Synthesis and biological evaluation of a new class of anti-brucella compounds targeting histidinol dehydrogenase: α-O-arylketones and α-S-arylketones derived from histidine
François
Turtaut
a,
Safia
Ouahrani-Bettache
b,
Jean-Louis
Montero
a,
Stephan
Köhler
*b and
Jean-Yves
Winum
*a
aInstitut des Biomolécules Max Mousseron (IBMM), UMR 5247 CNRS-UM1-UM2, Bâtiment de Recherche Max Mousseron, Ecole Nationale Supérieure de Chimie de Montpellier, 8 rue de l′Ecole Normale, 34296, Montpellier, Cedex, France. E-mail: jean-yves.winum@univ-montp2.fr; Fax: +33 [0] 4 67 14 43 44; Tel: (+33) [0] 4 67 14 72 34
bCentre d'Etudes d'Agents Pathogènes et Biotechnologies pour la Santé (CPBS), UMR5236 CNRS-UM1-UM2, 1919 route de Mende, F-34293, Montpellier Cedex 5, France. E-mail: stephan.kohler@cpbs.cnrs.fr; Fax: +33 [0]4 34 35 94 11; Tel: +33 [0]4 34 35 94 05
First published on 25th August 2011
Abstract
Bacterial infections are commonly treated with antibiotic chemotherapy. The biological targets of these antibiotics are at the origin of appearance of resistant bacterial strains. Recently, the development of new approaches, such as the antivirulence strategy leads the medicinal chemist to describe novel targets with a reduced probability of resistance development. Virulence factors, such as enzymes involved in amino acids biosynthesis, have been validated as such targets. In this paper, we describe the synthesis of a new class of histidinol dehydrogenase inhibitors: α-O-arylketones and α-S-aryl ketones derived from histidine.
Brucellosis,1 most commonly known as Malta fever or undulant fever, is the most widespread bacterial zoonosis worldwide. Its causative agent, Brucella spp., is a facultative intracellular pathogen developing inside the host's macrophages, and pathogenesis is linked to this intramacrophagic replication. Due to its intracellular localization, eradication of Brucella spp. with standard chemotherapy strategies such as antibiotic treatment is delicate.2 Moreover, clinical isolates show that drug-resistant Brucella strains are developing. The absence of a vaccine for humans3 and the appearing resistance of Brucella spp. to antibiotic chemotherapy points to the necessity to develop new therapeutic strategies to eradicate this reemerging pathogen. The virulome analysis of Brucella suis has shown that among others, genes involved in the biosynthesis of amino acids are essential for the virulence of the bacteria.4 This new approach consists in targeting a virulence factor of this pathogen, the histidinol dehydrogenase (HDH, EC. 1.1.23).5–7 This metalloenzyme is involved in the final two steps of the biosynthesis of histidine where it catalyses the NAD dependent oxidation of histidinol to histidineviahistidinaldehyde. Inhibition of its enzymatic activity with specific inhibitors will prevent intramacrophagic multiplication of Brucella. HDH being essential exclusively for the growth of the bacteria inside the macrophage of the host, and having no counterpart in mammalians, it constitutes a therapeutic target for the development of an anti-infectious treatment against intracellular pathogens.
In our previous investigations,7 we showed that α-arylketones derived from histidine (Fig. 1), synthesised by a modified Claisen alkylation-decarboxylation step, were potent inhibitors of histidinol dehydrogenase (HDH, EC 1.1.1.23), with activities in the low nanomolar range on the purified enzyme. These investigations validated a new target-directed therapeutic approach which will prevent Brucella to synthesize some essential amino-acids in the macrophage. This strategy will specifically block the development of the bacteria inside the host cell niche, without affecting the host itself and the commensal flora.
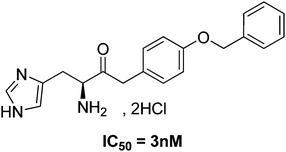 | ||
| Fig. 1 Structure of the leader α-arylketone compound. | ||
In this paper, we extend the methodology of the alkylation-decarboxylation key-step of the synthesis to new substrates: α-O-arylacetic acids and α-S-arylacetic acids. By this extension of methodology, we obtained new inhibitors: α-O-arylketones and α-S-arylketones derived from histidine (Fig. 2). Those newly synthesized compounds were assayed against the purified bacterial enzyme in order to evaluate their inhibitory activity.
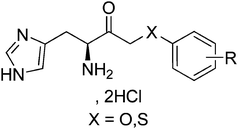 | ||
| Fig. 2 General structure of the α-O-arylketones and α-S-arylketones derived from histidine. | ||
Results and discussion
Chemistry
The protected histidine methyl ester was prepared starting from commercially available L-histidine as previously described by our group7,8 (Fig. 3).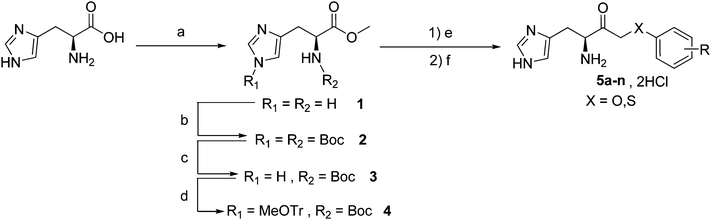 | ||
| Fig. 3 Synthesis of the different inhibitors. Reagents and conditions: a) SOCl2, MeOH, 0 °C to reflux of MeOH; b) Boc2O, NEt3, MeOH, rt; c) K2CO3, MeOH, reflux; d) MeOTrCl, NEt3, DMF, r.t.; e) ArXCH2COOH, LHMDS, THF, −78 °C to r.t.; f) HCl 4N in dioxane. | ||
The histidine methyl ester 1 was obtained by reacting thionyl chloride to a suspension of L-histidine in methanol. The obtained compound was then placed in presence of triethylamine and tert-butyldicarbonate to yield the Boc protected histidine methylester 2 after purification by column chromatography. The selective deprotection of NτBoc group was performed in presence of potassium carbonate with methanol as a solvent. Compound 3 was then placed in presence of methoxytritylchloride and triethylamine to yield compound 4 after purification by column chromatography.
Compounds 5a–n were obtained by extension of the modified Claisen alkylation/decarboxylation step, previously described by our group for the preparation of substituted benzylic ketones derived from histidine.7,8 The existing protocol was thus extended to a new class of substrates: 2-(O-aryl)acetic acids and 2-(S-aryl)acetic acids. As few of them were available commercially, we performed the synthesis of these acetic acid derivatives starting from commercially available tert-butyl bromoacetate and aromatic compounds (Fig. 4).9 The expected compounds 5a–n were obtained by a 5–15 min deprotection step with a 4N solution of HCl in dioxane.7,8 When this reaction is proceeded overnight, formation of compounds 5a and 5h was observed, resulting from the acidic cleavage of respectively tolyl and phenyl moieties.
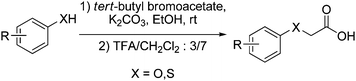 | ||
| Fig. 4 Synthesis of the α-O-arylacetic and α-S-arylacetic acids. | ||
The obtained compounds were analysed by mass spectrometry, NMR and polarimetry in order to confirm their purity and optical activity.
Biological assay
The compounds 5a–n were assessed10 for their inhibitory activity against the purified B. suisHDH enzyme. The following should be noted regarding HDH inhibitory data of Table 1: all compounds investigated here showed weak to strong inhibitory properties. It is worth to point out that inhibitors in α-O-aryl ketone gave better inhibitory results as compared to the compounds in S-arylketone series. Most of these latter inhibitors behaved as weak to moderate inhibitors whereas some very effective inhibitors in O-arylketones series were detected such as 5h, 5j and 5l, with IC50 values ranging from 24.3nM, to 208nM.| Compound | X | Ar | IC50 (μM) |
|---|---|---|---|
| 5a | SH | — | 2.100 ± 0.1659 |
| 5b | S | Ph | 7.100 ± 0.355 |
| 5c | S | Tolyl | 39.460 ± 2.000 |
| 5d | S | 4-F Ph | 3.100 ± 0.2046 |
| 5e | S | 2-Naphtyl | 3.300 ± 0.3267 |
| 5f | S | 4-Br Ph | 2.800 ± 0.,280 |
| 5g | S | 2,3,4,5-F Ph | 19.230 ± 0.960 |
| 5h | OH | — | 0.0243 ± 0.,0014 |
| 5i | O | Ph | 3.650 ± 0.,150 |
| 5j | O | Tolyl | 0.2083 ± 0.0207 |
| 5k | O | 4-F Ph | 4.700 ± 0.,2303 |
| 5l | O | 2-Naphtyl | 0.108 ± 0.0081 |
| 5m | O | 4-Br Ph | 1.600 ± 0.1328 |
| 5n | O | 2,3,4,5-F Ph | 2.780 ± 0.160 |
It can be evidenced from data of Table 1 that the presence and the substitution pattern of the aromatic scaffold is an important parameter. The most effective inhibitor was obtained with compound 5h (IC50 = 24.3nM) which does not have an aromatic moiety, whereas its homolog in S-arylketone series 5a was a much weaker inhibitor (IC50 = 2100 nM). This constitutes a nice example of how a very small structural difference in the molecule of the inhibitors leads to a strong (roughly a 100-fold) decrease of inhibitory power. It is rather difficult to rationalize this behavior without X-ray crystal structures of these adducts with the enzyme.
From the group of inhibitors bearing aromatic scaffold, it can be noted that bulky naphtyl group seems more beneficial than the phenyl group as suggested by the IC50 values between 5b and 5e, for the S-aryl series, or 5i and 5l, for the O-aryl series.
Substitution on the para position on the phenyl ring allows modulation of the activity of the inhibitors. All para-substituted compounds show better activity than the simple phenyl group in O or S-arylketone series. The better halogenpara-substituent was Br (5f, 5m) giving the lower IC50 in both series whereas the pentafluorophenyl group decreases activity leading to low potency HDH inhibitors (5g). It is also important to see that the tolyl moiety appears to be more beneficial than any para-substituted phenyl in the O-aryl series (5j).
Conclusion
We prepared small series of α-O-arylketones and α-S-arylketones derived from histidine and assayed them for the inhibition of HDH. New synthetic methodology was applied for the synthesis of these compounds, extending a modified Claisen alkylation/decarboxylation to new types of substrates. Most of the new compounds showed weak to moderate inhibitory activity whereas α-O-arylketones 5h, 5j and 5l demonstrated interesting nanomolar range activities against HDH and could represent valuable candidates for the potential development of novel non-classical antibacterial agents.Experimental
All chemicals, reagents and solvents for the synthesis of the compounds were of analytical grade, purchased from commercial sources and used without further purification, unless otherwise specified. TLC analyses were performed on silica gel 60 F254 plates (Merck Art.1.05554). Spots were visualized at 254 nm under UV illumination, or by ninhydrin solution spraying. Melting points were determined on a Büchi Melting Point 510 apparatus and are uncorrected. Specific rotations were determined on a Perkin-Elmer 241 polarimeter and are uncorrected. 1H and 13C NMR spectra were recorded on Brücker DRX-400 spectrometer using DMSO-d6 and MeOD-d4 as solvent and tetramethylsilane as internal standard. For 1H NMR spectra, chemical shifts are expressed in δ (ppm) downfield from tetramethylsilane, and coupling constants (J) are expressed in Hertz. Electron Ionization mass spectra (10–30 eV) were recorded in positive or negative mode on a Water MicroMass ZQ.Methyl L-histidinate (1)
To a suspension of L-histidine (5 g; 32.2 mmol) in methanol, thionyl chloride (2.8 mL; 38.6 mmol) was added dropwise at 0 °C. The reaction was then refluxed for 16 h. After completion of the reaction (TLC monitoring (MeOH/CH2Cl2 : 3/7)) the mixture was concentrated to dryness and coevaporated several times with methanol to yield methyl L-histidinate 1 as a white powder (7.76 g; 32.06 mmol; 99.5%); Rf 0.45 (MeOH/CH2Cl2:3/7); mp = 188–190 °C; ESI+MS: m/z 170 (M + H)+, 339 (2M + H)+; ESI−MS: m/z 204 (M + Cl)−; 1H NMR (DMSO-d6): δ = 9.05 (d, J = 1.3, 1H); 7.51 (d, J = 1.22, 1H); 4.47 (t, J = 7.1, 1H); 3.72 (s, 3H); 3.31 (dd, J = 1.2, J = 7.1, 2H).Methyl Nα-Nτ-bis-(tert-butoxycarbonyl)-L-histidinate (2)
Methyl L-histidinate 1 (7.76 g; 32 mmol), and di-tert-butyl-dicarbonate (13.97 g; 64 mmol) were placed in solution in methanol. Triethylamine (7.12 g; 70 mmol) was then added dropwise at 0 °C and the mixture was vigorously stirred at room temperature for 2 h (TLC monitoring: Et2O). The mixture was then diluted with ethyl acetate and washed with water. The organic layer was then dried over anhydrous sodium sulfate, concentrated under reduced pressure and the residue purified on column chromatography (Et2O/petroleum ether : 9/1) to yield the methyl Nα-Nτ-bis-(tert-butoxycarbonyl)-L-histidinate 2 as a white powder (10.17 g; 27 mmol; 86%); Rf 0.39 (Et2O); mp = 105–107 °C; ESI+MS: m/z 370 (M + H)+, 392 (M + Na)+; 1H NMR (DMSO-d6): δ = 8.11 (d, J = 1.2, 1H); 7.25 (d, J = 0.8, 1H); 7.18 (d, J = 8.1, 1H); 4.25 (m, 1H); 3.62 (s, 3H); 2.83 (m, 2H); 1.56 (s, 9H); 1.35 (s, 9H).Methyl Nα-(tert-butoxycarbonyl)-L-histidinate (3)
Methyl Nα-Nτ-bis-(tert-butoxycarbonyl)-L-histidinate 2 (10.17 g; 27 mmol) was dissolved in methanol, and potassium carbonate (0.37 g; 2.7 mmol) was then added. The mixture was then refluxed for 2 h. The reaction was monitored by TLC (Et2O) and after completion the mixture was diluted with ethyl acetate and washed twice with water. The organic layer is then dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to yield methyl Nα-(tert-butoxycarbonyl)-L-histidinate 3 as a white powder (4.27 g; 15.9 mmol, 58.9%); Rf 0.76 (MeOH/CH2Cl2:3/7); mp = 164–166 °C; ESI+MS: m/z 270 (M + H)+, 292 (M + Na)+; 1H NMR (DMSO-d6): δ = 7.54 (d, J = 1.2, 1H); 7.15 (d, J = 7.8, 1H); 6.79 (s, 1H); 4.23 (dt, J = 5.7, J = 7.8, 1H); 3.58 (s, 3H); 2.86 (m, 2H); 1.36 (s, 9H).Methyl Nα-(tert-butoxycarbonyl)- Nτ-[(4-methoxyphenyl) (diphenyl)methyl]-L-histidinate (4)
Mono methoxytrityl chloride (5.404 g; 17.5 mmol) and triethylamine (3.3 mL; 23.8 mmol) were added to a solution of methyl Nα-(tert-butoxycarbonyl)-L-histidinate 3 (4.27 g; 15.9 mmol) in DMF. The reaction mixture was stirred at room temperature for 4 h. After completion (TLC monitoring), the reaction was diluted with ethyl acetate and washed several times with water. The organic layer was then dried over anhydrous sodium sulfate, filtered and concentrated under vacuum. The crude was purified by column chromatography (CH2Cl2 100% to Et2O 100%) to yield methyl Nα-(tert-butoxycarbonyl)-Nτ-[(4-methoxyphenyl)(diphenyl) methyl]-L-histidinate 4 as a white solid (6.88 g; 12.7 mmol; 79.8%); Rf 0.29 (Et2O); [α]20D: −19,6° (MeOH) c = 1.65g L−1; mp= 135–137 °C; ESI+MS: m/z 542 (M + H)+, 564 (M + Na)+; 1H NMR (DMSO-d6): δ = 7.38–6.95 (m, 15H); 6.65 (s, 1H); 4.21 (m, 1H); 3.76 (s, 3H); 3.55 (s, 3H); 2.80 (m, 2H); 1.34 (s, 9H).General procedure for the preparation of α-O-aryl and α-S-arylketones derivatives (5a–n)
The phenoxyacetic acid or (phenylthio)acetic acid derivative (6a–n) was dissolved in THF in a Schlenk tube under argon atmosphere. The solution was then cooled to 0 °C with an ice-bath and, once thermic equilibrium was reached, a solution of bis-(trimethylsilyl)-lithium amide 1M in THF (12mmol) was added dropwise. A rigorous control of stirring is necessary during the addition due to the augmentation of viscosity of the mixture in the tube. Once the addition is over, the reaction mixture is cooled to −78 °C and a solution of methyl Nα-(tert-butyloxycarbonyl)- Nτ-[(4-methoxyphenyl)(diphenyl)methyl]-L-histidinate 4 (2mmol) in dry THF is added to the mixture. The reaction is allowed to warm up to room temperature overnight, then diluted with ethyl acetate and washed with water. The organic layer is dried over anhydrous sodium sulfate, filtered, concentrated in vacuo and the obtained residue was purified on silica-gel column chromatography (petroleum ether/CH2Cl2 : 2/8 to Et2O/Ethyl acetate : 9/1). The resulting oil is dissolved in a 4N solution of HCl in dioxane. The reaction was monitored by TLC (Et2O). Once the starting material was totally consumed (10 min to 16 h), the mixture was filtered and washed with diethyl ether. The desired compound is obtained as a hydrochloride salt.(3S)-3-Amino-1-thio-4-(1H-imidazol-4-yl)butan-2-one dihydro chloride (5a)
This compound is obtained starting from 5b after 16h in HCl 4N, yielding 1.02 mmol (51%) of 5a as a brown oil; [α]20D: −1.7° (MeOH) c = 1.9 g L−1; ESI+MS: m/z 185 (M + H)+; ESI−MS: m/z 220 (M + Cl)−; 1H NMR (MeOD-d4): δ = 8.81 (s, 1H); 7.44 (s, 1H); 4.37 (t, J = 6.9, 1H); 4.21 (q, J = 7.1, 1H); 3.77 (s, 1H); 3.43–3.26 (m, 2H); 3.21 (dt, J = 3.2, 1.6, 2H); 1.18 (t, J = 7.1, 1H); 13C NMR (DMSO-d6): δ = 199.48 (1C); 169.41 (1C); 168.96 (1C); 64.31 (1C); 54.26 (1C); 53.05 (1C); 26.57 (1C).(3S)-3-Amino-4-(1H-imidazol-4-yl)-1-(phenylthio)butan-2-one dihydrochloride (5b)
Yield 1.1 mmol (55%) as a yellowish-brown powder; [α]20D: +2.3° (MeOH) c = 1.3 g L−1; ESI+MS: m/z 262 (M + H)+, 294 (M + Na)+, 523(2M + H+); 1H NMR (DMSO-d6): δ =14.70 (s, 2H); 9.10 (d, J = 1.3, 1H); 8.71 (s, 3H); 7.48 (d, J = 1.1, 1H); 7.28 (m, 5H); 4.77 (dd, J = 4.6, 8.6, 1H); 4.48 (m, 2H); 3.56 (dd, J = 4.6, 15.5, 1H); 3.22 (dd, J = 8.7, 15.5, 1H); 13C NMR (DMSO-d6): δ = 199.5 (1C); 134.7 (1C); 133.9 (1C); 128.9 (1C); 128.2 (1C); 126.4 (1C); 126 (1C); 118.2 (1C); 56.3 (1C); 40.4 (1C); 24.2 (1C).(3S)-3-Amino-4-(1H-imidazol-4-yl)-1-(tolylthio)butan-2-one di hydrochloride (5c)
Yield 1.2 mmol (60%) as a brown powder; [α]20D: +3.4° (MeOH) c = 1.15 g L−1; ESI+MS: m/z 276 (M + H)+, 298 (M + Na)+; 1H NMR (MeOD-d4): δ = 8.92 (s, 1H); 7.15 (dd, J = 18.6, 6.9, 4H); 4.12 (q, J = 15.8, 1H); 3.75 (d, J = 4.8, 1H); 3.61 (s, 1H); 2.32 (d, J = 17.3, 1H); 1.32 (s, 3H); 13C NMR (MeOD-d4): δ = 200.08 (1C); 160.13 (1C); 145.89 (1C); 136.79 (1C); 132.08 (1C); 131.58 (1C); 131.07 (1C); 130.56 (1C); 114.02 (1C); 73.54 (1C); 57.12 (1C); 55.81 (1C); 21.21 (1C).(3S)-3-Amino-1-(4-fluorophenylthio)-4-(1H-imidazol-4-yl)butan-2-one dihydrochloride (5d)
Yield 0.98 mmol (49%) as a white powder; [α]20D: −1.3° (MeOH) c = 1.0 g L−1; mp = 110–115 °C; ESI+MS:m/z 280 (M + H)+; ESI−MS: m/z 278 (M–H)−, 314 (M + Cl)−; 1H NMR (MeOD-d4): δ = 8.84 (d, J = 1.1, 1H); 7.47–7.33 (m, 3H); 7.07–6.90 (m, 2H); 4.04 (q, J = 16.0, 2H); 3.55 (dt, J = 30.5, 11.4, 1H); 3.28–3.11 (m, 3H); 13C NMR (MeOD-d4): δ = 199.84 (s, 1C); 165.17 (s, 1C); 134.72 (d, J = 8.3, 1C); 127.93 (s, 1C); 120.00 (s, 1C); 117.42 (s, 1C); 117.29 (s, 1C); 117.20 (s, 1C); 57.29 (s, 1C); 42.91 (s, 1C); 25.98 (s, 1C); 24.69 (s, 1C).(3S)-3-Amino-4-(1H-imidazol-4-yl)-1-(naphthalen-2-ylthio)butan-2-one dihydrochloride (5e)
Yield 1 mmol (50%) as a brown oil; [α]20D: −31° (MeOH) c = 1.0 g L−1; ESI+MS: m/z 312 (M + H)+; ESI−MS: 310 (M–H)−, 346 (M + Cl)−; 1H NMR (DMSO-d6): δ =15.21–14.13 (m, 1H); 9.10 (s, 1H); 8.68 (s, 2H); 7.89 (dd, J = 14.4, 7.0, 2H); 7.57 − 7.43 (m, 2H); 4.83 (d, J = 4.0, 1H); 4.67 − 4.49 (m, 1H); 3.62 (dd, J = 15.5, 4.3, 1H); 3.24 (dd, J = 15.6, 8.8, 1H); 13C NMR (DMSO-d6): δ =199.57 (1C); 133.30 (1C); 132.34 (1C); 131.23 (1C); 128.37 (1C); 127.57 (1C); 127.19 − 126.84 (1C); 126.70 (1C); 126.50 (1C); 126.35 (1C); 125.80 (1C); 118.33 (1C); 56.44 (1C); 51.01 (1C); 24.33 (1C); CLogP: 1.7846(3S)-3-Amino-1-(4-bromophenylthio)-4-(1H-imidazol-4-yl) butan-2-one dihydrochloride (5f)
Yield 1.2 mmol (60%) as a brown oil; [α]20D: +4.4°(MeOH) c = 1.6 g L−1; ESI+MS: m/z 340 (M + H)+, 342 (M + H)+; 1H NMR (DMSO-d6): δ = 9.09 (s, 1H); 8.63 (s, 3H); 7.52–7.47 (m, 3H); 7.34–7.29 (m, 2H); 4.75 (s, 1H); 4.48 (q, J = 17.4, 2H); 3.56 (dd, J = 4.4, 15.6, 1H); 3.19 (dd, J = 8.7, 15.5, 1H); 13C NMR (DMSO-d6): δ = 199.49 (1C); 134.54 (1C); 131.80 (3C); 130.20 (2C); 126.49 (1C); 119.12 (1C); 56.38 (1C); 40.25 (1C); 24.33 (1C).(3S)-3-Amino-1-(2,3,4,5,6-pentafluorophenylthio)-4-(1H-imida zol-4-yl)butan-2-one dihydrochloride (5g)
Yield 0.92 mmol (46%) as a yellowish-white powder; [α]20D: +4.0° (MeOH) c = 1.75 g L−1; ESI+MS: m/z 352 (M + H)+; 1H NMR (MeOD-d4): δ = 8.86 (d, J = 16.8, 1H); 7.43 (d, J = 11.3, 1H); 3.68 (s, 2H); 3.63 (d, J = 5.9, 2H); 3.49 (d, J = 5.2, 1H); 13C NMR (MeOD-d4): δ = 145.95 (1C); 131.58 (1C); 129.57 (1C); 128.74 (1C); 127.88 (1C); 114.03 (1C); 73.58 (1C); 72.46 (1C); 62.19 (1C); 57.80 (1C); 55.72 (1C); 43.77 (1C); 24.23 (1C).(3S)-3-Amino-1-hydroxy-4-(1H-imidazol-4-yl)butan-2-one dihydrochloride (5h)
This compound is obtained starting from 5j after 16 h in HCl 4N, yielding 0.64 mmol (32%) as a yellow powder; [α]20D: +0.9° (MeOH) c = 1.5 g L−1; mp = 175–180 °C; ESI+MS: m/z 170 (M + H)+; 1H NMR (MeOD-d4): δ = 8.86 (s, 1H); 7.49 (s, 1H); 4.40 (t, J = 6.6, 1H); 3.77 (s, 2H); 3.37 (qd, J = 15.7, 6.7, 2H); 13C NMR (MeOD-d4): δ = 169.36 (1C); 128.54 (1C); 119.91 (1C); 54.24 (1C); 53.07 (1C); 26.57 (1C); 26.47 (1C).(3S)-3-Amino-4-(1H-imidazol-4-yl)-1-(phenyloxy)butan-2-one dihydrochloride (5i)
Yield 0.6 mmol (30%) as a yellow oil; [α]20D: +2.2° (MeOH) c = 1.76 g L−1; ESI+MS: m/z 246 (M + H)+, 268 (M + Na)+; 1H NMR (MeOD-d4): δ =8.74 (d, J = 18, 1H); 7.56 (d, J = 1.1, 1H); 6.91 (m, 5H); 4.63 (dd, J = 4.6, 8.6, 1H); 4.18 (m, 2H); 3.83 (m, 1H); 3.33 (d, J = 18.6, 1H); 13C NMR (MeOD-d4): δ = 160.11 (1C); 136.80 (1C); 130.82 (1C); 130.56 (1C); 129.23 (1C); 128.69 (1C); 122.91 (1C); 115.66 (1C); 55.84 (1C); 43.93 (1C); 30.98 (1C).(3S)-3-Amino-4-(1H-imidazol-4-yl)-1-(p-tolyloxy)butan-2-one dihydrochloride (5j)
Yield 0.7 mmol (35%) as a brown oil; [α]20D: +5.02° (MeOH) c = 1.19 g L−1; ESI+MS: m/z 260 (M + H)+; 1H NMR (MeOD-d4): δ =8.91 (s, 1H); 7.55 (s, 4H); 4.56 − 4.18 (m, 1H); 3.85 (d, J = 25.7, 1H); 3.33 (s, 1H); 2.25 (d, J = 17.1, 1H); 1.30 (s, 3H); 13C NMR (MeOD-d4): δ =183.07 (1C); 150.96 (1C); 148.30 (1C); 138.01 (1C); 131.10 (1C); 130.86 (1C); 117.07 (1C); 116.11 (1C); 115.59 (1C); 89.04 (1C); 57.36 (1C); 55.79 (1C); 20.58 (1C).(3S)-3-Amino-1-(4-fluorophenoxy)-4-(1H-imidazol-4-yl)butan-2-one dihydrochloride (5k)
Yield 0.6 mmol (30%) as a orange-brown oil; [α]20D: −9.7° (MeOH) c = 1.34 g L−1; ESI+MS: m/z 264 (M + H)+; 1H NMR (MeOD-d4): δ =7.44 − 7.38 (m, 2H); 7.32 − 7.24 (m, 3H); 7.24 −7.18 (m, 1H); 6.90 − 6.82 (m, 1H); 3.78 (d, J = 1.8, 2H); 3.01 (d, J = 2.9, 1H); 2.66 (s, 1H); 1.44 (d, J = 5.5, 2H); 1.40 (s, 2H); 13C NMR (MeOD-d4): δ = 131.58 (s, 1C); 129.59 (s, 1C); 128.67 (d, J = 12.9, 1C); 127.89 (s, 1C); 114.05 (s, 1C); 55.74 (s, 1C); 52.33 (s, 1C); 28.67 (s, 2C).(3S)-3-Amino-4-(1H-imidazol-4-yl)-1-(naphthalen-2-yloxy)butan-2-one dihydrochloride (5l)
Yield 0.56 mmol (28%) as a yellow oil; [α]20D: +3.7° (MeOH) c = 0.82 g L−1; ESI+MS: m/z 296 (M + H)+; 1H NMR (DMSO-d6): δ =8.75 (s, 1H); 7.48 (ddd, J = 14.12, 8.19, 4.35, 9H); 4.43 (d, J = 9.58, 2H); 4.16 − 3,93 (m, 1H); 3.88 (d, J = 7.3, 1H); 3.47 (s, 1H); 3.22 (d, J = 7.1, 1H); 1.32 (dd, J = 14.6, 7.4, 2H); 13C NMR (MeOD-d4): δ = 204.88 (1C); 135.88 (1C); 130.73 (1C); 130.44 (1C); 128.70 (1C); 127.70 (1C); 127.21 (1C); 125.22 (1C); 123.93 (1C); 119.35 (1C); 109.95 (1C); 108.83 (1C); 56.43 (1C); 53.09 (1C); 48.00 (1C); 9.34 (1C).(3S)-3-Amino-1-(4-bromophenoxy)-4-(1H-imidazol-4-yl)butan-2-one dihydrochloride (5m)
Yield 0.92 mmol (46%) as a yellowish-brown oil; [α]20D: −21.4° (MeOH) c = 1.4 g L−1; ESI+MS: m/z 324 (M + H)+, 326 (M + H)+; ESI−MS: 358 (M + Cl)−, 360 (M + Cl)−; 1H NMR (MeOD-d4): δ =8.82 (s, 1H); 7.45 (s, 1H); 7.33 (s, 1H); 7.17 (d, J = 8.9, 1H); 6.82 (d, J = 9.1, 1H); 6.61 (d, J = 8.9, 1H); 4.38 (t, J = 6.9, 1H); 4.29 (s, 1H); 3.77 (s, 2H); 3.35 (qd, J = 15.7, 6.9, 2H); 3.22 (dt, J = 3.3, 1.6, 2H); 2.07 (s, 1H); 13C NMR (MeOD-d4): δ =201.95 (1C); 169.40 (1C); 147.61 (1C); 133.55 (1C); 133.23 (1C); 128.39 (1C); 119.83 (1C); 118.30 (1C); 104.08 (1C); 54.23 (1C); 53.04 (1C); 26.60 (1C); 26.49 (1C).(3S)-3-Amino-1-(2,3,4,5,6-pentafluorophenyloxy)-4-(1H-imida zol-4-yl)butan-2-one dihydrochloride (5n)
Yield 1.2 mmol (60%) as a white powder; [α]20D: +1.8° (MeOH) c = 1.6 g L−1; ESI+MS: m/z 336 (M + H)+, 358 (M + Na)+; 1H NMR (MeOD-d4): δ = 8.80 (d, J = 3.52, 1H); 7.44 (d, J = 6.5, 1H); 4.25 (t, J = 7.6, 1H); 3.64 (t, J = 5.0, 1H); 3.57 (dd, J = 4.68, J = 9.6, 2H); 3.48 (t, J = 5.0, 1H); 13C NMR (MeOD-d4): δ =145.95 (1C); 131.58 (1C); 129.58 (1C); 128.76 (1C); 127.83 (1C); 114.04 (1C); 73.58 (1C); 72.47 (1C); 62.19 (1C); 55.75 (1C); 43.84 (1C).Cloning and overexpression of the HDH-encoding gene from B. suis, and purification of the enzyme
The HDH-encoding gene, BR0252, was specifically amplified by PCR using B. suis 1330 chromosomal DNA as template and OPJ5-foward primer (5′-GCGGG![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif)
![[T with combining low line]](https://www.rsc.org/images/entities/char_0054_0332.gif)
![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif)
![[T with combining low line]](https://www.rsc.org/images/entities/char_0054_0332.gif)
![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif) GTCACAACGCTCAGACAGACCG-3′) and OPJ6-reverse primer (5′-GCGCG
GTCACAACGCTCAGACAGACCG-3′) and OPJ6-reverse primer (5′-GCGCG![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif)
![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif)
![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif)
![[T with combining low line]](https://www.rsc.org/images/entities/char_0054_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) TCATAGGTT CAGACGAATGGCGACG-3′) which contain BamHI and NdeI recognition sequences (underlined), respectively. The PCR products were digested with BamHI and NdeI and ligated to BamHI- and NdeI-digested pET15b (Novagen) prior to introduction into E. coli strain DH5α. The integrity of the cloned gene was verified by sequencing, using primers OPJ5 and OPJ6 described above. The construct pET15bHDH was then transformed into E. coli strain BL21(DE3) for production of the 6x(His)-HDH fusion protein. E. coliBL21(DE3) cells harbouring pET15bHDH were grown at 37 °C in one liter of Luria-Bertani medium supplemented with 50 μg mL−1ampicillin. When the culture reached an optical density at 600 nm (OD600) of approximately 0.6, expression of 6x(His)-HDH protein was induced by the addition of isopropyl-thio-β-D-galactoside (IPTG) to a final concentration of 1 mM and growth was continued for 5 h. Cells were then harvested by centrifugation at 3500 rpm at 4 °C for 20 min and broken by sonication in buffer A (200 mM KCl, 50 mM Tris–Cl [pH 7.5], 10% glycerol, 0.1% Nonidet P-40, 1 mM phenylmethylsulfonyl fluoride, 2 μM pepstatin A) supplemented with 0.5 mM dithiothreitol and 0.2 mM disodium EDTA. All subsequent steps were performed at 4 °C. After centrifugation (13000 rpm, 20 min), the soluble extract was treated with streptomycin sulfate to remove ribosomes and nucleic acids. The suspension was then centrifuged at 13000 rpm for 15 min, and the supernatants were dialysed against 2 L of sonication buffer A for 1 h. The dialysed lysates were mixed with Talon Co+-affinity resin (Clontech) that had been equilibrated with buffer I (20 mM Tris–HCl [pH 8.0], 5 mM β-mercaptoethanol, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, 2 μM pepstatin A and 0.1% Nonidet P-40) supplemented with 150 mM KCl. The resin and bound His-tagged protein were collected by centrifugation at 2500 rpm for 10 min and washed with buffer I containing 500 mM KCl and 10 mM imidazole. The 6x(His)-HDH protein was eluted with buffer I containing 125 mM KCl and 75 mM imidazole without Nonidet P-40. Elution fractions were free of detectable contaminating proteins as determined by Coomassie blue staining of sodium dodecyl sulfate (SDS)–polyacrylamide gels. The fractions containing the His-tagged proteins (estimated purity, >95%) were pooled and stored in elution buffer supplemented with 50% (v/v) glycerol at −20 °C. Protein concentration was determined with the bicinchoninic acid reagent (Pierce) using bovine serum albumin as a standard.
TCATAGGTT CAGACGAATGGCGACG-3′) which contain BamHI and NdeI recognition sequences (underlined), respectively. The PCR products were digested with BamHI and NdeI and ligated to BamHI- and NdeI-digested pET15b (Novagen) prior to introduction into E. coli strain DH5α. The integrity of the cloned gene was verified by sequencing, using primers OPJ5 and OPJ6 described above. The construct pET15bHDH was then transformed into E. coli strain BL21(DE3) for production of the 6x(His)-HDH fusion protein. E. coliBL21(DE3) cells harbouring pET15bHDH were grown at 37 °C in one liter of Luria-Bertani medium supplemented with 50 μg mL−1ampicillin. When the culture reached an optical density at 600 nm (OD600) of approximately 0.6, expression of 6x(His)-HDH protein was induced by the addition of isopropyl-thio-β-D-galactoside (IPTG) to a final concentration of 1 mM and growth was continued for 5 h. Cells were then harvested by centrifugation at 3500 rpm at 4 °C for 20 min and broken by sonication in buffer A (200 mM KCl, 50 mM Tris–Cl [pH 7.5], 10% glycerol, 0.1% Nonidet P-40, 1 mM phenylmethylsulfonyl fluoride, 2 μM pepstatin A) supplemented with 0.5 mM dithiothreitol and 0.2 mM disodium EDTA. All subsequent steps were performed at 4 °C. After centrifugation (13000 rpm, 20 min), the soluble extract was treated with streptomycin sulfate to remove ribosomes and nucleic acids. The suspension was then centrifuged at 13000 rpm for 15 min, and the supernatants were dialysed against 2 L of sonication buffer A for 1 h. The dialysed lysates were mixed with Talon Co+-affinity resin (Clontech) that had been equilibrated with buffer I (20 mM Tris–HCl [pH 8.0], 5 mM β-mercaptoethanol, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, 2 μM pepstatin A and 0.1% Nonidet P-40) supplemented with 150 mM KCl. The resin and bound His-tagged protein were collected by centrifugation at 2500 rpm for 10 min and washed with buffer I containing 500 mM KCl and 10 mM imidazole. The 6x(His)-HDH protein was eluted with buffer I containing 125 mM KCl and 75 mM imidazole without Nonidet P-40. Elution fractions were free of detectable contaminating proteins as determined by Coomassie blue staining of sodium dodecyl sulfate (SDS)–polyacrylamide gels. The fractions containing the His-tagged proteins (estimated purity, >95%) were pooled and stored in elution buffer supplemented with 50% (v/v) glycerol at −20 °C. Protein concentration was determined with the bicinchoninic acid reagent (Pierce) using bovine serum albumin as a standard.
HDH inhibition assay
Inhibition assays were performed by kinetics measurement. The formation of NADH was followed by UV measurement at 340nm. Each inhibitor was tested at 3 different concentrations and each measurement was repeated three times. A control measurement of enzyme activity was performed in the absence of inhibitor. This positive control was also performed in triplicate. For each experiment, 884-x μL of pH 9.2 150mM glycine buffer, 50 μL of a 10 mM MnCl2 solution, 40 μL of a 50 mM NAD solution, 1 μL of purified HDH (0.0405 μM, final concentration), and x μL of inhibitor solution were introduced in the measurement cell and then incubated for 5 min at 30 °C. 25 μL of a 2 mM L-histidinol solution were then added and the measurement started. IC50 values were determined by calculation of the inhibition rate for each concentration tested.Acknowledgements
This work was supported by the French Ministry of Research (MESR) for PhD Fellowship (to F.T.) and the National Research Agency (ANR) for ANR grant ANR-09-MIEN-001 (to S.K. and J.-Y. W).Notes and references
- M. N. Seleem, S. M. Boyle and N. Sriranganathan, Vet. Microbiol., 2010, 140, 392–398 CrossRef.
- C. Gamazo, M. C. Lecàroz, S. Prior, A. I. Vitas, M. A. Campanero, J. M. Irache and M. J. Blanco-Prieto, Curr. Drug Delivery, 2006, 3, 359–365 CrossRef CAS.
- S. D. Perkins, S. J. Smither and H. S. Atkins, FEMS Microbiol. Rev., 2010, 1–16 Search PubMed.
- (a) S. Köhler, V. Foulongne, S. Ouahrani-Bettache, G. Bourg, J. Teyssier, M. Ramuz and J.-P. Liautard, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 15711–15716 CrossRef; (b) S. Köhler, S. Michaux-Charachon, F. Porte, M. Ramuz and J.-P. Liautard, Trends Microbiol., 2003, 11, 215–219 CrossRef.
- (a) D. A. Rasko and V. Sperandio, Nat. Rev. Drug Discovery, 2010, 9, 117–128 CrossRef CAS; (b) J.-F. Desnotes, Antibiotiques, 2009, 11, e1–e12 CrossRef (in French).
- J. E. Dancer, M. J. Ford, K. Hamilton, M. Kilkelly, S. D. Lindell, M. J. O′Mahony and E. A. Saville-Stones, Bioorg. Med. Chem. Lett., 1996, 6, 2131–2136 CrossRef CAS.
- (a) M.-R. Abdo, P. Joseph, R.-A. Boigegrain, J.-P. Liautard, J.-L. Montero, S. Köhler and J.-Y. Winum, Bioorg. Med. Chem., 2007, 15, 4427–4433 CrossRef CAS; (b) P. Joseph, M. R. Abdo, R. A Boigegrain, J.-L. Montero, J.-Y. Winum and S. Köhler, Antimicrob. Agents Chemother., 2007, 51, 3752–3755 CrossRef CAS; (c) M. R. Abdo, P. Joseph, R. A. Boigegrain, J.-P. Montero, S. Köhler and J.-Y. Winum, J. Enzyme Inhib. Med. Chem., 2008, 23, 357–361 CrossRef CAS; (d) C. T. Supuran, J.-Y. Winum, Introduction to zinc enzymes as drug targets. In Drug Design of Zinc-Enzyme Inhibitors: Functional, Structural, and Disease Applications, Supuran, C. T.; Winum, J. Y. ed., Wiley, Hoboken, 2009, pp. 3–12 Search PubMed; (e) J.-Y. Winum, S. Köhler, A. Scozzafava, J.-L. Montero and C. T. Supuran, Anti-Infect. Agents Med. Chem., 2008, 7, 169–179 CAS.
- M.-R. Abdo, P. Joseph, J. Mortier, F. Turtaut, J.-L. Montero, B. Masereel, S. Köhler and J.-Y. Winum, Org. Biomol. Chem., 2011, 9, 3681–3690 CAS.
- K. Hee-Doo, S. Young-Ger, PCT Int. Appl. WO 2007/120012.
- J. C. Loper and E. Adams, J. Biol. Chem., 1965, 240, 788–795 CAS.
| This journal is © The Royal Society of Chemistry 2011 |
