Discovery and structural modification of novel inhibitors of PTP1B inspired by the ACT fragment of scleritodermin A†
Yi
Wei
,
Yue-Ting
Chen
,
Lei
Shi
,
Li-Xin
Gao
,
Shen
Liu
,
Yong-Mei
Cui
,
Wei
Zhang
,
Qiang
Shen
,
Jia
Li
* and
Fa-Jun
Nan
*
Chinese National Center for Drug Screening, State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 189 Guoshoujing Road, Zhangjiang Hi-Tech Park, Shanghai, 201203, People's Republic of China. E-mail: fjnan@mail.shcnc.ac.cn; jli@mail.shcnc.ac.cn
First published on 23rd September 2011
Abstract
A series of compounds synthesized from a key intermediate in the total synthesis of Scleritodermin A containing a novel conjugated thiazole moiety, 2-(1-amino-2-p-hydroxyphenylethane)-4-(4-carboxy-2,4-dimethyl-2Z,4E-propadiene)-thiazole (ACT), were discovered to be potent inhibitors of protein tyrosine phosphatase 1B, with IC50 values in the low micromolar range. Structure–activity relationships around the scaffold were investigated and some compounds exhibited more potent PTP1B inhibitory activity and improved specificities compared with the original hit.
Natural products play a significant role in drug discovery.1,2 Nature provides not only numerous active compounds, but also the attributes of rich structural diversity, abundant conformational complexity, and many stereogenic centers, beyond the wildest thoughts of organic chemists.3,4 Leads obtained by optimization of natural products usually have higher quality than those from classical combinatorial chemistry because natural products always provide more drug-like scaffolds for chemical space exploration.5 Increasing attention has been paid to diversity-oriented synthesis (DOS) of natural product-like libraries,6,7 and several successful examples have been reported recently.8–10
Protein tyrosine phosphatase 1B (PTP1B), an intracellular nonreceptor PTPase, has been shown to play an essential role in signal transduction for both insulin and leptin pathways11,12 by dephosphorylating the insulin receptor (IR),13insulin receptor substrate-1 (IRS-1),14 and Janus kinase 2 (JAK2),15,16downstream of the leptin receptor. Recent knockout studies have demonstrated that PTP1B-deficient mice display increased insulin sensitivity and resistance to diet-induced obesity.17,18 Furthermore, diabetic mice treated with specific PTP1B antisense oligonucleotide exhibit blood glucose level normalization, greater insulin sensitivity,19 and modulated fat storage and lipogenesis in adipose tissue.20 Therefore, PTP1B inhibitors are recognized as potential therapeutic agents for treatment of type-2 diabetes and obesity.21–23
Scleritodermin A (Fig. 1) is a cyclic peptide isolated by Schmidt et al.24 from the lithistid sponge Scleritoderma nodosum that shows several interesting activities including disruption of microtubules through tubulin polymerization inhibition and in vitro cytotoxicity against human tumor cell lines. It was also reported that several key fragments and open analogs of Scleritodermin A showed cytotoxic activity on HCT-15 cells.25 Scleritodermin A has a novel conjugated thiazole moiety, 2-(1-amino-2-p-hydroxyphenylethane)-4-(4-carboxy-2,4-dimethyl-2Z, 4E-propadiene)-thiazole (ACT), and this unique moiety presents a useful molecular scaffold in diversified library design for lead discovery. In this paper, we report the diversity-oriented synthesis and structure–activity relationships (SAR) of ACT skeleton compounds as novel PTP1B inhibitors.
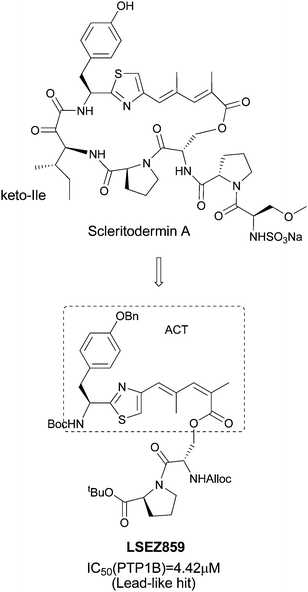 | ||
| Fig. 1 Discovery of PTP1B inhibitors. | ||
Following our first total synthesis of Scleritodermin A,26 several intermediates containing the ACT scaffold were found to possess varying degrees of inhibition against PTP1B in preliminary screening (data not shown). One of these, LSEZ859 (Fig. 1), showed potent inhibition against PTP1B (IC50 = 4.42 μM). This compound presented a novel scaffold for PTP1B inhibitors, and therefore provided a new starting point for further modification. To simplify the fragment, we modified the Ser-Pro side chain to an ethoxy group and gram quantities of compound 1 were synthesized and used as the key intermediate for further derivatization. The Boc group was removed using trifluoroacetic acid (TFA) and the resulting amine 2 was derivatized to give various amides (3a–i) in 58% to 99% yields (as outlined in Scheme 1), most of which showed moderate inhibition against PTP1B (IC50 values 9–20 μM, Table 1). Increasing the size of R1 from cyclopropyl (3a) to cyclopentyl (3b) and to cyclohexyl (3f) decreased the IC50 value. Incorporation of a 4-fluorophenyl group (3c) resulted in a slight improvement in potency compared with the corresponding phenyl-substituted compound 3g. Considering both activity and synthetic facility, compound 3f was selected as a new starting point for further optimization.
 | ||
| Scheme 1 Reagents and conditions: (a) TFA, CH2Cl2; (b) R1COCl, DIPEA, CH2Cl2; (c) R1COOH, EDCI, DIPEA, DMAP, CH2Cl2. | ||
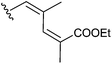
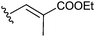
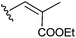
To investigate the importance of the conjugated double bond, cis–trans isomers of the diene 3f (6a–c), the fully saturated analogue 6d, the monoene analogues 6e,f and compound 6g with no aliphatic side chains were synthesized (Scheme 2). Deletion of either one (6e,f) or two (6g) double bonds or saturation of the two double bonds (6d) caused a loss of activity. However, the dienes with different configurations (3f, 6a–c) exhibited moderately different activities and the E,Z-isomer 3f showed the lowest IC50 within the group (Table 2).
 | ||
| Scheme 2 Reagents and conditions: (a) (EtO)2P(O)CH(CH3)COOEt, NaH, THF; (b) (CF3CH2O)2P(O)CH(CH3)COOEt, KN(TMS)2, THF, −78 °C; (c) DIBAL–H, CH2Cl2, −78 °C; (d) DMSO, (COCl)2, Et3N, CH2Cl2, −65 °C; (e) TFA, CH2Cl2; (f) cyclohexanecarbonyl chloride, DIPEA, CH2Cl2; (g) Pd/C, H2, MeOH. | ||
| Compd. | R3 | PTP1B IC50 (μM) |
|---|---|---|
| 6a |

|
19.02 ± 0.19 |
| 6b |

|
23.88 ± 4.84 |
| 6c |
|
13.00 ± 2.80 |
| 6d |

|
>40 |
| 6e |
|
>40 |
| 6f |
|
>40 |
| 6g | COOEt | >40 |
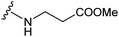
Several amides and esters of the E,Z-conjugated thiazole acid 9 were prepared according to the procedures in Scheme 3. Lengthening either the amide or ester side chains decreased the inhibitory activity, as shown in Table 3. Esters showed lower IC50 values than the corresponding amides. Based on these results, 10e was selected to undergo further modification.
 | ||
| Scheme 3 Reagents and conditions: (a) LiOH, MeOH/H2O, rt; (b) DCC, HOBt, R4NH2, DMF, rt; (c) Cs2CO3, R4Br, DMF, rt. | ||
| Compd. | R4 | IC50 (μM) |
|---|---|---|
| 10a |

|
12.39 ± 0.92 |
| 10b |
|
15.86 ± 0.02 |
| 10c |

|
— |
| 10d |

|
— |
| 10e |
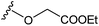
|
7.10 ± 0.35 |
| 10f |

|
12.48 ± 1.94 |
| 10g |

|
9.66 ± 0.17 |
Amine 11, the free amine form of 10e, (Scheme 4) was reacted first with several protected natural amino acids (data not shown) and the tryptophan-substituted product 12a (IC50 = 5.60 μM) showed the lowest IC50 value within the group. Hydrolysis of 12a afforded 12b showing activity three times greater than the ester. We then investigated the effect of the amino side chain of tryptophan, as shown in Table 4.
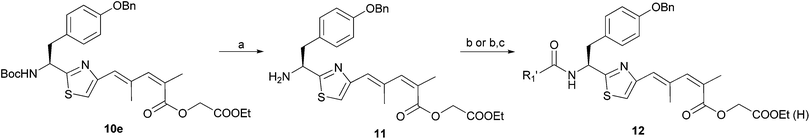 | ||
| Scheme 4 Reagents and conditions: (a) TFA, CH2Cl2; (b) EDCI, DMAP, R1′COOH, CH2Cl2, (c) LiOH, MeOH/H2O, rt. | ||
|
|
|||
|---|---|---|---|
| Compd. | R1′ | R2 | IC50 (μM) |
| 12a |

|
Et | 5.601 ± 0.84 |
| 12b |
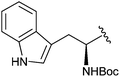
|
H | 1.60 ± 0.21 |
| 12c |

|
Et | — |
| 12d |

|
Et | 2.08 ± 0.03 |
| 12e |

|
H | 3.01 ± 0.21 |
| 12f |
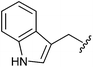
|
Et | 5.94 ± 0.29 |
| 12g |

|
H | 3.68 ± 0.14 |
| 12h |
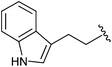
|
Et | 5.43 ± 0.43 |
| 12i |

|
H | 2.32 ± 0.17 |
| 12j |

|
Et | 3.05 ± 0.47 |
| 12k |

|
H | 2.45 ± 0.34 |
Compound 12c, the free amine form of 12a, displayed a loss of activity but acetylation of the amino group recovered the activity (12d). Inhibitory activity was maintained following simplification by deletion of the amino side chain (12h). Several analogues of compound 12h with different side chain lengths (12f, 12g, and 12i–k) also maintained activity.
The inhibitory activity of some ACT derivatives was evaluated against a broad range of PTPs including T-cell protein tyrosine phosphatase (TCPTP), cell division cycle 25 homolog B (CDC25B), leukocyte antigen-related tyrosine phosphatase (LAR), SH2-containing protein tyrosine phosphatase-1 (SHP-1) and SH2-containing protein tyrosine phosphatase-2 (SHP-2) (Table 5). The selected ACT derivatives showed improved specificities for PTP1B over TCPTP (1.7- to 5.9-fold) relative to LSEZ859 (IC50 = 3.95 μM against TCPTP) and displayed several-fold greater specificities over SHP-1, SHP-2, and LAR. Among the selected compounds, the acids showed better specificities against TCPTP and CDC25B compared with their corresponding esters.
| IC50 (μM) | ||||||
|---|---|---|---|---|---|---|
| PTP1B | TCPTP | CDC25B | SHP-1 | SHP-2 | LAR | |
| 3f | 9.32 ± 0.42 | 15.63 ± 3.00 | 3.92 ± 0.86 | >20 | >20 | >20 |
| 12b | 1.60 ± 0.21 | 4.53 ± 0.33 | 3.38 ± 0.23 | >20 | >20 | >20 |
| 12d | 2.08 ± 0.03 | 8.84 ± 1.51 | 2.40 ± 0.45 | >20 | >20 | >20 |
| 12e | 3.01 ± 0.21 | 17.74 ± 2.26 | 5.41 ± 1.01 | >20 | >20 | >20 |
| 12f | 5.94 ± 0.29 | 13.97 ± 1.39 | 2.83 ± 0.38 | >20 | >20 | >20 |
| 12g | 3.68 ± 0.14 | 9.98 ± 1.17 | 4.88 ± 1.28 | >20 | >20 | >20 |
| 12h | 5.43 ± 0.43 | 12.83 ± 0.85 | 3.66 ± 1.17 | >20 | >20 | >20 |
| 12i | 2.32 ± 0.17 | 8.72 ± 0.89 | 4.82 ± 0.26 | 15.02 ± 1.60 | 19.14 ± 2.98 | >20 |
| 12j | 3.05 ± 0.47 | 12.68 ± 1.44 | 2.55 ± 0.31 | >20 | >20 | >20 |
| 12k | 2.45 ± 0.34 | 11.06 ± 0.50 | 4.38 ± 0.59 | >20 | >20 | >20 |
Conclusions
We prepared a series of ACT-containing structures from a key intermediate in the total synthesis of Scleritodermin A with inhibitory activity against PTP1B. Some compounds showed increased activities and specificities compared with the original hit and preliminary SAR studies were performed. These compounds present a novel scaffold worthy of further detailed SAR studies that are in progress in our lab.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grants 30725049 and 30901850) and the National Science & Technology Major Project “Key New Drug Creation and Manufacturing Program,” China (No. 2009Z × 09301-001, No. 2009Z × 09302-001).Notes and references
- R. A. Bauer, J. M. Wurst and D. S. Tan, Curr. Opin. Chem. Biol., 2010, 14, 308 CrossRef CAS.
- K.-H. Lee, J. Nat. Prod., 2010, 73, 500 CrossRef CAS.
- K. Grabowski, K.-H. Baringhaus and G. Schneider, Nat. Prod. Rep., 2008, 25, 892 RSC.
- M.-Q. Zhang and B. Wilkinson, Curr. Opin. Biotechnol., 2007, 18, 478 CrossRef CAS.
- C. M. Dobson, Nature, 2004, 432, 824 CrossRef CAS.
- D. S. Tan, Nat. Chem. Biol., 2005, 1, 74 CrossRef CAS.
- C. Cordier, D. Morton, S. Murrison, A. Nelson and C. O'Leary-Steele, Nat. Prod. Rep., 2008, 25, 719 RSC.
- H. E. Pelish, N. J. Westwood, Y. Feng, T. Kirchhausen and M. D. Shair, J. Am. Chem. Soc., 2001, 123, 6740 CrossRef CAS.
- B. B. Metaferia, L. Chen, H. L. Baker, X.-Y. Huang and C. A. Bewley, J. Am. Chem. Soc., 2007, 129, 2434 CrossRef CAS.
- H.-J. Chen, W.-L. Wang, G.-F. Wang, L.-P. Shi, M. Gu, Y.-D. Ren, L.-F. Hou, P.-L. He, F.-H. Zhu, X.-G. Zhong, W. Tang, J.-P. Zou and F.-J. Nan, ChemMedChem, 2008, 3, 1316 CrossRef CAS.
- J. N. Andersen, O. H. Mortensen, G. H. Peters, P. G. Drake, L. F. Iversen, O. H. Olsen, P. G. Jansen, H. S. Andersen, N. K. Tonks and N. P. H. Møller, Mol. Cell. Biol., 2001, 21, 7117 CrossRef CAS.
- S.-C. Yip, S. Saha and J. Chernoff, Trends Biochem. Sci., 2010, 35, 442 CrossRef CAS.
- S. Wälchli, M.-L. Curchod, R. P. Gobert, S. Arkinstall and R. Hooft van Huijsduijnen, J. Biol. Chem., 2000, 275, 9792 CrossRef.
- B. J. Goldstein, A. Bittner-Kowalczyk, M. F. White and M. Harbeck, J. Biol. Chem., 2000, 275, 4283 CrossRef CAS.
- A. Cheng, N. Uetani, P. D. Simoncic, V. P. Chaubey, A. Lee-Loy, C. J. McGlade, B. P. Kennedy and M. L. Tremblay, Dev. Cell, 2002, 2, 497 CrossRef CAS.
- J. M. Zabolotny, K. K. Bence-Hanulec, A. Stricker-Krongrad, F. Haj, Y.-P. Wang, Y. Minokoshi, Y.-B. Kim, J. K. Elmquist, L. A. Tartaglia, B. B. Kahn and B. G. Neel, Dev. Cell, 2002, 2, 489 CrossRef CAS.
- M. Elchebly, P. Payette, E. Michaliszyn, W. Cromlish, S. Collins, A. L. Loy, D. Normandin, A. Cheng, J. Himms-Hagen, C.-C. Chan, C. Ramachandran, M. J. Gresser, M. L. Tremblay and B. P. Kennedy, Science, 1999, 283, 1544 CrossRef CAS.
- L. D. Klaman, O. Boss, O. D. Peroni, J. K. Kim, J. L. Martino, J. M. Zabolotny, N. Moghal, M. Lubkin, Y. B. Kim, A. H. Sharpe, A. Stricker- Krongrad, G. I. Shulman, B. G. Neel and B. B. Kahn, Mol. Cell. Biol., 2000, 20, 5479 CrossRef CAS.
- B. A. Zinker, C. M. Rondinone, J. Trevillyan, R. J. Gum, J. E. Clampit, J. F. Waring, N. Xie, D. Wilcox, P. Jacobson, L. Frost, P. E. Kroeger, R. M. Reilly, S. Koterski, T. J. Opgenorth, R. G. Ulrich, S. Crosby, M. Butler, S. F. Murray, R. A. McKay, S. Bhano, B. P. Monia and M. R. Jirousek, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 11357 CrossRef CAS.
- C. M. Rondinone, J. M. Trevillyan, J. Clampit, R. J. Gum, C. Berg, P. Kroeger, L. Frost, B. A. Zinker, R. Reilly, R. Ulrich, M. Butler, B. P. Monia, M. R. Jirousek and J. F. Waring, Diabetes, 2002, 51, 2405 CrossRef CAS.
- S. Zhang and Z.-Y. Zhang, Drug Discovery Today, 2007, 12, 373 CrossRef CAS.
- R. H. Van Huijsduijnen, A. Bombrun and D. Swinnen, Drug Discovery Today, 2002, 7, 1013 CrossRef.
- T. O. Johnson, J. Ermolieff and M. R. Jirousek, Nat. Rev. Drug Discovery, 2002, 1, 696 CrossRef CAS.
- E. W. Schmidt, C. Raventos-Suarez, M. Bifano, A. T. Menendez, C. R. Fairchild and D. J. Faulkner, J. Nat. Prod., 2004, 67, 475 CrossRef CAS.
- D. Sellanes, F. Campot, I. Núñez, G. Lin, P. Espósito, S. Dematteis, J. Saldaña, L. Domínguez, E. Manta and G. Serra, Tetrahedron, 2010, 66, 5384 CrossRef CAS.
- S. Liu, Y.-M. Cui and F.-J. Nan, Org. Lett., 2008, 10, 3765 CrossRef CAS.
- Y.-N. Zhang, W. Zhang, D. Hong, L. Shi, Q. Shen, Y.-Y. Li, J. Li and L.-H. Hu, Bioorg. Med. Chem., 2008, 16, 8697 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and compound characterization data. See DOI: 10.1039/c1md00153a |
| This journal is © The Royal Society of Chemistry 2011 |