A new route to indolesvia in situdesilylation–Sonogashira strategy: identification of novel small molecules as potential anti-tuberculosis agents†
Ali
Nakhi
a,
Bagineni
Prasad
a,
Uppender
Reddy
a,
Raja Mohan
Rao
a,
Sandhya
Sandra
a,
Ravikumar
Kapavarapu
a,
D.
Rambabu
a,
G.
Rama Krishna
b,
C. Malla
Reddy
b,
Kishore
Ravada
c,
Parimal
Misra
a,
Javed
Iqbal
*a and
Manojit
Pal
*a
aInstitute of Life Sciences, University of Hyderabad Campus, Gachibowli, Hyderabad, 500 046, India. E-mail: manojitpal@rediffmail.com
bDepartment of Chemical Sciences, Indian Institute of Science Education and Research, Kolkata, West Bengal 741252, India
cSchool of Chemistry, University of Hyderabad, Gachibowli, Hyderabad, 500 046, India
First published on 26th August 2011
Abstract
A new Pd/C-mediated tandem reaction has been developed for the one pot synthesis of indoles containing an o-(RSO2NH)C6H4 group at the C-2 position. The methodology provided novel indoles as inhibitors of Mycobacterium tuberculosis H37Rv chorismate mutasein vitro representing the first example of chorismate mutase inhibition by a heteroarene based small molecule.
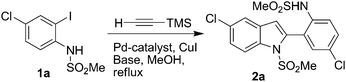
The indole ring is considered as one of the privileged structures in the area of drug discovery. Indole derivatives display a range of valuable pharmacological properties.1 For example, indole (2, Scheme 1) containing an o-(MeSO2NH)C6H4 group at C-2 has been reported to be useful for the potential treatment of multiple disorders.2 While a number of methods are available for the preparation of 2-aryl indoles,3 a direct and general method for the construction of the key indole moiety of 2 has not been reported yet. The existing method for 2 requires a multi-step process.2 Recently, because of their environmental and economic advantages, one-pot multi-step syntheses which do not require the typical purification and isolation of products in each step have gained considerable interest. Thus, a one-pot, four-step synthesis of 2-substituted indoles has been reported.4,5 More recently, we have observed that coupling of o-iodoanilide with trimethylsilylacetylene (TMSA) directly provides the indole derivatives unexpectedly without the requirement of any additional catalyst or reagent for the removal of a Me3Si– group or a cyclization step, respectively. This resulted in the development of the first Pd/C-mediated one-pot synthesis of bioactive indoles possessing an o-(RSO2NH)C6H4 group at C-2 (2, Scheme 1) via in situdesilylation–Sonogashira strategy. While a similar type of strategy i.e. the Sila-Sonogashira reaction6 found applications in the preparation of internal alkynes, its use as a key step in heterocyclic synthesis remained unexplored.
 | ||
| Scheme 1 Pd/C-mediated one-pot synthesis of indoles containing an o-(RSO2)C6H4 group at C-2. | ||
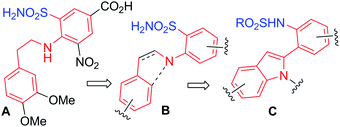
Tuberculosis (TB), though curable, remains a deadly disease that kills more than two million people a year worldwide. The growing incidences of multi-drug-resistance TB and its synergism with HIV is an emerging threat which requires immediate attention. Thus characterization of new enzyme targets and the identification of new drugs are highly desirable. The shikimate pathway for the biosynthesis of aromatic amino acids such as phenylalanine and tyrosine involves the Claisen rearrangement of chorismate to prephenate in the presence of chorismate mutase or CM (EC 5.4.99.5). Due to the absence of this pathway in animals but not in bacteria CM is considered as a novel target for the identification of effective antibacterial agents.7 However, to our knowledge only a few small molecules8 have been reported to possess inhibitory activity against CM including a sulfonamide derivative A (Fig. 1).8a In this communication we wish to present our preliminary work on the discovery of novel small molecules as inhibitors of CM synthesis of which was carried out via a new tandem reaction. The key role played by the sulfonamide moiety of A during its interaction with the active site8a of CM prompted us to design the indole 2 containing the o-(RSO2NH)C6H4 group at C-2 (C) via the structure B (Fig. 1).
 | ||
| Fig. 1 Design of novel inhibitors of chorismate mutase. | ||
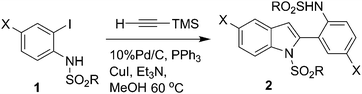
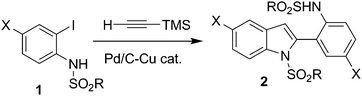
Based on the earlier report that the trimethylsilyl group participates in Pd-mediated C–C bond forming reaction,6 we examined the reaction of TMSA with N-(4-chloro-2-iodophenyl)methanesulfonamide (1a) under Pd/C–Cu catalysis. While the formation of a 1,2-diarylethyne via a Sila-Sonogashira type reaction was expected, the isolated product however was identified as an indole derivative 2a. To this end, we conducted the reaction of 1a with TMSA under various conditions to establish the optimized conditions (Table 1).
|
|
||||
|---|---|---|---|---|
| Entry | Pd-catalysts | Base | Timeb/h | Yieldc (%) |
| a All the reactions were carried out using iodide 1a (1.6835 mmol), TMSA (6.734 mmol), 10% Pd/C (0.0168 mmol), PPh3 (0.0673 mmol), CuI (0.1685 mmol), and Et3N (4.0287 mmol) in MeOH (5.0 mL), at 60 °C. b After adding 2a. c Isolated yield. d The reaction was carried out without the Pd-catalyst. e The reaction was carried out without CuI. | ||||
| 1 | 10%Pd/C–PPh3 | Et3N | 4 | 39 |
| 2 | 10%Pd/C–PPh3 | Et3N | 6 | 62 |
| 3 | 10%Pd/C | Et3N | 6 | 15 |
| 4 | PPh3d | Et3N | 6 | 33 |
| 5 | 10%Pd/C–PPh3e | Et3N | 8 | 10 |
| 6 | Pd(PPh3)2Cl2e | Et3N | 6 | 18 |
| 7 | Pd(PPh3)2Cl2e | Piperidine | 6 | 55 |
We performed the coupling of 1a with TMSA using a Pd catalyst, CuI and a base in methanol at 60 °C. Due to our long term interest in the Pd/C-mediated alkynylation reaction9 we conducted the present reaction using 10% Pd/C–PPh3 as the catalyst system. The reaction afforded 2a in low yield after 4 h (entry 1, Table 1). However, a longer reaction time i.e. 6 h improved the product yield significantly (entry 2, Table 1). The omission of any component of the catalyst 10% Pd/C–PPh3–CuI decreased the product yield significantly (entries 3, 4 and 5, Table 1). Notably, the use of Pd(PPh3)2Cl2 was found to be effective even in the absence of CuI when piperidine was used in place of Et3N (entry 6 vs. 7, Table 1). To assess the generality of this reaction we treated TMSA with other iodoarenes (Scheme 1 and Table 2). The present single pot tandem reaction proceeded well affording a variety of indoles. Both electron donating groups such as Cl (1a, 1l), F (1b, 1k), Me (1d, 1m) and OMe (1f) and electron withdrawing groupse.g.CF3 (1e, 1n), COCH3 (1g), CN (1h) and NO2 (1i) were tolerated. All the indole derivatives (2) synthesized were characterized by spectral data and this was supported by the molecular structure of a representative compound 2e being confirmed by X-ray analysis (Fig. 2).10
|
|
||||
|---|---|---|---|---|
| Entry | o-Iodoanilides (1) X; R = | Indoles (2) | Time/h | Yieldb (%) |
| a All the reactions were carried out using o-iodoanilide 1 (1.6835 mmol), TMSA (6.734 mmol), 10% Pd/C (0.0168 mmol), PPh3 (0.0673 mmol), CuI (0.1685 mmol), and Et3N (4.0287 mmol) in MeOH (5.0 mL), at 60 °C. b Isolated yield. | ||||
| 1 | Cl; Me | 2a | 6 | 62 |
| 1a | ||||
| 2 | F; Me | 2b | 5 | 65 |
| 1b | ||||
| 3 | H; Me | 2c | 8 | 60 |
| 1c | ||||
| 4 | Me; Me | 2d | 6 | 63 |
| 1d | ||||
| 5 | CF3; Me | 2e | 7 | 60 |
| 1e | ||||
| 6 | CH3O; Me | 2f | 6 | 58 |
| 1f | ||||
| 7 | CH3CO; Me | 2g | 9 | 56 |
| 1g | ||||
| 8 | CN; Me | 2h | 9 | 64 |
| 1h | ||||
| 9 | NO2; Me | 2i | 8 | 55 |
| 1i | ||||
| 10 | H; C6H4Me-p | 2j | 4 | 64 |
| 1j | ||||
| 11 | F; C6H4Me-p | 2k | 6 | 58 |
| 1k | ||||
| 12 | Cl; C6H4Me-p | 2l | 4 | 60 |
| 1l | ||||
| 13 | CH3; C6H4Me-p | 2m | 7 | 63 |
| 1m | ||||
| 14 | CF3; C6H4Me-p | 2n | 5 | 62 |
| 1n | ||||
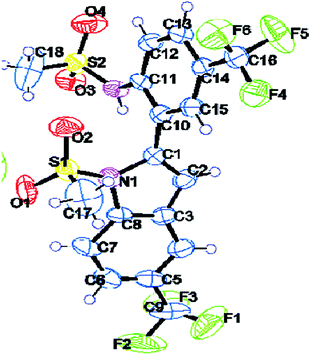 | ||
| Fig. 2 X-Ray crystal structure of 2e (ORTEP diagram). Thermal ellipsoids are drawn at 50% probability level. | ||
Mechanistically, the present reaction seems to proceed viaPd/C–Cu mediated11Sonogashira coupling followed by in situdesilylation–Sonogashira coupling and subsequent cyclization step in a single pot (Scheme 2). Thus, the alkyne E-1 generated in situ viaPd(0) mediated stepwise formation of a C–C bond between 1 and TMS–acetylene undergoes the cleavage of C–Si bond facilitated by CuI to generate the corresponding Cu-acetylide E-212 which on subsequent coupling with 1 provides the internal alkyne E-3. The Cu-mediated ring closure of E-3 in an intramolecular fashion provides the desired indole (2). Notably, the o-sulfonamide moiety facilitated the CuI-mediated desilylation of E-1 leading to the generation of Cu-acetylide (E-2) in the present case whereas a separate desilylation step in the presence of a suitable reagent was required when this substituent was absent at the o-position in the aryl iodides employed.4,5 In other words 1,2-diphenylethyne was not isolated when iodobenzene was reacted with TMSA under Pd/C–Cu catalysis in the presence of Et3N in MeOH at refluxing temperature.5 Nonetheless, to gain further evidence on the intermediacy of E-1, the reaction of 1c with TMSA was carried out at 25 °C for 3 h under the condition of Entry 2 of Table 1 when the uncyclized product i.e. N-[2-{(trimethylsilyl)ethynyl} phenyl]methanesulfonamide (E) was isolated (Scheme 3). The alkyne E when reacted with 1c in the presence of 10% Pd/C, PPh3, CuI, and Et3N in MeOH at 60 °C for 4 h provided 2c confirming the intermediacy of E-1 in the present reaction.
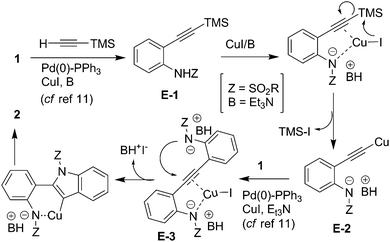 | ||
| Scheme 2 Proposed mechanism for the synthesis of indoles (2) via in situdesilylation–Sonogashira strategy. | ||
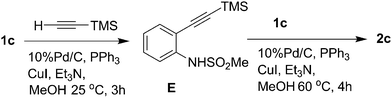 | ||
| Scheme 3 Synthesis of indole 2cvia the isolation of the intermediate E. | ||
In order to demonstrate the further scope of this methodology,11N-mesyl indoles 2d and 2e were converted to indoles 3a and 3b in good yields (Scheme 4). Compound 2d was also converted to an oxime derivative 5viaTFAA–H3PO4 mediated13benzoylation to give N-(2-(6-benzoyl-5-methyl-1-(methylsulfonyl)-1H-indol-2-yl)-4-methylphenyl)methane sulfonamide 4 as the major product which was then reacted with hydroxyl amine (Scheme 5). The molecular structure of 4 was confirmed by spectral data followed by X-ray analysis (Fig. 3).14
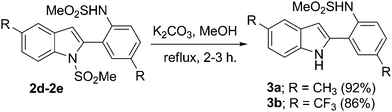 | ||
| Scheme 4 N-Demesylation of the indole ring. | ||
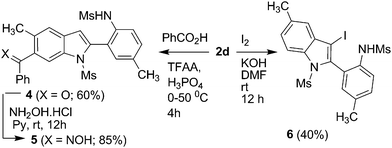 | ||
| Scheme 5 Structural elaboration of 2d (Ms = mesyl). | ||
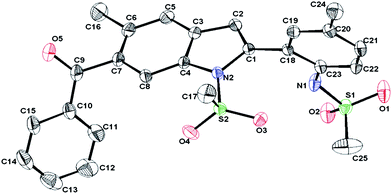 | ||
| Fig. 3 X-Ray crystal structure of 4 (ORTEP diagram). Thermal ellipsoids are drawn at 50% probability level. | ||
A regioisomer of 4i.e.(N-(2-(4-benzoyl-5-methylsulfonyl)-1H-indol-2-yl)-4-methylphenyl)methanesulfonamide was also isolated during the acylation of 2d (see ESI†). Iodination of 2d provided 3-iodoindole derivative 6 (Scheme 5) for further functionalization via transition metal catalyzed reactions.15
Due to our interest in bioactive molecules16 all the compounds synthesized were tested for CM inhibiting properties in vitro and the results are summarized in Table 3. In general, N-methylsulfonyl derivatives were found to be superior to N-tosyl indoles whereas the presence of an electron donating groupe.g. F or MeO was beneficial in terms of CM inhibition. Compounds 2a, 2b, 2c, 2d, and 2f showed significant inhibition of CM at 50 μM whereas the compound 2b showed dose dependent inhibition with an IC50 value 17.02 μM (Fig. 4).17 This was supported by the docking results of 2b with CM protein (binding energy −6.23 kcal mol−1) which showed H-bonding interactions between the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of 2b and the NH moiety of LYS60, ARG134, GLN76, ARG72 residues of CM (Fig. 5). This interaction was further strengthened by the additional H-bonding between the NH of 2b and C
O group of 2b and the NH moiety of LYS60, ARG134, GLN76, ARG72 residues of CM (Fig. 5). This interaction was further strengthened by the additional H-bonding between the NH of 2b and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of GLN76 residue of CM. Thus a new chemical class based on indoles has been identified that possesses inhibitory activity against CM. Being the first example of CM inhibition by a heteroarene based small molecule, indole 2b represents the lead structure for the development of potential agents for the treatment of tuberculosis.
O of GLN76 residue of CM. Thus a new chemical class based on indoles has been identified that possesses inhibitory activity against CM. Being the first example of CM inhibition by a heteroarene based small molecule, indole 2b represents the lead structure for the development of potential agents for the treatment of tuberculosis.
|
|
||
|---|---|---|
| Entry | Indoles (2) X; R = | % Inhibitiona @ 50 μM |
| a Average of three experiments. | ||
| 1 | Cl; Me (2a) | 45 |
| 2 | F; Me (2b) | 69 |
| 3 | H; Me (2c) | 40 |
| 4 | Me; Me (2d) | 42 |
| 5 | CF3; Me (2e) | 25 |
| 6 | CH3O; Me (2f) | 50 |
| 7 | CH3CO; Me (2g) | 30 |
| 8 | CN; Me (2h) | 20 |
| 9 | NO2; Me (2i) | 15 |
| 10 | H; C6H4Me-p (2j) | 12 |
| 11 | F; C6H4Me-p (2k) | 15 |
| 12 | Cl; C6H4Me-p (2l) | 10 |
| 13 | Me; C6H4Me-p (2m) | 15 |
| 14 | CF3; C6H4Me-p (2n) | 12 |
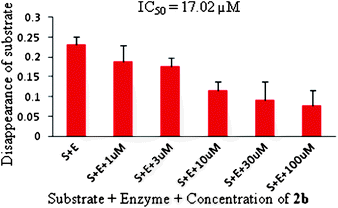 | ||
| Fig. 4 Dose dependent chorismate mutase inhibition and IC50 value of indole 2b. | ||
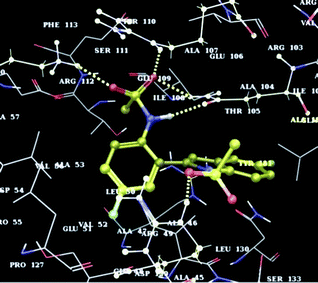 | ||
| Fig. 5 Docking of indole 2b at the active site of CM. | ||
In conclusion, a new and general synthesis of novel indole derivatives has been accomplished via a tandem coupling–cyclization strategy. This research has led to the identification of a small-molecule based inhibitor of CM for the potential treatment of tuberculosis.
The authors thank Professor Seyed E. Hasnain for encouragement and DBT, New Delhi, India for financial support (Grant NO BT/01/COE/07/02). B. Prasad thanks CSIR, New Delhi, India for a Junior Research Fellowship.
Notes and references
- For recent reviews, see: (a) G. R. Humphrey and J. T. Kuethe, Chem. Rev., 2006, 106, 2875 CrossRef CAS; (b) S. Cacchi and G. Fabrizi, Chem. Rev., 2005, 105, 2873 CrossRef CAS; (c) G. W. Gribble, in Comprehensive Heterocyclic Chemistry II, ed. A. R. Katrizky, C. W. Rees and E. F. V. Scriven, Pergamon, Oxford, 1996, vol. 2, p. 20 Search PubMed.
- A. V. Purandare, H. Wan and T. N. Huynh, US2006/235037, 2006, A1.
- For selected references, see: (a) S. Fukuoka, T. Naito, H. Sekiguchi, T. Somete and A. Mori, Heterocycles, 2008, 78, 819 Search PubMed; (b) O. Leogane and H. Lebel, Angew. Chem., Int. Ed., 2008, 47, 350 CrossRef CAS; (c) M. McLaughlin, M. Palucki and I. W. Davies, Org. Lett., 2006, 8, 3307 CrossRef CAS; (d) S. S. Palimkar, P. H. Kumar, R. J. Lahoti and K. V. Srinivasan, Tetrahedron, 2006, 62, 5109 CrossRef CAS; (e) M. Pal, V. Subramanian, V. R. Batchu and I. Dager, Synlett, 2004, 11, 1965 Search PubMed; (f) M. Layek, U. Lakshmi, D. Kalita, D. K. Barange, A. Islam, K. Mukkanti and M. Pal, Beilstein J. Org. Chem., 2009, 5(46) DOI:10.3762/bjoc.5.46 , for fused indole derivatives, see; (g) M. Layek, M. A. Reddy, A. V. D. Rao, M. Alvala, M. K. Arunasree, A. Islam, K. Mukkanti, J. Iqbal and M. Pal, Org. Biomol. Chem., 2011, 9, 1004 RSC; (h) M. Layek, A. V. D. Rao, V. Gajare, D. Kalita, D. K. Barange, A. Islam, K. Mukkanti and M. Pal, Tetrahedron Lett., 2009, 50, 4878 CrossRef CAS; (i) M. Layek, V. Gajare, D. Kalita, A. Islam, K. Mukkanti and M. Pal, Tetrahedron Lett., 2009, 50, 3867 CrossRef CAS.
- H. Sakai, K. Tsutsumi, T. Morimoto and K. Kakiuchi, Adv. Synth. Catal., 2008, 350, 2498 CrossRef CAS.
- R. M. Rao, U. Reddy CH, A. Nakhi, N. Mulakayala, M. Alvala, M. K. Arunasree, R. R. Poondra, J. Iqbal and M. Pal, Org. Biomol. Chem., 2011, 9, 3808 RSC.
- For selected references, see: (a) Y. Hatanaka, K. Matsui and T. Hiyama, Tetrahedron Lett., 1989, 30, 2403 CrossRef CAS; (b) S. Chang, S. H. Yang and P. H. Lee, Tetrahedron Lett., 2001, 42, 4833 CrossRef CAS; (c) G. Li, X. Wang and F. Wang, Tetrahedron Lett., 2005, 46, 8971 CrossRef CAS; (d) U. S. Sorensen and E. Pombo-Villar, Tetrahedron, 2005, 61, 2697 CrossRef CAS; (e) J. Gil-Molto and C. Najera, Adv. Synth. Catal., 2006, 348, 1874 CrossRef CAS; (f) Y. Nishihara, E. Inoue, D. Ogawa, D. Okada, S. Noyori and K. Takagi, Tetrahedron Lett., 2009, 50, 4643 CrossRef CAS.
- E. Haslam, Shikimic Acid: Metabolism and Metabolites, Wiley, New York, 1993 Search PubMed.
- For selected references, see: (a) H. Agarwal, A. Kumar, N. C. Bal, M. I. Siddiqi and A. Arora, Bioorg. Med. Chem. Lett., 2007, 17, 3053 Search PubMed; (b) M. E. Hediger, Bioorg. Med. Chem., 2004, 12, 4995 Search PubMed; (c) P. A. Bartlett, Y. Nakagawa, C. R. Johnson, S. H. Reich and A. Luis, J. Org. Chem., 1988, 53, 3195 CrossRef CAS.
- M. Pal, Synlett, 2009, 2896 CrossRef CAS.
-
Crystal data of 2e: Molecular formula = C18H14F6N2O4S2, formula weight = 500.45, crystal system = monoclinic, space group = Pn, a = 9.922 (5) Å, b = 14.243 (7) Å, c = 14.847 (7) Å, V = 2092.7 (18) Å3, T = 296 K, Z = 4, Dc = 1.550 Mg m−3, μ(Mo-Kα) = 0.71073 mm−1, 10
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 680 reflections measured, 5285 independent reflections, 3766 observed reflections [I > 2.0σ(I)], R1_obs = 0.072, goodness of fit =1.357. Crystallographic data (excluding structure factors) for 2e have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication number CCDC 818602.
680 reflections measured, 5285 independent reflections, 3766 observed reflections [I > 2.0σ(I)], R1_obs = 0.072, goodness of fit =1.357. Crystallographic data (excluding structure factors) for 2e have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication number CCDC 818602. - (a) D. R. Gorja, K. S. Kumar, K. Mukkanti and M. Pal, Beilstein J. Org. Chem., 2011, 7, 338 Search PubMed; (b) M. Layek, Y. S. Kumar, A. Islam, R. Karavarapu, A. Sengupta, D. Halder, K. Mukkanti and M. Pal, Med. Chem. Commun., 2011, 2, 478 RSC.
- (a) Y. Nishihara, K. Ikegashira, K. Hirabayashi, J. Ando, A. Mori and T. Hiyama, J. Org. Chem., 2000, 65, 1780 CrossRef CAS; (b) Y. Nishihara, M. Takemura, A. Mori and K. Osakada, J. Organomet. Chem., 2001, 620, 282 Search PubMed.
- (a) V. R. Veeramaneni, M. Pal and K. R. Yeleswarapu, Tetrahedron, 2003, 5(9), 3283 Search PubMed; (b) S. Pal, P. Bindu, P. R. Vinna and P. K. Dubey, Lett. Org. Chem., 2007, 4, 292 Search PubMed; (c) S. Pal, M. A. Khan, P. Bindu and P. K. Dubey, Beilstein J. Org. Chem., 2007, 3, 35, DOI:10.1186/1860-5397-3-35; (d) K. Kankanala, L. V. Reddy, V. R. Reddy, K. Mukkanti and S. Pal, Lett. Org. Chem., 2011, 8, 53 Search PubMed.
-
Crystal data of 4: molecular formula = C25H24N2O5S2, formula weight = 496.58, crystal system = triclinic, space group = P
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 8.271 (4) Å, b = 12.010 (6) Å, c = 13.310 (6) Å, V = 1237.00 (10) Å3, T = 298 K, Z = 2, Dc = 1.333 Mg m−3, μ(Mo-Kα) = 0.71073 mm−1, 8168 reflections were measured with 4219 unique reflections (Rint = 0.0199), of which 4219 (I > 2σ(I)) were used for the structure solution. Final R1(wR2) = 0.0429 (0.1053), 311 parameters. The final Fourier difference synthesis showed minimum and maximum peaks of −0.273 and +0.246 e Å−3 respectively. Goodness of fit = 1.061. Crystallographic data (excluding structure factors) for 4 have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication number CCDC 818615.
, a = 8.271 (4) Å, b = 12.010 (6) Å, c = 13.310 (6) Å, V = 1237.00 (10) Å3, T = 298 K, Z = 2, Dc = 1.333 Mg m−3, μ(Mo-Kα) = 0.71073 mm−1, 8168 reflections were measured with 4219 unique reflections (Rint = 0.0199), of which 4219 (I > 2σ(I)) were used for the structure solution. Final R1(wR2) = 0.0429 (0.1053), 311 parameters. The final Fourier difference synthesis showed minimum and maximum peaks of −0.273 and +0.246 e Å−3 respectively. Goodness of fit = 1.061. Crystallographic data (excluding structure factors) for 4 have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication number CCDC 818615. - For Suzuki and Sonogashira coupling of 2-aryl-3-iodoindoles, see: S. A. Worlikar, B. Neuenswander, G. H. Lushington and R. C. Larock, J. Comb. Chem., 2009, 11, 875 Search PubMed.
- (a) M. Pal, Drug Discovery Today, 2009, 14, 784 CrossRef CAS; (b) M. Pal, Curr. Med. Chem., 2009, 16, 3858 CrossRef CAS; (c) S. H. Havale and M. Pal, Bioorg. Med. Chem., 2009, 17, 1783 CrossRef CAS; (d) R. Gupta, S. S. Walunj, R. K. Tokala, K. V. L. Parsa, S. K. Singh and M. Pal, Curr. Drug Targets, 2009, 10, 71 Search PubMed; (e) A. Kodimuthali, S. S. L. Jabaris and M. Pal, J. Med. Chem., 2008, 18, 5471 CrossRef; (f) M. Pal, S. Angaru, A. Kodimuthali and N. Dhingra, Curr. Pharm. Des., 2009, 15, 1008 CrossRef CAS; (g) M. Pal and S. Pillarisetti, Curr. Med. Chem.: Cardiovasc. Hematol. Agents, 2007, 5, 55 Search PubMed; (h) M. Pal, Tetrahedron, 2009, 65, 433 Search PubMed; (i) N. Mulakayala, U. Reddy CH, J. Iqbal and M. Pal, Tetrahedron, 2010, 66, 4919 CrossRef CAS; (j) K. V. L. Parsa and M. Pal, Expert Opin. Drug Discovery, 2011, 6, 855 Search PubMed.
- The IC50 value of compound A was found to be less than 10 μM in the same assay.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, spectral data for all new compounds, and results of docking study. CCDC reference numbers 818602 (2e) and 818615 (4). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c1md00148e |
| This journal is © The Royal Society of Chemistry 2011 |