Identification of 3-aminothieno[2,3-b]pyridine-2-carboxamides and 4-aminobenzothieno[3,2-d]pyrimidines as LIMK1 inhibitors†
Brad E.
Sleebs
abc,
Alla
Levit
abc,
Ian P.
Street
abc,
Hendrik
Falk
abc,
Tim
Hammonds
d,
Ai Ching
Wong
d,
Mark D.
Charles
d,
Michael F.
Olson
e and
Jonathan B.
Baell
*abc
aThe Walter and Eliza Hall Institute of Medical Research, 1G Royal Parade, Parkville, Victoria, Australia 3052. E-mail: jbaell@wehi.edu.au.; Tel: +613 9345 2108
bCancer Therapeutics-CRC P/L, 4 Research Ave, La Trobe R&D Park, Bundoora, Victoria, Australia 3086
cDepartment of Medical Biology, The University of Melbourne, Parkville, Victoria 3010, Australia
dCancer Research Technology Ltd, The Cruciform Building, Gower Street London, UK WC1E 6BT
eThe Beatson Institute for Cancer Research, Garscube Estate, Switchback Road, Glasgow, Scotland G61 1BD, UK
First published on 12th August 2011
Abstract
A high throughput chemical screening campaign has led to the identification of 3-aminobenzo[b]thiophene-2-carboxamides as LIMK1 inhibitors. Evolution of bicyclic hits to the tricyclic 4-aminobenzothieno[3,2-d]pyrimidine, using a traditional medicinal chemistry SAR guided approach, resulted in a significant increase in potency. Further elaboration has seen the 7-phenyl-4-aminobenzothieno[3,2-d]pyrimidine emerge as a LIMK1 inhibitor lead candidate.
Introduction
The LIM-kinase (LIMK) family proteins are serine-only protein kinases that possess a LIM1 zinc finger, a PDZ2 domain, and a protein-protein kinase domain. The LIMK family has two members LIMK1 and LIMK2, and both are reported to have widespread tissue distribution. A LIMK2 isoform, known as LIMK2t, also exists that lacks the LIM domain, and is endemic to spermatogenic cells found in the testis.3LIMK1 and 2 are found downstream of the Rho family GTPase cascade and aid in the regulation of the actin cytoskeleton. LIMK family proteins mediate the actin cytoskeleton by phosphorylation of cofilin, an actin depolymerization factor. Non-phosphorylated cofilin is able to bind globular actin preventing actin polymerisation while promoting filamentous–actin depolymerisation. Phosphorylation of cofilin by LIMK family proteins renders cofilin inactive or unable to bind to actin, leading to actin polymerisation. Sub-cellular de-localisation of LIMK family proteins, allows sub-cellular vicinal filamentous actin formation. This induces formation of actin-based cellular protrusions, such as filopodia, lamellipodia and stress fibers. These actin-organised structures allow for cellular motility toward an extracellular chemoattractant.
It has recently been postulated that the LIMK regulation of actin dynamics determines the metastatic potential of tumour cells.4 To support this theory, there is evidence that LIMK1 is up-regulated in a number of highly invasive and metastatic cell lines.5 Furthermore, in various of LIMK1 and 2 knockdown models, cellular motility, invasiveness and metastasis were shown to be nullified.6 It is thought that inhibition of LIMK1 and LIMK2 will disrupt actin polymerisation, and thus prevent the metastatic potential of tumor cell lines where LIMK is over expressed. It is this hypothesis that has seen LIMK family proteins emerge as a potential target for cancer therapies.
While much of the research surrounding the LIMK family proteins has been to elucidate biological function, a number of recent publications have disclosed dual inhibitors of LIMK 1 and 2.7 Bristol-Myers-Squibb (BMS) have disclosed two series of potent LIMK inhibitors, a pyrazolo series and a 5-thiazolopyrimidine series.8–10 Some of these were shown to be potently cytotoxic but it was determined that this was due to off-target (anti-microtubule) activity and that LIMK inhibition per se did not result in inhibition of cellular proliferation. As BMS were not interested in a purely antimetastatic agent, this program was dropped. More recently, Lexicon Pharmaceuticals revealed a pyrrolopyrimidine series as potent inhibitors of LIMK1 and 2 for treatment for ocular hypertension and associated glaucoma.11–13
We were interested in LIMK1 inhibitors as antimetastatic agents. Moreover, we had internal data that suggested LIMK1 inhibition might not inhibit cell proliferation in vitro but that it could inhibit primary tumour growth in vivo. Herein we report on the preliminary disclosure of a novel series of compounds that exhibit moderate inhibition of LIMK1.
Results and discussion
A high throughput screen (HTS) of a diverse 40000 compound library and verification of resulting hits identified the 3-aminothieno[2,3-b]pyridine-2-carboxamide 1 scaffold. A substructure search of 1 in the HTS library revealed a number of compounds with a lower activity than HTS cut-off with the aminothieno[2,3-b]pyridine-2-carboxamide core. A representation of the hits from the HTS screen and library SAR probes are given in Table 1. From the data there were clear signs of early SAR. For example, a hydrophobic substituent in the 4 position, either a methyl (2) but particularly a phenylamino (4) increased inhibitory activity. It was also observed that N-substitution on the 2-carboxamide was not tolerated. For example, relatively good inhibition by 4 was completely abrogated in 6.| # | Structure | % Inhib @ 30 μM | # | Structure | % Inhib @ 30 μM |
|---|---|---|---|---|---|
| a Assays performed as described in the supplementary information.† | |||||
| 1 |
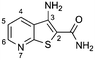
|
40 | 6 |
|
0 |
| 2 |
|
79 | 7 |
|
50 |
| 3 |
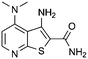
|
67 | 8 |
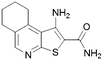
|
16 |
| 4 |

|
98 | 9 |
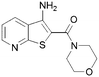
|
0 |
| 5 |
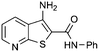
|
15 | 10 |
|
2 |
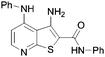
For this reason, commercially available analogues were sought and tested and the results are shown in Table 2. Here, it can be seen that a 6-aryl group can give rise to improved kinase inhibition (11, 12, 15–18) though neither a benzyl (13) nor furan (14) are well tolerated. In the 4 position a variety of substituents except for a bulky phenyl group (19 and 20) are well tolerated.
| # | Structure | IC50 (μM) | # | Structure | IC50 (μM) |
|---|---|---|---|---|---|
| a Assays performed as described in the supplementary information.† | |||||
| 11 |

|
7.1 | 16 |

|
3.0 |
| 12 |

|
1.3 | 17 |

|
2.0 |
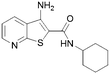
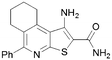
| 13 |
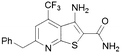
|
30 | 18 |
|
2.8 |
| 14 |
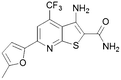
|
41 | 19 |
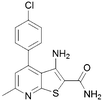
|
37 |
| 15 |

|
1.6 | 20 |
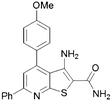
|
44 |
These promising results suggested to us that this system was amenable to optimization of potency. As a prelude to this we investigated the optimum requirement of the core heterocycle and the simplicity of the hit allowed for easy synthesis of relevant analogues as shown in Scheme 1. Thus, sulfurisation of 2-chloro-3-cyanopyridine (21) with thiourea gave 22, the sulfur atom of which was alkylated with either bromoacetamide or ethyl bromoacetate followed by cyclization in the one pot to give respective thienopyridines 1 and 23. The ester of 23 was further manipulated to give the respective mono- and dimethylamides 24 and 25 and hydrazide 26. On the other hand, treatment of 2-fluorobenzonitrile with ethyl mercaptoacetate followed by potassium carbonate to induce cyclization gave benzothiophene ester 28. This was readily hydrolysed to give acid 31, which in turn allowed synthesis of the 3-N-methylated derivative 33via the isatoic anhydride 32, after methylation with methyl iodide and ring opening with aqueous ammonia. Benzofuran 35 was readily obtained after cyclization of the alkylation product of 2-hydroxybenzonitrile (34) with ethyl bromoacetate. The 2-thiol benzoate 38 was similarly alkylated to give rise to the amide 40. The acid 36 gave the des-3-aminobenzothiophene-2-carboxamide 37, via a HBTU coupling. The biological results are shown in Table 3.
![Synthesis of 3-aminothieno[2,3-b]pyridine-2-carboxamide and 4-aminobenzothieno[3,2-d]pyrimidine derivatives. Reagents and conditions: a) thiourea, EtOH; b) Et3N, DMF, (for 1 BrCH2CONH2; for 23 BrCH2CO2Et), then K2CO3; c) From 23: 1. NaOH; 2. HBTU, DIPEA, for 24 HNMe2, for 25 H2NMe; For 26 NH2NH2 (from 23); d) SHCH2CO2Et, Et3N, DMF, then K2CO3; e) ClCH2CONH2, Et3N, DMSO; f) aq. NaOH, EtOH; g) triphosgene, dioxane, 90 °C; h) NaH, MeI DMF; i) NH4OH j) DMF, BrCH2CONH2, K2CO3; k) HBTU, Et3N, DMF, NH4OH.](/image/article/2011/MD/c1md00137j/c1md00137j-s1.gif) | ||
| Scheme 1 Synthesis of 3-aminothieno[2,3-b]pyridine-2-carboxamide and 4-aminobenzothieno[3,2-d]pyrimidine derivatives. Reagents and conditions: a) thiourea, EtOH; b) Et3N, DMF, (for 1 BrCH2CONH2; for 23 BrCH2CO2Et), then K2CO3; c) From 23: 1. NaOH; 2. HBTU, DIPEA, for 24 HNMe2, for 25 H2NMe; For 26 NH2NH2 (from 23); d) SHCH2CO2Et, Et3N, DMF, then K2CO3; e) ClCH2CONH2, Et3N, DMSO; f) aq. NaOH, EtOH; g) triphosgene, dioxane, 90 °C; h) NaH, MeI DMF; i) NH4OH j) DMF, BrCH2CONH2, K2CO3; k) HBTU, Et3N, DMF, NH4OH. | ||
| # | Structure | IC50 (μM) | # | Structure | IC50 (μM) |
|---|---|---|---|---|---|
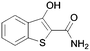
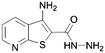
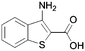
| a Compounds 41, 42, 43, 44 were purchased from commercial vendors. Assays performed as described in the supplementary information.† | |||||
| 1 |

|
37 | 33 |

|
34 |
| 2 |
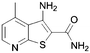
|
11 | 35 |

|
>100 |
| 24 |

|
>100 | 37 |
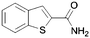
|
>100 |
| 25 |

|
>100 | 40 |
|
>100 |
| 26 |
|
>100 | 41 |

|
9 |
| 28 |

|
>100 | 42 |

|
56 |
| 30 |

|
33 | 43 |

|
92 |
| 31 |
|
>100 | 44 |

|
>100 |
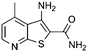
Here it is revealed that the endocyclic 7-N in 1 is not essential and replacement with a carbon atom to give the benzothiophene (30) is well tolerated without loss of activity. Assuming crossover of SAR between benzo- and pyridothiophenes, it is clear that N-methylation (33) of the 3-amino group is tolerated, but its removal (37) or its replacement with a hydroxy (40) is not. No modification of the 2-carboxamide (24–26, 28, 31) is tolerated. The thiophene sulfur is important and the benzofuran (35) is inactive. A methyl group in the 6-position (42) is tolerated but in the 4-position (2, 41) appears to improve activity. Moving the endocyclic nitrogen atom from the 7-position to the 4 position (43) was less well tolerated.
At this point we had identified 3-aminobenzothiophene-2-carboxamides and their thienopyridine counterparts as the minimal, preferred core structures. However, we were concerned with the number of hydrogen bond donors and the implied difficulty for optimization with retention of permeability and hence cell-based activity. For this reason, we targeted a pyrimidine as a putative isostere of the pseudo six-membered ring that would be formed by virtue of an intramolecular hydrogen bond between the 3-amino and 2-carboxamide groups in our core structures.
The required 4-aminopyrimidine system was accessed conveniently via extension of current chemistry, as shown in Scheme 2. Here, we have continued to investigate the benzofuran and indole systems in addition to the benzothiophene. Thus alkylation of 3-cyano-2-mercaptopyridine with chloroacetonitrile afforded 45, followed by treatment with formamidine acetate gave the desired aza-4-aminobenzothieno[3,2-d]pyrimidine target 49. Alternatively, cyclization of ethyl 3-amino-2-carboxylates 28 and 53 (the latter made by analogy to 28 but using ethyl 2-mercaptoacetate) using formamidine acetate afforded the 4-hydroxypyrimidines 54, 55 respectively. Further transformation via the activated halide afforded the desired 4-aminopyrimidines 56 and 57 respectively. In an analogous manner aza-4-aminobenzothieno[3,2-d]pyrimidines 50 and 51, were respectively made from the esters 46 and 47. The corresponding indole 61 was readily made by acylating anthranilonitrile 58 to give 59, then alkylating with chloroacetonitrile followed by in situ cyclization to give 60 and finally reaction with formamidine acetate.
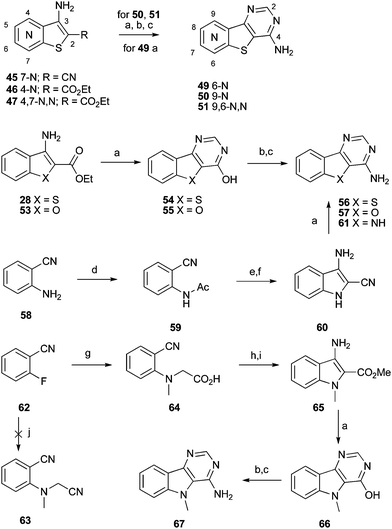 | ||
| Scheme 2 Synthesis of 4-aminopyrimidine derivatives. Reagents and conditions: a) formamidine acetate, formamide, 150 °C; b) POCl3, 90 °C; c) NH4OH, DMSO, 100 °C; d) Ac2O, DMAP; e) 1. ClCH2CN, KOtBu; 2. aq. K2CO3; f) aq. K2CO3, EtOH; g) sarcosine, K2CO3, Cu(OAc)2, DMSO; h) SOCl2, MeOH; i) K2CO3, DMF, 60 °C; j) N-methylacetonitrile, K2CO3, Cu(OAc)2, DMSO. | ||
Synthesis of the N-Me indole 67 started from SNAr addition of sarcosine to 2-fluorobenzonitrile (62) to give 64. Initial attempts to react 62 more directly with N-methylaminoacetonitrile were unsuccessful. To aid purification, the acid 64 was converted to the ester before cyclization to the indole 65. Cyclocondensation of 65 with formamidine acetate gave 66 and this was converted to the activated chloride and aminated to give 67, in three high yielding steps.
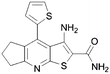
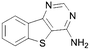
A summary of the LIMK1 inhibition of the synthesised compounds is given in Table 4. Our first striking observation was that the simple tricyclic cores 49 and 56 were remarkably potent and returned IC50 values of 7–9 μM. Changing the position of the endocyclic nitrogen atom (50) or inclusion of another (51) led to significant losses of activity. However, changing to a benzofuran scaffold in 57 was better tolerated with an IC50 of 15 μM. Further, indole scaffolds in 61 and 67 led if anything to a slight increase in inhibitory potency with IC50 values of 4 μM.
| # | Structure | IC50 (μM) | # | Structure | IC50 (μM) |
|---|---|---|---|---|---|
| a Assays performed as described in the supplementary information. | |||||
| 49 |

|
9 | 56 |
|
7 |
| 50 |

|
43 | 57 |
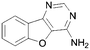
|
15 |
| 51 |
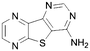
|
>100 | 61 |
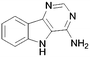
|
4 |
| 67 |
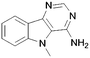
|
4 |
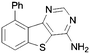
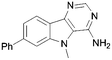
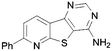
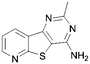
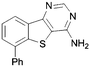
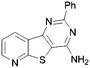
In order to probe the space around this core, we targeted the construction of a set of aryl-substituted analogues. The phenyl analogues 73–76 were easily obtained from the respective bromo precursors 68–71 (made in analogy to 56 in Scheme 2) via a Suzuki reaction. Similarly, indole 77 was made from 72, which in turn was made in analogy to 61 in Scheme 2. However, the 7-phenyl aza-derivative 83 utilized an alternative strategy starting from the nicotinic acid 78. A regioselective Suzuki reaction was undertaken first and then the acid 79 was then converted to the nitrile 80, and the 2-chloro converted to the thione 81. The 2-thione nitrile 81 was now setup for alkylation as before with bromoacetonitrile, followed by cyclocondensation to give 82. Finally cyclocondensation with formamidine acetate yielded the desired 7-phenyl analogue 83. The 2-methyl substituted derivative 84 was afforded from aminonitrile 45, via reaction with trimethylorthoformate, followed by reaction with ammonium acetate. The 2-substituted phenyl analogue 85 was obtained from a base-mediated cyclisation of the aminonitrile 45 with benzonitrile (Scheme 3).
 | ||
| Scheme 3 Synthesis of substituted 4-aminopyrimidine derivatives. Reagents and conditions: a) PhB(OH)2, PdCl2(PPh3)2, K2CO3, dioxane, H2O, 90 °C; b) formamidine acetate, formamide, 150 °C; c) i) SOCl2, ii) NH4OH; d) Ac2O, 120 °C; e) thiourea, EtOH; f) Et3N, DMF, BrCH2CN, then K2CO3; g) For 84, 1. CH(OMe)3, 130 °C 2. NH4OAc, 150 °C; For 85, PhCN, cat. KtOBu, DMSO, 200 °C. | ||
A summary of the LIMK1 inhibition of the synthesised compounds is given in Table 5. Substitution in the 2-position was detrimental to activity, as observed with 84 and 85. However, even though phenyl substitution in positions 6 (76), 8 (74) and 9 (73) did not give an increase in potency, the substitution was tolerated.
| # | Structure | IC50 (μM) | # | Structure | IC50 (μM) |
|---|---|---|---|---|---|
| a Assays performed as described in the supplementary information.† | |||||
| 73 |
|
5.5 | 77 |
|
0.26 |
| 74 |

|
5.7 | 83 |
|
0.70 |
| 75 |

|
0.30 | 84 |
|
43 |
| 76 |
|
6.1 | 85 |
|
>50 |
Conversely, a substantial increase in potency was observed with 7-phenyl substitution and the benzo compound 75 and its aza counterpart 83 returned respective IC50 values of 0.30 and 0.70 μM. Intriguingly, the indole-based scaffold 77 was also very active for such a simple compound (mw 274) with an IC50 of 0.26 μM.
Conclusions
In summary, from a HTS of a 40000 diverse compound library we identified the hit series of 3-aminothieno [2,3-b]pyridine-2-carboxamides as inhibitors of LIMK1. Guided by SAR, the active pharmacophore was transformed into a tricyclic 4-aminobenzothieno[3,2-d]pyrimidine with low micromolar activity. It was then determined that phenyl substitution in the 7-position led to sub-micromolar inhibitory activity. This level of activity was maintained with endocyclic carbon to nitrogen replacement and in cores containing an indole scaffold. These simple compounds form an excellent basis on which to further optimize physicochemical properties and potency.Acknowledgements
The authors acknowledge the financial support of the Cancer Therapeutics CRC, established and supported under the Australian Government's Cooperative Research Centres Program; NHMRC IRIISS grant number 361646 and Victorian State Government OIS grant.References
- LIMK stands for LIM-kinase, where LIM is an acronym of the three gene products Lin-11, Isl-1 and Mec-3.
- The PDZ domain is a common structural domain of 80–90 amino acids found in the signalling proteins of bacteria, yeast, plants, viruses and animals. PDZ is an acronym combining the first letters of the three proteins - post synaptic density protein (PSD95), Drosophila disc large tumor suppressor (DlgA), and zonula occludens-1 protein (zo-1) - which were first discovered to share the domain.
- H. Takahashi, H. Funakoshi and T. Nakamura, Cytogenet. Genome Res., 2003, 103, 290–298 CrossRef CAS.
- K. Yoshioka, V. Foletta, O. Bernard and K. Itoh, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 7247–7252 CrossRef CAS.
- O. Bernard, Int. J. Biochem. Cell Biol., 2007, 39, 1071–1076 CrossRef CAS.
- R. W. Scott and M. F. Olson, J. Mol. Med., 2007, 85, 555–568 CrossRef CAS.
- F. Manetti, Med. Res. Rev., 2011 DOI:10.1002/med.20230.
- P. Ross-Macdonald, H. de Silva, Q. Guo, H. Xiao, C.-Y. Hung, B. Penhallow, J. Markwalder, L. He, R. M. Attar, T.-a. Lin, S. Seitz, C. Tilford, J. Wardwell-Swanson and D. Jackson, Mol. Cancer Ther., 2008, 7, 3490–3498 CrossRef CAS.
- S. T. Wrobleski, S. Lin, K. Leftheris, L. He, S. P. Seitz, T.-a. Lin and W. Vaccaro, 2006 US Applic 0178388.
- B. E. Sleebs, I. P. Street, X. Bu and J. B. Baell, Synthesis, 2010, 1091–1096 CrossRef CAS.
-
H. A. Burgoon, N. C. Goodwin, B. A. Harrison, J. P. Healy, Y. Liu, R. Mabon, B. Marinelli, D. B. Rawlins, D. S. Rice and N. A. Whitlock, WO 2009264450 Chem Abstr 2009 151
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 491144 28–16.
491144 28–16. -
B. A. Harrison, S. D. Kimball, R. Mabon, D. B. Rawlins, D. S. Rice, M. V. Voronkov and Y. Zhang, WO 2009176829 CAN 150
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 237638 28–17 2009.
237638 28–17 2009. - B. A. Harrison, N. A. Whitlock, M. V. Voronkov, Z. Y. Almstead, K.-J. Gu, R. Mabon, M. Gardyan, B. D. Hamman, J. Allen, S. Gopinathan, B. McKnight, M. Crist, Y. Zhang, Y. Liu, L. F. Courtney, B. Key, J. Zhou, N. Patel, P. W. Yates, Q. Liu, A. G. E. Wilson, S. D. Kimball, C. E. Crosson, D. S. Rice and D. B. Rawlins, J. Med. Chem., 2009, 52, 6515–6518 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Details of assay protocols, synthetic procedures and compound characterisation. See DOI: 10.1039/c1md00137j |
| This journal is © The Royal Society of Chemistry 2011 |
