Design, synthesis and antiproliferative activity of urocanic-chalcone hybrid derivatives†
Alexander
Ciupa
,
Natalie J.
Griffiths
,
Stephanie K.
Light
,
Pauline J.
Wood
and
Lorenzo
Caggiano
*
Medicinal Chemistry, Department of Pharmacy and Pharmacology, University of Bath, Claverton Down, Bath BA2 7AY, UK. E-mail: l.caggiano@bath.ac.uk; Fax: +44 1225 386114; Tel: +44 1225 385709
First published on 5th September 2011
Abstract
Inspired by biologically active natural products, a hybrid analogue that combines the N-Me urocanic side chain of the sarcodictyin family of compounds with the chalcone motif has been proposed, synthesised and examined for antiproliferative activity in three cancer cell lines and one normal primary cell line. The analogues are all synthesised in one or two steps from commercially available materials and, of the compounds examined, the proposed hybrid analogue displays the most active and selective inhibition of cell proliferation in human colon cancer cell line HT29 (IC50 2.9 μM) and highly metastatic human breast carcinoma MDA-MB-231 (IC50 4.8 μM).
Introduction
Eleutherobin 11,2 and the structurally related sarcodictyin family of compounds 23,4 are natural products originally isolated from soft coral. They possess potent anti-tumour activity with a mechanism of action similar to paclitaxel (Taxol®) through the stabilisation of microtubules.5–8 They have attracted great interest as they display nanomolar cytotoxicity against a range of cancer cell lines including Taxol®-resistant cell lines5,9,10 (Fig. 1).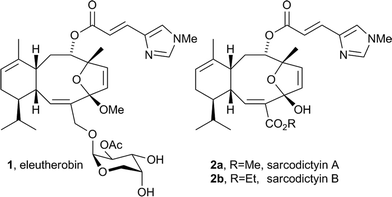 | ||
| Fig. 1 Eleutherobin 1 and sarcodictyin A (2a) and B (2b). | ||
Owing to their limited availability from corals, chemical synthesis is required to explore potential clinical application. The total syntheses of eleutherobin 111–14 and sarcodictyins A (2a) and B (2b)8,15–17 have been reported. Extensive structure–activity relationships of the sarcodictyins were conducted and found that the α,β unsaturated urocanic ester side chain was vital for biological activity.8,17,18 Removal of this pharmacophore resulted in complete loss of cytotoxicity and even subtle substitutions of the imidazole ring were not tolerated and resulted in decreased activity.
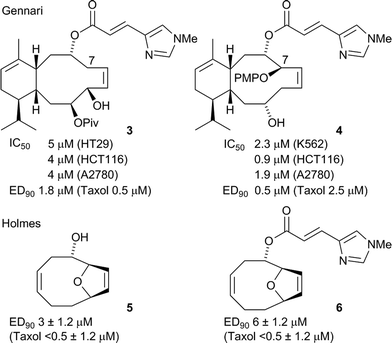
Gennari has reported the synthesis of a variety of simplified eleutherobin analogues, using enantioselective allyltitanation reactions and ring closing metathesis (RCM) as key-steps,19–23 culminating in the formal synthesis of eleutherobin.24,25 The simplified analogues, exemplified by compounds 3 and 4, retained potent microtubule-stabilising properties (ED90 effective dose that induces 90% tubulin polymerisation) but exhibited reduced inhibition of tumour cell proliferation (IC50 concentration inhibiting cell proliferation by 50%) (Fig. 2). It was suggested that this may be the result of hydrolytic cleavage of the N-methylurocanic ester due to a lack of substitution at C7, resulting in diminished activities, as the natural products are fully substituted at the equivalent position.23 Although most studies show that the ester is essential to biological activity,8,17,18 Holmes reported simplified eleutherobin analogues 5 and 6 which displayed improved tubulin binding activities when the N-methylurocanic ester side chain was absent.26
Since the urocanic ester is required for potency in the natural products and can be susceptible to hydrolysis in simplified analogues, we considered using ketone isosteres. Substituting the tricyclic core of the sarcodictyins with an aromatic group would generate compounds closely related to the structure of chalcones 7 that are biologically active compounds themselves.27 We therefore proposed to investigate chalcone derivatives that feature the N-methylurocanic moiety, such as hybrid 8 shown in Fig. 3.
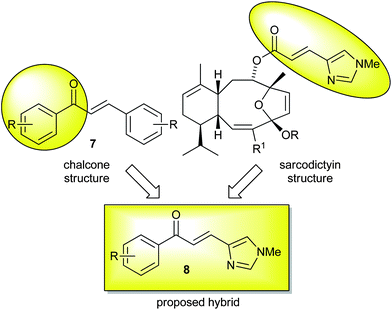 | ||
| Fig. 3 Rationale for urocanic-chalcone hybrid. | ||
Despite the wealth of research in the area of chalcones and related compounds, very few examples have been reported which possess the framework of the proposed hybrid compound 8. These include compound 8a (R = H) generated as an intermediate in the synthesis of γ-lactam alkaloids.28 The structurally similar compound 9 in which the enone is conformationally constrained has been previously reported and exhibits potent and selective inhibition of P450 aromatase (P450 arom, CYP19), with possible therapeutic applications in various hormone-dependent cancers, such as breast, prostate and uterine (Fig. 4).29–33 Similar compounds have also been investigated as agonists of the α2B and α2C adrenergic receptors for the treatment of chronic pain.34,35
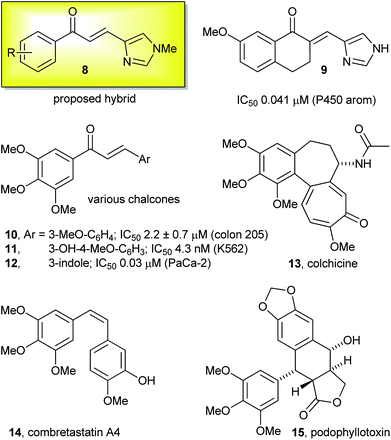 | ||
| Fig. 4 Examples of biologically active arylmethylethers. | ||
The chalcone motif is a privileged structure present in an extensive range of biologically active molecules that display anti-inflammatory, anti-infective, anti-oxidative, anti-malarial and anti-cancer activities.27,36 Recent reports have shown chalcones having a trimethoxyphenyl group exhibit potent biological activities against various cancer cell lines, selected examples of which are shown by compounds 10,371138 and 1239 in Fig. 4. The trimethoxyphenyl group is also present in a range of biologically active compounds such as colchicine 13,40 combretastatin A4 1441 and podophyllotoxin 15,42,43 all of which also bind to tubulin but induce depolymerisation (Fig. 4).
Colchicine 13 and podophyllotoxin 15 display limited clinical potential due to severe toxicity; however, the phosphate prodrug of combretastatin (CA4P) is a promising lead compound under clinical evaluation.40,41,44 The success of combretastatin 14 demonstrates that structural complexity is not necessary for potent biological activity and that simple molecular scaffolds can provide new lead compounds. One emerging strategy in the development of new drugs involves combining key pharmacophores from existing natural products to generate novel chimeric compounds with improved biological properties.45–49
Combining the pharmacophores of the trimethoxyaryl motif and the urocanic ester side chain, the synthesis and antiproliferative activity of various urocanic-chalcone hybrid derivatives is now described.
Results and discussion
Chemical synthesis
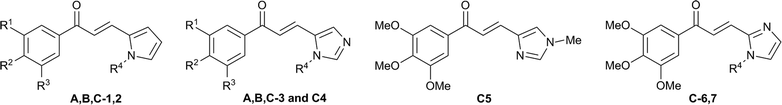
The chalcone hybrid derivatives were obtained by a Claisen-Schmidt condensation between the methoxy-substituted acetophenones A, B and/or C and the heteroarylcarboxaldehyde 1,2,3,4 and 6-CHO (Scheme 1). N-Me imidazole derivatives C5 and C7 were obtained by methylation of the corresponding N-H imidazole, compounds C3 and C6 respectively.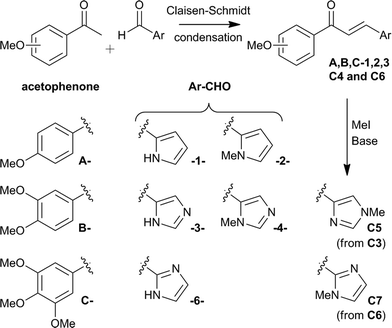 | ||
| Scheme 1 Synthesis of proposed urocanic-chalcone hybrid derivatives. | ||
LiOH·H2O has been reported to catalyse the Claisen-Schmidt reaction of various aryl methyl ketones with heteroaryl aldehydes under mild conditions.50 The reactions of 2-pyrrolecarboxaldehyde 1-CHO with acetophenones A, B and C using 10 mol% of LiOH·H2O, as described, were unsuccessful, although using 1 equivalent gave the products A1, B1 and C1 in moderate yields (Method (i), entries 1–3, Table 1). Following recently reported solvent-free conditions,51grinding1-methyl-2-pyrrolecarboxaldehyde 2-CHO with acetophenones A, B and C in the presence of NaOH with a mortar and pestle gave the corresponding products A2, B2 and C2 after only 5 min. in moderate to good yields (Method (ii), entries 4–6, Table 1). Unfortunately, attempts to apply this method to other substrates failed.
|
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Entry | Compound | R1 | R2 | R3 | R4 | Methoda | Yield (%) | IC50 (μM)b | |||
| HT29 | MDA-MB-231 | LNCaP | FEK-4 | ||||||||
| a Methods (i) 1 equiv. LiOH·H2O, (ii) NaOH, grinding, (iii) 2 equiv. BF3·OEt2, (iv) H2, Pd/C, (v) NaH, MeI, DMF and (vi) Cs2CO3, MeI. b IC50 is the concentration that inhibits 50% cell proliferation. Values are the mean from three independent experiments, except c two and d one experiment, with quadruplicate readings in each. e Ref. 21. f Paclitaxel IC50 < 5nM. g Doxorubicin was used as a positive control. n.d not determined. | |||||||||||
| 1 | A1 | H | OMe | H | H | i | 43 | >500 | >500 | >500 | >500 |
| 2 | B1 | OMe | OMe | H | H | i | 53 | 86.0 ± 3.2 | 102.4 ± 3.7 | 104.6 ± 9.8 | 135.1 ± 37.2 |
| 3 | C1 | OMe | OMe | OMe | H | i | 74 | 43.0 ± 8.0 | 49.9 ± 11.7 | 59.9 ± 9.2 | 156.1 ± 37.7 |
| 4 | A2 | H | OMe | H | Me | ii | 63 | 61.8 ± 2.9 | 53.5 ± 5.7 | 75.5 ± 6.5 | 188.2 ± 91.8 |
| 5 | B2 | OMe | OMe | H | Me | ii | 79 | 60.4 ± 13.1 | 42.8 ± 11.1 | 56.3 ± 12.4 | 161.7 ± 48.2 |
| 6 | C2 | OMe | OMe | OMe | Me | ii | 83 | 12.5 ± 4.8 | 18.0 ± 7.7 | 69.5 ± 11.0 | 117.5 ± 24.6 |
| 7 | A3 | H | OMe | H | H | iii | 53 | 23.6 ± 4.9 | 17.6 ± 4.7 | 33.5 ± 9.6 | 87.1 ± 14.1c |
| 8 | B3 | OMe | OMe | H | H | iii | 74 | 37.9 ± 10.5 | 18.2 ± 2.1 | 46.2 ± 6.7 | 84.2 ± 19.0 |
| 9 | C3 | OMe | OMe | OMe | H | iii | 74 | 19.5 ± 0.6 | 22.9 ± 3.7 | 48.1 ± 7.6 | 53.2 ± 7.4 |
| 10 | C3-H2 | OMe | OMe | OMe | H | iv | 57 | >500 | 223.4 ± 20.6 | 367.7 ± 141.8 | >500d |
| 11 | C4 | OMe | OMe | OMe | Me | iii | 54 | 15.9 ± 1.9 | 16.9 ± 1.5 | 30.6 ± 8.4 | 49.9 ± 1.9 |
| 12 | C5 | OMe | OMe | OMe | Me | v | 36 | 2.9 ± 1.2 | 4.8 ± 2.3 | 48.4 ± 13.5 | 85.0 ± 25.9 |
| 13 | C6 | OMe | OMe | OMe | H | iii | 38 | 5.0 ± 0.5 | 4.9 ± 0.9 | 17.1 ± 3.4 | 28.6 ± 10.7 |
| 14 | C7 | OMe | OMe | OMe | Me | vi | 54 | 4.2 ± 0.6 | 4.9 ± 0.2 | 11.0 ± 3.6 | 17.5 ± 2.3 |
| 15 e | 3 | — | — | — | — | — | — | 5f | n.d | n.d | n.d |
| 16 g | Doxorubicin | — | — | — | — | — | — | 0.164d | 0.120d | 0.154d | n.d |
The imidazole derivatives gave variable results under these conditions and treatment with 50 mol% of BF3·OEt2, as described by Narender,52 only gave starting materials, presumably because of unproductive co-ordination to the basic nitrogen in imidazole. Use of two equivalents of BF3·OEt2, followed by decomplexation with NaOH, gave the desired urocanic-chalcone derivatives A3, B3 and C3 in moderate to good yields (Method (iii), entries 7–9, Table 1).
To examine the effect of the enone double bond, the dihydro-derivative C3-H2 was obtained in 57% yield by hydrogenation of the conjugated double bond of compound C3 under standard conditions (entry 10, Table 1). Having obtained the core framework of the urocanic-chalcone hybrid with acetophenones A, B and C, further derivatives were focused on the trimethoxyaryl motif C. 1-Methylimidazole-2-carboxaldehyde 4-CHO is commercially available and gave the non-natural urocanic-chalcone 1-Me derivative C4 in the presence of BF3·OEt2 in 54% yield (entry 11). Methylation of the imidazole group present in C3 using Cs2CO3 and MeI gave a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 ratio of 1-Me C4 and 3-Me C5 isomers, which proved difficult to separate. Using the conditions reported for the selective methylation of the N-urocanic methyl ester,53NaH and MeI in DMF at 0 °C selectively gave the desired 3-Me isomer C5 in a 75
50 ratio of 1-Me C4 and 3-Me C5 isomers, which proved difficult to separate. Using the conditions reported for the selective methylation of the N-urocanic methyl ester,53NaH and MeI in DMF at 0 °C selectively gave the desired 3-Me isomer C5 in a 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 ratio of C5:C4 that could be separated by silica gel column chromatography (entry 12). The 2-substituted imidazole derivative C6 was obtained using excess BF3·OEt2 and was methylated under standard conditions to afford compound C7 without the formation of isomers due to the symmetry of the imidazole substituent (entries 13 and 14).
25 ratio of C5:C4 that could be separated by silica gel column chromatography (entry 12). The 2-substituted imidazole derivative C6 was obtained using excess BF3·OEt2 and was methylated under standard conditions to afford compound C7 without the formation of isomers due to the symmetry of the imidazole substituent (entries 13 and 14).
Cell proliferation assays
In all cases, the only stereoisomer observed was the thermodynamic trans-product, identified by a characteristic 3J coupling of ca. 15 Hz. All compounds were determined to be ≥95% pure by HPLC and were examined for inhibition of cell proliferationin vitro using the MTS assay54 with various cell lines and the results are reported as the concentration required to inhibit 50% cell proliferation (IC50) (Table 1). The results are the mean of three experiments, except where indicated in Table 1, and were performed on different days with different batches of cells. Three cancer cell lines were investigated, human colon carcinoma HT29, highly metastatic human breast carcinoma MDA-MB-231 and an androgen-dependent human prostate carcinoma LNCaP. In addition, a normal primary cell line, human skin fibroblasts FEK-4, were also investigated in the MTS assay to examine selectivity.The addition of methoxy substituents significantly increased activity of the N-H pyrrole derivatives (A1-C1) and was also observed to a lesser extent with N-Me pyrrole hybrids (A2-C2) (entries 1–3 and 4–6, Table 1). The effect of the substituents was not evident with N-H 4-imidazole derivatives (A3-C3), which showed moderate levels of activity against all three cancer cell lines, but without much selectivity (entries 7–9). The importance of the enone was demonstrated by the dramatic loss of activity of the saturated derivative C3-H2 (entry 10). The non-natural methylated analogue C4 gave similar levels of activity to the unsubstituted compound C3, although the proposed N-methylurocanic trimethoxy chalcone hybrid C5, which has the side chain present in the sarcodictyins, did exhibit enhanced antiproliferative activity and selectivity, especially with HT29 and MDA-MB-231 (entries 11 and 12). Interestingly, the symmetrical N-H 2-imidazole hybrid C6 and the methylated derivative C7 showed similar levels of potency as hybrid C5 against HT29 and MDA-MB-231 and displayed improved activity against LNCaP, although they are much less selective and also inhibited cell proliferation in the normal primary human skin fibroblasts FEK-4 (entries 13 and 14).
Conclusions
Combining pharmacophores present in the sarcodictyin and chalcone family of compounds, the hybrid analogue 8 was proposed and synthesised together with related isomers. It is of interest that both sarcodictyin and chalcones interact with tubulin, although they have opposing effects on microtubule assembly. From our investigations however, the lead compound is indeed hybrid chalcone C5 which includes the N-methylurocanic side chain present in the sarcodictyins. It is the most active and most selective compound in the series investigated, displaying selective antiproliferative activity against HT29 and MDA-MB-231 cell lines.It is worthy of note that the urocanic-chalcone derivative C5 can be obtained in only two synthetic steps and displays similar levels of antiproliferative activity against HT29 as the simplified eleutherobin analogue 3 previously reported21 (Fig. 2 and Table 1, entry 15). Owing to their ease of synthesis, low molecular weight and promising initial results, these hybrid compounds could be used in a fragment-based lead discovery (FBLD) approach to achieve more potent compounds.
Hybrid C5, together with others examined in this series, will be further investigated to determine the biological mode of action and will be reported in due course.
The simple chalcone scaffold combined with the N-methylurocanic ester side chain present in the sarcodictyin family of compounds has afforded biologically active hybrid analogues with the potential for improved biological activities and drug-like properties due to their ease of synthesis and the diverse range of inexpensive and commercially available starting materials.
Acknowledgements
We wish to thank Dr Timothy J. Woodman (University of Bath) for his assistance with the NMR spectra, Dr Anneke Lubben (University of Bath) for the mass spectra, Dr Ian M. Eggleston (University of Bath) for use of the HPLC, Prof. Rex M. Tyrrell (University of Bath) for the gift of FEK-4 cells and Prof. Michael D. Threadgill (University of Bath) for providing access to the MTS assays. We are grateful to the University of Bath for providing financial support and studentships for AC and NJG. We also wish to acknowledge RCUK and the University of Bath for the fellowship to LC. LC is a member of Cancer Research at Bath (CR@B).Notes and references
- W. H. Fenical, P. R. Jensen and T. Lindel, 1995, US Patent 5473057.
- T. Lindel, P. R. Jensen, W. Fenical, B. H. Long, A. M. Casazza, J. Carboni and C. R. Fairchild, J. Am. Chem. Soc., 1997, 119, 8744 CrossRef CAS.
- M. D'Ambrosio, A. Guerriero and F. Pietra, Helv. Chim. Acta, 1987, 70, 2019 CrossRef CAS.
- M. D'Ambrosio, A. Guerriero and F. Pietra, Helv. Chim. Acta, 1988, 71, 964 CrossRef CAS.
- M. Ciomei, C. Albanese, W. Pastori, M. Grandi, F. Pietra, M. D'Ambrosio, A. Guerriero and C. Battistini, Proc. Amer. Ass. Can. Res., 1997, 38, 5 Search PubMed.
- K.-H. Altmann and J. Gertsch, Nat. Prod. Rep., 2007, 24, 327 RSC.
- Y. Zhao, W.-S. Fang and K. Pors, Expert Opin. Ther. Pat., 2009, 19, 607 Search PubMed.
- K. C. Nicolaou, J. Pfefferkorn, J. Xu, N. Winssinger, T. Ohshima, S. Kim, S. Hosokawa, D. Vourloumis, F. van Delft and T. Li, Chem. Pharm. Bull., 1999, 47, 1199 CAS.
- B. H. Long, J. M. Carboni, A. J. Wasserman, L. A. Cornell, A. M. Casazza, P. R. Jensen, T. Lindel, W. Fenical and C. R. Fairchild, Cancer Res., 1998, 58, 1111 CAS.
- E. Hamel, D. L. Sackett, D. Vourloumis and K. C. Nicolaou, Biochemistry, 1999, 38, 5490 CrossRef CAS.
- K. C. Nicolaou, F. van Delft, T. Ohshima, D. Vourloumis, J. Xu, S. Hosokawa, J. Pfefferkorn, S. Kim and T. Li, Angew. Chem., Int. Ed. Engl., 1997, 36, 2520 CrossRef CAS.
- K. C. Nicolaou, T. Ohshima, S. Hosokawa, F. L. van Delft, D. Vourloumis, J. Y. Xu, J. Pfefferkorn and S. Kim, J. Am. Chem. Soc., 1998, 120, 8674 CrossRef CAS.
- X.-T. Chen, C. E. Gutteridge, S. K. Bhattacharya, B. Zhou, T. R. R. Pettus, T. Hascall and S. J. Danishefsky, Angew. Chem., Int. Ed., 1998, 37, 185 CrossRef CAS.
- X.-T. Chen, B. Zhou, S. K. Bhattacharya, C. E. Gutteridge, T. R. R. Pettus and S. J. Danishefsky, Angew. Chem., Int. Ed., 1998, 37, 789 CrossRef CAS.
- K. C. Nicolaou, J. Y. Xu, S. Kim, T. Ohshima, S. Hosokawa and J. Pfefferkorn, J. Am. Chem. Soc., 1997, 119, 11353 CrossRef CAS.
- K. C. Nicolaou, J. Y. Xu, S. Kim, J. Pfefferkorn, T. Ohshima, D. Vourloumis and S. Hosokawa, J. Am. Chem. Soc., 1998, 120, 8661 CrossRef CAS.
- K. C. Nicolaou, S. Kim, J. Pfefferkorn, J. Xu, T. Ohshima, S. Hosokawa, D. Vourloumis and T. Li, Angew. Chem., Int. Ed., 1998, 37, 1418 CrossRef CAS.
- K. C. Nicolaou, N. Winssinger, D. Vourloumis, T. Ohshima, S. Kim, J. Pfefferkorn, J. Y. Xu and T. Li, J. Am. Chem. Soc., 1998, 120, 10814 CrossRef CAS.
- J. Telser, R. Beumer, A. A. Bell, S. M. Ceccarelli, D. Monti and C. Gennari, Tetrahedron Lett., 2001, 42, 9187 CrossRef CAS.
- R. Beumer, P. Bayón, P. Bugada, S. Ducki, N. Mongelli, F. R. Sirtori, J. Telser and C. Gennari, Tetrahedron Lett., 2003, 44, 681 CrossRef CAS.
- R. Beumer, P. Bayón, P. Bugada, S. Ducki, N. Mongelli, F. R. Sirtori, J. Telser and C. Gennari, Tetrahedron, 2003, 59, 8803 CrossRef CAS.
- L. Caggiano, D. Castoldi, R. Beumer, P. Bayón, J. Tesler and C. Gennari, Tetrahedron Lett., 2003, 44, 7913 CrossRef CAS.
- D. Castoldi, L. Caggiano, P. Bayón, A. M. Costa, P. Cappella, O. Sharon and C. Gennari, Tetrahedron, 2005, 61, 2123 CrossRef CAS.
- D. Castoldi, L. Caggiano, L. Panigada, O. Sharon, A. M. Costa and C. Gennari, Angew. Chem., Int. Ed., 2005, 44, 588 CrossRef CAS.
- D. Castoldi, L. Caggiano, L. Panigada, O. Sharon, A. M. Costa and C. Gennari, Chem.–Eur. J., 2006, 12, 51 CrossRef CAS.
- (a) G. C. H. Chiang, A. D. Bond, A. Ayscough, G. Pain, S. Ducki and A. B. Holmes, Chem. Commun., 2005, 1860 RSC; (b) S. Y. Frankie Mak, G. C. H. Chiang, J. E. P. Davidson, J. E. Davies, A. Ayscough, G. Pain, J. W. Burton and A. B. Holmes, Tetrahedron: Asymmetry, 2009, 20, 921 CrossRef CAS.
- D. I. Batovska and I. T. Todorova, Curr. Clin. Pharmacol., 2010, 5, 1 Search PubMed.
- C. W. G. Fishwick, R. J. Foster and R. E. Carr, Tetrahedron Lett., 1996, 37, 3915 CrossRef CAS.
- R. W. Hartmann, M. Frotscher, D. Ledergerber, G. A. Wächter, G. L. Grün and T. F. Sergejew, Arch. Pharm., 1996, 329, 251 CrossRef CAS.
- G. A. Wächter, R. W. Hartmann, T. Sergejew, G. L. Grün and D. Ledergerber, J. Med. Chem., 1996, 39, 834 CrossRef CAS.
- R. W. Hartmann, B. Wachall, M. Yoshihama, M. Nakakoshi, S. Nomoto and Y. Ikeda, 2003, US 6559157.
- M. Recanatini, A. Cavalli and P. Valenti, Med. Res. Rev., 2002, 22, 282 CrossRef CAS.
- S. Grubjesic, R. M. Moriarty and J. M. Pezzuto, Expert Opin. Ther. Pat., 2002, 12, 1647 Search PubMed.
- D. W. Gil and J. E. Donello, 2003, WO 03/099289.
- K. Chow, T. Heidelbaugh, D. Gil, M. Garst, L. A. Wheeler, P. X. Nguyen and D. G. Gomez, 2003, WO 03/099795.
- L. M. Ni, C. Q. Meng and J. A. Sikorski, Expert Opin. Ther. Pat., 2004, 14, 1669 Search PubMed.
- Y. K. Rao, S.-H. Fang and Y.-M. Tzeng, Bioorg. Med. Chem., 2009, 17, 7909 CrossRef.
- S. Ducki, D. Rennison, M. Woo, A. Kendall, J. F. D. Chabert, A. T. McGown and N. J. Lawrence, Bioorg. Med. Chem., 2009, 17, 7698 CrossRef CAS.
- D. Kumar, N. M. Kumar, K. Akamatsu, E. Kusaka, H. Harada and T. Ito, Bioorg. Med. Chem. Lett., 2010, 20, 3916 CrossRef CAS.
- A. L. Risinger, F. J. Giles and S. L. Mooberry, Cancer Treat. Rev., 2009, 35, 255 CrossRef CAS.
- G. C. Tron, T. Pirali, G. Sorba, F. Pagliai, S. Busacca and A. A. Genazzani, J. Med. Chem., 2006, 49, 3033 CrossRef CAS.
- D. L. Sackett, Pharmacol. Ther., 1993, 59, 163 CrossRef CAS.
- V. Srivastava, A. S. Negi, J. K. Kumar, M. M. Gupta and S. P. S. Khanuja, Bioorg. Med. Chem., 2005, 13, 5892 CrossRef CAS.
- D. W. Siemann, D. J. Chaplin and P. A. Walicke, Expert Opin. Invest. Drugs, 2009, 18, 189 CrossRef CAS.
- J. Bourdron, L. Commeiras, P. Barbier, V. Bourgarel-Rey, E. Pasquier, N. Vanthuyne, J. C. Hubaud, V. Peyrot and J. L. Parrain, Bioorg. Med. Chem., 2006, 14, 5540 CrossRef CAS.
- M. P. Leese, F. Jourdan, M. R. Kimberley, G. E. Cozier, N. Thiyagarajan, C. Stengel, S. Regis-Lydi, P. A. Foster, S. P. Newman, K. R. Acharya, E. Ferrandis, A. Purohit, M. J. Reed and B. V. L. Potter, Chem. Commun., 2010, 46, 2907 RSC.
- E. C. Breen and J. J. Walsh, Curr. Med. Chem., 2010, 17, 609 CrossRef CAS.
- Z. Wang, E. M. Bennett, D. J. Wilson, C. Salomon and R. Vince, J. Med. Chem., 2007, 50, 3416 CrossRef CAS.
- H. S. Bodiwala, S. Sabde, P. Gupta, R. Mukherjee, R. Kumar, P. Garg, K. K. Bhutani, D. Mitra and I. P. Singh, Bioorg. Med. Chem., 2011, 19, 1256 CrossRef CAS.
- S. Bhagat, R. Sharma, D. M. Sawant, L. Sharma and A. K. Chakraborti, J. Mol. Catal. A: Chem., 2006, 244, 20 CrossRef CAS.
- N. M. Rateb and H. F. Zohdi, Synth. Commun., 2009, 39, 2789 CrossRef CAS.
- T. Narender and K. Papi Reddy, Tetrahedron Lett., 2007, 48, 3177 CrossRef CAS.
- N. Lauth-de Viguerie, N. Sergueeva, M. Damiot, H. Mawlawi, M. Riviere and A. Lattes, Heterocycles, 1994, 37, 1561.
- MTS is 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium. A. H. Cory, T. C. Owen, J. A. Barltrop and J. G. Cory, Cancer Commun., 1991, 3, 207 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterisation data, 1H NMR and 13C NMR spectra, HPLC traces and MTS assay results are provided for all compounds. See DOI: 10.1039/c1md00155h |
| This journal is © The Royal Society of Chemistry 2011 |