargylamine pharmacophore contained in the structure of rasagiline (PD, MAOB inhibition and neuroprotection) with a carbamate cholinesterase inhibitory moiety (AD). This drug is now in clinical trials for AD and eventually for PD. This multifunctional approach could advance the discovery of novel therapeutics to prevent or slow down the progression of human neurodegenerative disorders more efficiently and effectively.
Recent interest in utilizing multifunctional small molecules to address various facets of AD and PD pathoetiologies have, herein, prompted the investigation of a chemical library derived from the stilbene-like families (Fig. 1) as MAO inhibitors. In general, a survey of small molecules represented in our chemical series suggested structural features similar to those found in other MAO inhibitors.17,18 Some of the compounds (L1-b, L2-a, and L2-b) were originally designed as bifunctional chemical reagents capable of both metal chelation and amyloid-β (Aβ) interaction in order to target metal-associated Aβ species and regulate metal-induced Aβ events in AD.19–22 Using these bifunctional compounds, the attenuation of metal-triggered Aβ aggregation and neurotoxicity including ROS generation in vitro and in living cells were achieved, which suggests their potential applications in AD. These promising results, suggesting inherent neuroprotective properties, guided a similar inquiry in using these small molecules for other neurodegenerative diseases such as PD. Taken together, this defines a possible new aspect of multifunctional compounds, a dual MAO inhibitor/metal-associated Aβ species modulator.
 | ||
| Fig. 1 A chemical library of stilbene-like derivatives. L1-a = N-(pyridin-2-ylmethylene)aniline; L1-b = N1,N1-dimethyl-N4-(pyridin-2-ylmethylene)benzene-1,4-diamine; L2-a = N-(pyridin-2-ylmethyl)aniline; L2-b = N1,N1-dimethyl-N4-(pyridin-2-ylmethyl)benzene-1,4-diamine; L2-c = N1-(pyridin-2-ylmethyl)benzene-1,4-diamine; L3-a = 2-(phenyldiazenyl)pyridine; L3-b = N,N-dimethyl-4-(pyridin-2-yldiazenyl)aniline. | ||
From our chemical family, the small molecules L1-b, L2-b, and L3-b were capable of providing relatively greater inhibitory effects on MAOB over MAOA in a reversible and competitive fashion showing nM values of IC50 and Ki [IC50, the half maximal inhibitory concentration of compounds; Ki, the binding affinity of the compound (inhibitor) to the target]. Furthermore, docking studies suggest that L2-b may occupy both the entrance and substrate cavity of MAOB and showed possible hydrophobic and/or hydrogen bonding interactions which may be crucial for inhibitory activity. An investigation into a possible structure–activity relationship of our family of compounds based on MAO selectivity (MAOBversusMAOA) and inhibitory activity demonstrated that the stilbene-like architecture could be considered for further development of a new class of multifunctional, neuroprotective drug candidates for PD and AD. Overall, these compounds introduce a new approach over more traditional MAO inhibitors in that the multifunctionality defined in these compounds are novel (MAO inhibitor (PD)/metal-Aβ species modulator (AD)) which have not been incorporated in previous multifunctional MAO inhibitors.
Results and discussion
MAO inhibition
The MAO inhibition assay was performed using recombinant human enzyme as described previously with minor modifications, and the results are summarized in Table 1 and Fig. 2.18 Inhibitory activity of the stilbene-like derivatives (Fig. 1) were determined by monitoring kynuramine metabolism by MAO (detection of the metabolite 4-hydroxyquinoline as a product) in the presence of various concentrations of compounds. The selectivity index (SI; Ki (MAOA)/Ki (MAOB)) for each compound was also obtained from Ki values expressed from IC50 values using the Cheng-Prussoff equation.23| Entry | Compound | IC50 (μM)a | K i (= IC50/(1 + [S]/Km); μM)b | SI (= Ki (MAOA)/Ki (MAOB)) | ||
|---|---|---|---|---|---|---|
| MAOB | MAOA | MAOB | MAOA | |||
| a The values are means from triplicate data. b K m = 16 μM and 23 μM for MAOA and MAOB, respectively; [S] = 40 μM and 20 μM for MAOA and MAOB, respectively. | ||||||
| 1 | L1-a | 1300 (±630) | 129 (±2) | 695 | 37 | 0.05 |
| 2 | L1-b | 0.068 (±0.014) | 0.36 (±0.10) | 0.040 | 0.10 | 2.8 |
| 3 | L2-a | 182 (±2) | 20 (±2) | 97 | 5.7 | 0.06 |
| 4 | L2-b | 0.052 (±0.011) | 0.67 (±0.13) | 0.028 | 0.19 | 6.8 |
| 5 | L2-c | 18 (±1) | 8.7 (±1.1) | 9.6 | 2.5 | 0.26 |
| 6 | L3-a | 10 (±1) | 18 (±1) | 5.3 | 5.1 | 0.96 |
| 7 | L3-b | 0.14 (±0.11) | 7.9 (±1.1) | 0.070 | 2.3 | 31 |
![Dose-dependent inhibition of MAOA and MAOB with small molecules depicted in Fig. 1. Kynuramine was used as a substrate ([S] = 40 μM and 20 μM for MAOA and MAOB, respectively). The final concentration of MAOA was 6 μg mL−1 and for MAOB 15 μg mL−1. Data were fitted to a one-site binding model using Prism 5.](/image/article/2011/MD/c1md00176k/c1md00176k-f2.gif) | ||
| Fig. 2 Dose-dependent inhibition of MAOA and MAOB with small molecules depicted in Fig. 1. Kynuramine was used as a substrate ([S] = 40 μM and 20 μM for MAOA and MAOB, respectively). The final concentration of MAOA was 6 μg mL−1 and for MAOB 15 μg mL−1. Data were fitted to a one-site binding model using Prism 5. | ||
As shown in Table 1, the stilbene-like derivatives (L1-b, L2-b, and L3-b) showed potent inhibitory activity toward MAO (MAOA and MAOB). Compared to other small molecules shown in the chemical library, it was observed that these compounds demonstrated more effective and relatively selective inhibition of MAOB over MAOA (IC50 and Ki in the nM range and high SI values; IC50/Ki/SI for L1-b, L2-b, and L3-b; 68 nM/40 nM/2.8, 52 nM/28 nM/6.8, 140 nM/70 nM/31, respectively). In addition, based on time course and Michaelis–Menten kinetic studies, L2-b could be classified as a reversible and competitive inhibitor of MAOB (Fig. 3). Thus, compared to known MAOB inhibitors such as rasagiline (irreversible, IC50 = 14 nM; Ki = 700 nM) and safinamide (reversible, IC50 = 80 nM; Ki = 450 nM), these stilbene-like derivatives (L1-b, L2-b, and L3-b) could be classified as potent, relatively selective, and reversible MAOB inhibitors.17,24
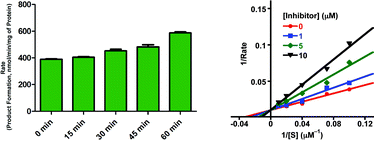 | ||
| Fig. 3 Time effect experiments (left) and Lineweaver–Burk plot (right). Left: Time effect on catalytic activity of MAOB in the presence of L2-b (120 nM, 2 × IC50). Time points on the figure indicate pre-incubation time of MAOB with L2-b. Production formation (nmmol/min mg−1 of protein) was measured after 20 min treatment with the substrate kynuramine. The effect of inhibition by L2-b was reversible over the period of 1 h. Data is represented as mean ± S.D. where N = 8. The experimental procedures and conditions are described in the experimental section. Right: Effect of L2-b on the rate of MAOB activity. These data suggest that L2-b interacted with MAOB in a competitive fashion. The experimental procedures and conditions are described in the experimental section. | ||
In Table 1, several trends emerged showing the correlation between the chemical structure of each molecule and their MAOA/B inhibitory activity (IC50) and binding affinity (Ki). The importance of the incorporated amine functionality in the stilbene-like framework, especially the dimethylamino moiety, in general, afforded greater efficiency and binding affinity in MAO inhibition. For instance, L2-a, L2-b, and L2-c displayed IC50 (Ki) values for MAOB of 182 μM (97 μM), 0.052 μM (0.028 μM), and 18 μM (9.6 μM), respectively, which contain structural variations from hydrogen, dimethylamino functionality, and primary amino group in that order, on the phenyl ring. Therefore, the increased potency and binding affinity following the structural pattern H < NH2 < N(CH3)2 suggest that hydrophobic and/or steric effects may play a role in the inhibitory activity of small molecules toward MAO. In addition to the IC50 and Ki trend, the SI values of our chemical library suggests that the substitution at the para position of the phenyl ring may be responsible for preferential affinities and activity of compounds with either MAOB or MAOA. The stilbene-like derivatives possessing the dimethylamino group presented relatively selective inhibition of MAOB over MAOA [e.g., the SI values of L1-b, L2-b, and L3-b were 2.8, 6.8, and 31, respectively (Table 1)]. Further observations also revealed trends between the different stilbene-like derivatives series. The amine series was more effective for MAOA/B inhibition than the imines (L2-aversusL1-a; L2-bversusL1-b). Overall, the structural variations in the stilbene-like derivatives (e.g., the presence of imine/amine/azo moiety as well as the dimethylamino group) are suggested to be important for effective MAO inhibition showing relative selectivity toward MAOB over MAOA (structure–activity relationship).
Molecular modeling
In order to visualize the structure–activity relationship described above for L2-a, L2-b, and L2-c, docking studies of these stilbene-like derivatives series were carried out (Fig. 4). Overall, the molecules occupied both the entrance and substrate cavity (close to the FAD). The ability of these compounds to reside in both cavities may be a result of their interaction with the gate residues (Phe168, Leu171, Ile199, and Tyr326 which define the boundary between the two binding pockets) allowing for an orientation favoring an ‘open’ conformation that permits the fusion of both pockets into one cavity (Fig. 4c).5 The C–N bond between the pyridyl and phenyl rings of the stilbene-like derivatives may allow flexibility of the structure and thus independent orientation of each ring, permitting a better conformational fit to the different shapes of the hydrophobic entrance and substrate cavity (circular versus flattened, respectively, Fig. 4). This binding orientation was reminiscent of a previously reported inhibitor, 1,4-diphenyl-2-butene, possessing a flexible linker between two aromatic rings.25 | ||
| Fig. 4 Docking studies of L2-a (pink), L2-b (orange), and L2-c (yellow) with MAOB. The molecules were superimposed into the active site of the MAOB bound coumarin analog inhibitor crystal structure (PDB 2V61) which was selected based on similarities in the chemical structure between the coumarin analog and the chemical library investigated here. In the figure, nitrogen, hydrogen, and oxygen were coded blue, white, and red, respectively, with carbon coded with the various colors stated unless otherwise noted. (a) Residues within 5 Å of the substrate (some omitted for clarity) which comprise the active site of MAOB, which is divided into the entrance (green) and substrate (purple) cavities. The gate residues, Phe168, Leu171, Ile199, and Tyr326, which displayed the boundary between the two sites were marked entirely in blue with a darker blue representing nitrogen. The FAD (light gray) cofactor is situated at the end boundary of the substrate cavity. (b) Electrostatic surface representations of L2-a, L2-b, and L2-c bound to the active site. Moreover, this model demonstrates the increased surface area penetration by the dimethyamino group of L2-b. (c) Surface representations of the four gate residues which surround the small molecule and, depending on their conformation, allow the bound substrate to occupy both the entrance and substrate cavities. | ||
Further analysis of the docking studies also showed and corroborated that structural features between the stilbene-like derivatives (L2-a, L2-b, and L2-c) series may affect inhibitory activity. Docking investigations suggested that the dimethylamino or amino group in L2-b or L2-c may increase the surface area and crowd the substrate cavity to a larger degree than the hydrogen atom in L2-a (Fig. 4). Basically, the dimethylamino functionality may further penetrate into the substrate cavity leading to more hydrophobic interactions without sacrificing occupation of the entrance cavity. In addition, the dimethylamino group may provide suitable hydrogen bonding with the residues in the cavities. These observations may account for the lower IC50 and Ki values observed in compounds containing the dimethylamino functionality and may provide an explanation for a decreased inhibitory activity in order, L2-b > L2-c > L2-a.
Conclusions
MAO inhibitory effects of the stilbene-like series that have previously shown ability to target metal-Aβ species and modulate their interaction and reactivity in AD19–22 were investigated in order to examine their potential applications in PD. Using this chemical library, some of the stilbene-like derivatives were found as potent and relatively selective inhibitors for MAOB, a well-known drug target for PD (IC50 and Ki values in the nM range, compared to clinically used MAOB inhibitors). In addition, MAO inhibition studies–including docking investigations–with our chemical family suggest the importance of the dimethylamino functionality for effective MAOB inhibition in the stilbene-like analogues, thereby demonstrating a structure–activity relationship. Overall, combining previous19–22 and current studies of stilbene-like derivatives for AD and PD, these structural scaffolds could provide a foundation for the development of potential multifunctional drug candidates for various neurodegenerative disorders.Experimental section
Materials and methods
All reagents were purchased from commercial suppliers and used as received unless stated otherwise. The compounds L1-b, L2-a, and L2-b were synthesized by previously reported methods.21,22MAO enzyme assay
The MAO enzyme inhibition assay (MAOA and MAOB) was performed using recombinant human enzyme (BD Genetest) as described previously with minor modifications.21 For the measurement of enzyme activity for MAO, kynuramine was employed as a substrate.16 As kynuramine is metabolized by MAO, it forms a fluorescent metabolite 4-hydroxyquinoline. The buffer system used was a 0.1 M phosphate buffer (pH 7.4). The MAO enzyme assay was performed in a fluorescent 96-well plate assay format, using a BioTek Synergy 4 plate reader, λex = 310 nm; λem = 380 nm) of the monochromator. The final concentration of MAOA was 6 μg mL−1 and for MAOB 15 μg mL−1. The final concentrations for kynuramine were 40 μM for MAOA and 20 μM for MAOB. The small molecules (Fig. 1) were dissolved in DMSO, and the final concentration of DMSO in the assays was 2%. The compounds were incubated with MAO and substrate for 20 min, after which the reaction was quenched with the addition of 2 N NaOH. The IC50 values were determined using Prism 5 statistical software (http://www.graphpad.com). The IC50 values were calculated from one-site binding, using eight different concentrations which span five log units, each performed in duplicate. Data are reported as mean ± S.E.M. The Ki values were obtained using the Cheng-Prusoff equation, Ki = IC50/(1 + [S]/Km)23, where Km for MAOA is 16 μM and MAOB is 23 μM; [S] for MAOA is 40 μM and for MAOB is 20 μM.Time dependence of inhibition
To determine the reversibility of MAOB inhibition by L2-b, MAOB was incubated with L2-b ([L2-b] = 2 × 120 nM) for 0, 15, 30, 45, and 60 min before the addition of kynuramine. Measurements of the enzyme activity were measured as described above.Studies of inhibition mode
The inhibition mode was determined under steady state kinetics by monitoring the time course of kynuramine metabolism as described above in the presence of various concentrations of L2-b over a 10 min period. All assays were carried out at 37 °C and performed at least in triplicate using a final MAOB concentration of 2 μM.Docking studies of MAOB with L2-a, L2-b, and L2-c
Docking studies were performed using MOE 2010 (Chemical Computing Group; http://www.chemcomp.com). The protein structure of MAOB used was 2V61, where 7-(3-chlorobenyloxy)-4-(methylamino)methylcoumarin was co-crystallized. In the case of MAOB, chain B was deleted and docking was carried out using only one of the chains of MAOB. The protein was treated before docking by protonation at pH 7.4. The binding site was identified as the area where the co-crystallized ligand was located. Since MOE recognizes FAD as a part of the ligand set, we first designated the true ligand as such so that MOE could use it in the docking run. Only the top-returned binding pose of each ligand was further evaluated.Acknowledgements
This work was supported by the start-up funding from the University of Michigan (to M.H.L.), the Stark Community Foundation, Canton, OH (to W.J.G.), and NEOMED (to C.J.V.d.S.).References
- C. Binda, F. Hubálek, M. Li, D. E. Edmondson and A. Mattevi, FEBS Lett., 2004, 564, 225–228 CrossRef CAS.
- L. De Colibus, M. Li, C. Binda, A. Lustig, D. E. Edmondson and A. Mattevi, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 12684–12689 CrossRef CAS.
- C. Binda, F. Hubálek, M. Li, Y. Herzig, J. Sterling, D. E. Edmondson and A. Mattevi, J. Med. Chem., 2004, 47, 1767–1774 CrossRef CAS.
- D. E. Edmondson, A. Mattevi, C. Binda, M. Li and F. Hubálek, Curr. Med. Chem., 2004, 11, 1983–1993 CAS.
- M. B. H. Youdim, D. Edmondson and K. F. Tipton, Nat. Rev. Neurosci., 2006, 7, 295–309 CrossRef CAS.
- C. J. Van der Schyf, S. Mandel, W. J. Geldenhuys, T. Amit, Y. Avramovich, H. Zheng, M. Fridkin, S. Gal, O. Weinreb, O. Bar Am, Y. Sagi and M. B. Youdim, Curr. Alzheimer Res., 2007, 4, 522–536 CrossRef CAS.
- C. J. Van der Schyf, W. J. Geldenhuys and M. B. Youdim, J. Neurochem., 2006, 99, 1033–1048 CrossRef CAS.
- A. Bolasco, S. Carradori and R. Fioravanti, Expert Opin. Ther. Pat., 2010, 20, 909–939 CrossRef CAS.
- M. B. H. Youdim and Y. S. Bakhle, Br. J. Pharmacol., 2006, 147, S287–S296 CrossRef CAS.
- J. J. Chen and D. M. Swope, Pharmacotherapy, 2007, 27, 161S–173S CrossRef CAS.
- A. H. V. Schapira, Neurology, 2009, 72, S44–S50 CrossRef CAS.
- M. B. H. Youdim, O. Bar Am, M. Yogev-Falach, O. Weinreb, W. Maruyama, M. Naoi and T. Amit, J. Neurosci. Res., 2005, 79, 172–179 CrossRef CAS.
- C. J. Fowler, Å. Wiberg, L. Oreland, J. Marcusson and B. Winblad, J. Neural Transm., 1980, 49, 1–20 CrossRef CAS.
- S. Mandel, O. Weinreb, T. Amit and M. B. H. Youdim, Brain Res. Rev., 2005, 48, 379–387 CrossRef CAS.
- J. Sterling, Y. Herzig, T. Goren, N. Finkelstein, D. Lerner, W. Goldenberg, I. Miskolczi, S. Molnar, F. Rantal, T. Tamas, G. Toth, A. Zagyva, A. Zekany, G. Lavian, A. Gross, R. Friedman, M. Razin, W. Huang, B. Krais, M. Chorev, M. B. Youdim and M. Weinstock, J. Med. Chem., 2002, 45, 5260–5279 CrossRef CAS.
- M. Weinstock, E. Gorodetsky, T. Poltyrev, A. Gross, Y. Sagi and M. Youdim, Progress Neuro-Psychopharmacol. Biol. Psychiatry, 2003, 27, 555–561 CrossRef CAS.
- C. Binda, J. Wang, L. Pisani, C. Caccia, A. Carotti, P. Salvati, D. E. Edmondson and A. Mattevi, J. Med. Chem., 2007, 50, 5848–5852 CrossRef CAS.
- W. J. Geldenhuys, A. S. Darvesh, M. O. Funk, C. J. Van der Schyf and R. T. Carroll, Bioorg. Med. Chem. Lett., 2010, 20, 5295–5298 CrossRef CAS.
- J. J. Braymer, A. S. DeToma, J.-S. Choi, K. S. Ko and M. H. Lim, Int. J. Alzheimers Dis., 2011, 2011, 623051 Search PubMed.
- A. S. DeToma, S. Salamekh, A. Ramamoorthy and M. H. Lim, Chem. Soc. Rev., 2011 10.1039/C1CS15112F.
- S. S. Hindo, A. M. Mancino, J. J. Braymer, Y. Liu, S. Vivekanandan, A. Ramamoorthy and M. H. Lim, J. Am. Chem. Soc., 2009, 131, 16663–16665 CrossRef CAS.
- J.-S. Choi, J. J. Braymer, R. P. Nanga, A. Ramamoorthy and M. H. Lim, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 21990–21995 CrossRef CAS.
- Y.-C. Cheng and W. H. Prusoff, Biochem. Pharmacol., 1973, 22, 3099–3108 CrossRef CAS.
- F. Hubálek, C. Binda, M. Li, Y. Herzig, J. Sterling, M. B. H. Youdim, A. Mattevi and D. E. Edmondson, J. Med. Chem., 2004, 47, 1760–1766 CrossRef.
- C. Binda, M. Li, F. Hubálek, N. Restelli, D. E. Edmondson and A. Mattevi, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 9750–9755 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
