A pseudorotaxane umbrella thread with chloride transmembrane transport properties†‡
Christine
Chhun
and
Andreea R.
Schmitzer
*
Département de Chimie, Université de Montréal, CP 6128 Succursale Centre Ville, H3C3J7, Montréal, Québec, Canada. E-mail: ar.schmitzer@umontreal.ca
First published on 19th August 2011
Abstract
We present here the synthesis and the transmembrane transport properties of a cholic acid-functionalized pseudorotaxane thread. The anion transport properties of this amphiphilic thread can be changed by the assembly of a [2]-pseudorotaxane. Besides its interest and potential application as chloride transmembrane transporter, the amphiphilic properties of the umbrella thread presented here may be of interest to transport cyclic drugs across the cell membrane.
Membrane permeability is an important consideration in drug effectiveness and cell survival.1Ion transport across biological membranes in normal cells is ensured by proteins which provide specific pathways through this apolar barrier lined with polar groups, or by natural products. While the transport of cations and anions is essential to maintenance of the biological activity of a living cell, there are only few known anion-transporting natural products.2 In the past years a particular effort was devoted to the design and synthesis of synthetic anion transporters for fundamental understanding and practical applications, including conductance regulators.3Chloride transport by different classes of steroid-based cholapods1,4 was extensively studied. It was demonstrated that they act as mobile anion carriers. The rigid steroid unit of cholic acid generally improves the recognition process of anions in these cholapods.1,5 Bilayer-incorporated transmembrane channels for ion transport were also built on the cholic acid scaffold, where transmembrane ion-channels were formed by self-assembly and provided anion transport. Cholic acid derivatives have also been used for the synthesis of more flexible entities, as electroneutral molecular umbrellas or cationic steroids.6 In these examples, the facial amphiphilicity of cholic acid was used to shield the polar face of the molecule when inserted into the hydrophobic part of the phospholipid bilayer. Based on the use of cholic acid and spermine, a family of squalamine mimics with strong antibacterial activity against a broad spectrum of Gram-positive bacteria was synthesized.7 However, the structure-transport activity study of these compounds did not allow the authors to clearly demonstrate their assembly into pores or channels.
Beer et al. recently demonstrated Cl− templating in the synthesis of a pyridyl-containing macrocycle followed by assembly into a [2]-rotaxane or a [2]-catenane.8 The selective recognition of Cl− by interlocked entities and the current interest in development of transmembrane chloride transporters inspired our design and study of an umbrella thread as a synthetic membrane transporter. This amphiphilic thread presents the benefits of the membrane active molecular umbrellas developed by Regen et al.5,6,9 and the features required for the assembly of a pseudorotaxane with pyridyl-containing macrocycles; such an assembly can be used as a control element of the transmembrane activity, as we previously reported for imidazolium-based threads.10 The synthesis of the umbrella thread III containing two cholic acid ribs and a rigid core containing a quaternary ammonium recognition unit is shown in Scheme 1.
 | ||
| Scheme 1 Synthesis of compounds I–III and structure of macrocycle IV | ||
The template-directed condensation of 2,6-pyridinedicarboxaldehyde and tetraethyleneglycol bis(2-aminophenyl)ether for the synthesis of the pyridyl-containing macrocycle IV has previously been reported by Stoddart et al.11 To our knowledge this macrocycle has not been studied yet for its transport properties across membranes. To clarify the important structure-function relationship of our system with respect to membrane transport, we compared all different moieties of the umbrella thread for their ability to transport Cl− across phosphatidylcholine bilayers (Fig. 1).
 | ||
| Fig. 1 Relative activity of compounds I–IV in the lucigenin-based Cl- transport assay. Intravesicular conditions: 100 mM NaCl, 10 mM phosphate buffer, 2 mM lucigenin; extravesicular conditions: 100 mM NaNO3, 10 mM phosphate buffer (pH 6.4). 0.1 mM solutions of I, II, III and IV were injected at t = 50 s; aqueous 10% Triton X-100 was injected at t = 500 s. | ||
Firstly, we compared the anion transport properties of compounds I–IV in egg yolk L-alpha-phosphatidylcholine (EYPC) liposomes by using the lucigenin assay, a standard protocol in the evaluation of membrane-active transporters.2b,12Chloride efflux out of the vesicles was measured in this way, where the fluorescence traces show the change in emission when an aliquot of transporter is added to lucigenin-containing vesicles. In the typical fluorescence profile, a small burst in fluorescence is obtained after the injection of the transporter, followed by a dominant time-dependent increase. To our surprise, the rigid moiety of the umbrella thread I, containing a protonatable secondary amine is of itself a relatively slow Cl−transporter. However, the combination of I and II yields umbrella thread III an effective Cl−transport. This combination may change the global conformation of the transporter and its self-association properties. Secondly, compounds I–IV were tested using liposomes loaded with pH-sensitive 8-hydroxypyrene-1,3,6-trisulfonic acid trisodium salt (HPTS) (Fig. 2).13 In this assay the ion transport ability of the tested compounds is measured indirectly by monitoring the pH changes inside the liposome, but carried out under identical conditions as the lucigenin assay, it can be used to confirm the transport properties observed previously. In the absence of transmembrane pH gradient, the imposed anionic gradients obtained by the addition compounds I–IV, induces pH changes down the electrochemical potential gradient. Fig. 2 indicates that compounds I–IV are active in this assay, whereas compound IV has a different behaviour compared to compounds I–III, as at the end of the assay a second pH change can be observed. This result suggests that IV can easily cross the bilayer, especially when complexed with the anion, transporting simultaneously H+/Cl− by a symport process.
 | ||
| Fig. 2 Relative activity of compounds I–IV in the HPTS-based transport assay. Intravesicular conditions: 100 mM NaCl, 10 mM phosphate buffer, 0.1mM HPTS; extravesicular conditions: 100 mM NaNO3, 10 mM phosphate buffer (pH 6.4). 0.1 mM solutions of I, II, III and IV were injected at t = 50 s; aqueous 10% Triton X-100 was injected at t = 500 s. | ||
A detailed kinetic description requires more data and is under study in our group. However, the transport process of III is composed of a fast step, followed by a slower one, where the initial rate of chloride efflux induced by the umbrella thread III is concentration dependent (Fig. 3).
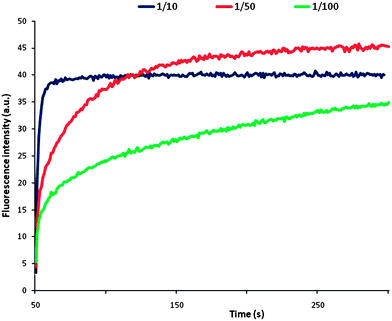 | ||
| Fig. 3 Chloride transport at different III/lipid ratios. | ||
Given the observed transport properties of III and IV individually and their recognition properties to form a [2]-pseudorotaxane by the complexation of IV on the quaternary ammonium site of III, it was of interest to investigate whether the [2]-pseudorotaxane possesses the same transport properties. The formation of the [2]-pseudorotaxane (followed by 1H NMR, see ESI) results in the blockage of the ammonium site, which becomes less available for Cl− complexation and transport. The residual Cl−transport properties of the [2]-pseudorotaxane is probably due to the complexation of Cl− by the pyridinium moiety of the macrocycle. As it can be observed in Fig. 4, the order of the addition of III and IV is not important and the transport properties of the obtained [2]-pseudorotaxane are identical to those of free IV, as well as the mechanically blocked [2]-rotaxane, used as a control for the [2]-pseudorotaxane formation (see ESI for the [2]-rotaxane synthesis). An interesting fluorescence quenching of lucigenin is observed when IV is added to III. As III itself has no effect on the lucigenin's fluorescence (see ESI), the obvious interpretation is that IV complexes the Cl− anions transported outside the liposome by III, and transport them back into the liposomes. By complexing the quaternary ammonium site on III, macrocycle IV releases Cl− in the bilayer or inside the liposomes.
![Relative activity of compounds III, IV, their complex and the mechanically blocked corresponding rotaxane in the lucigenin-based Cl−transport assay. Intravesicular conditions: 100 mM NaCl, 10 mM phosphate buffer, 2 mM lucigenin; extravesicular conditions: 100 mM NaNO3, 10 mM phosphate buffer (pH 6.4). 0.1 mM solutions of III or IV were injected at t = 300 s to form the [2]-pseudorotaxane.](/image/article/2011/MD/c1md00128k/c1md00128k-f4.gif) | ||
| Fig. 4 Relative activity of compounds III, IV, their complex and the mechanically blocked corresponding rotaxane in the lucigenin-based Cl−transport assay. Intravesicular conditions: 100 mM NaCl, 10 mM phosphate buffer, 2 mM lucigenin; extravesicular conditions: 100 mM NaNO3, 10 mM phosphate buffer (pH 6.4). 0.1 mM solutions of III or IV were injected at t = 300 s to form the [2]-pseudorotaxane. | ||
To gain an insight into the behavior of III in aqueous solution in the presence of a EYPC bilayer, we performed a computational study using the PM6/SCF-MO method for III, followed by a 200 ps molecular dynamics study in the presence of a bilayer of EYPC bilayer.14 As shown in Fig. 5A, after 19 ps, the umbrella thread III was adsorbed at the hydrophilic exterior surface of the bilayer, by favorable H-bonding interactions between the hydrophilic face of the cholic acid and the phosphate groups of the EYPC. After 50 ps (Fig. 5B), III completely spanned the bilayer with a concurrent flip of the umbrella structure where the cholic ribs expose the I moiety to the centre of the bilayer. A possible transport mechanism, in accordance with the one proposed by Kobuke et al.,15 is the formation of dimeric species of III in the bilayer (Fig. 5C), followed by their disassembly and the monomer acting as mobile carrier. Given the importance of this issue, further confirmation is required and extraction of EC5016 and the order reaction of the transport process may help to fully understand the transport mechanism with the umbrella thread and the [2]-pseudorotaxane.
 | ||
| Fig. 5 Molecular dynamics shaptshots at 19 ps (A) and 50 ps (B). Self association of compound III in hydrophobic media (C). | ||
Conclusions
In conclusion, we synthesized an amphiphilic pseudorotaxane umbrella thread containing an ammonium recognition site that can be used for transmembrane chloride transport. We also demonstrate that the assembly of a [2]-pseudorotaxane with this thread and a pyridyl-containing macrocycle can be used as a transmembrane transporter. These findings open the door to the assembly of amphiphilic [2]-rotaxanes where a biologically active macrocycle can be introduced in the rotaxane architecture and transported across the phospholipid bilayer.Notes and references
- P. R. Brotherhood and A. P. Davis, Chem. Soc. Rev., 2010, 39, 3633 RSC.
- (a) J. T. Davis, O. Okunola and R. Quesada, Chem. Soc. Rev., 2010, 39, 3843 RSC; (b) A. P. Davis, D. N. Sheppard and B. D. Smith, Chem. Soc. Rev., 2007, 36, 348 RSC; (c) S. K. Berezin and J. T. Davis, J. Am. Chem. Soc., 2009, 131, 2458 CrossRef CAS; (d) S. Hussain, P. R. Brotherhood, L. W. Judd and A. P. Davis, J. Am. Chem. Soc., 2011, 133, 1614 CrossRef CAS; (e) X. Li, B. Shen, X. Q. Yao and D. Yang, J. Am. Chem. Soc., 2009, 131, 13676 CrossRef CAS; (f) J. Mareda and S. Matile, Chem.–Eur. J., 2009, 15, 28 CrossRef CAS; (g) C. R. Yamnitz, S. Negin, I. A. Carasel, R. K. Winter and G. W. Gokel, Chem. Commun., 2010, 46, 2838 RSC; (h) N. J. Andrews, C. J. E. Haynes, M. E. Light, S. J. Moore, C. C. Tong, J. T. Davis, W. A. Harrell and P. A. Gale, Chem. Sci., 2011, 2, 256 RSC.
- G. W. Gokel and N. Barkey, New J. Chem., 2009, 33, 947 RSC.
- C. Goto, M. Yamamura, A. Satake and Y. Kobuke, J. Am. Chem. Soc., 2001, 123, 12152 CrossRef CAS.
- (a) P. Bandyopadhyay, V. Janout, L. H. Zhang and S. L. Regen, J. Am. Chem. Soc., 2001, 123, 7691 CrossRef CAS; (b) P. Bandyopadhyay, V. Janout, L. H. Zhang, J. A. Sawko and S. L. Regen, J. Am. Chem. Soc., 2000, 122, 12888 CrossRef CAS.
- (a) M. Merritt, M. Lanier, G. Deng and S. L. Regen, J. Am. Chem. Soc., 1998, 120, 8494 CrossRef CAS; (b) W. H. Chen, V. Janout, M. Kondo, A. Mosoian, G. Mosoyan, R. R. Petrov, M. E. Klotman and S. L. Regen, Bioconjugate Chem., 2009, 20, 1711 CrossRef CAS; (c) V. Janout, C. Di Giorgio and S. L. Regen, J. Am. Chem. Soc., 2000, 122, 2671 CrossRef CAS; (d) V. Janout, B. W. Jing and S. L. Regen, Bioconjugate Chem., 2002, 13, 351 CrossRef CAS; (e) V. Janout, B. W. Jing and S. L. Regen, J. Am. Chem. Soc., 2005, 127, 15862 CrossRef CAS; (f) V. Janout, B. W. Jing, I. V. Staina and S. L. Regen, J. Am. Chem. Soc., 2003, 125, 4436 CrossRef CAS; (g) V. Janout, M. Lanier, G. Deng and S. L. Regen, Bioconjugate Chem., 1997, 8, 891 CrossRef CAS; (h) V. Janout and S. L. Regen, J. Am. Chem. Soc., 2005, 127, 22 CrossRef CAS; (i) V. Janout, I. V. Staina, P. Bandyopadhyay and S. L. Regen, J. Am. Chem. Soc., 2001, 123, 9926 CrossRef CAS; (j) V. Janout, L. H. Zhang, I. V. Staina, C. Di Giorgio and S. L. Regen, J. Am. Chem. Soc., 2001, 123, 5401 CrossRef CAS; (k) M. Mehiri, W. H. Chen, V. Janout and S. L. Regen, J. Am. Chem. Soc., 2009, 131, 1338 CrossRef CAS; (l) M. Mehiri, B. Jing, D. Ringhoff, V. Janout, L. Cassimeris and S. L. Regen, Bioconjugate Chem., 2008, 19, 1510 CrossRef CAS.
- W. H. Chen, X. B. Shao, R. Moellering, C. Wennersten and S. L. Regen, Bioconjugate Chem., 2006, 17, 1582 CrossRef CAS.
- (a) L. M. Hancock and P. D. Beer, Chem.–Eur. J., 2009, 15, 42 CrossRef CAS; (b) L. M. Hancock, L. C. Gilday, S. Carvalho, P. J. Costa, V. Felix, C. J. Serpell, N. L. Kilah and P. D. Beer, Chem.–Eur. J., 2010, 16, 13082 CrossRef CAS; (c) K. Y. Ng, A. R. Cowley and P. D. Beer, Chem. Commun., 2006, 3676 RSC.
- (a) G. Deng, T. Dewa and S. L. Regen, J. Am. Chem. Soc., 1996, 118, 8975 CrossRef CAS; (b) G. Deng, M. Merritt, K. Yamashita, V. Janout, A. Sadownik and S. L. Regen, J. Am. Chem. Soc., 1996, 118, 3307 CrossRef CAS; (c) A. Sadownik, G. Deng, V. Janout, S. L. Regen, E. M. Bernard, K. Kikuchi and D. Armstrong, J. Am. Chem. Soc., 1995, 117, 6138 CrossRef CAS.
- C.-R. Elie, N. Noujeim, C. Pardin and A. R. Schmitzer, Chem. Commun., 2011, 47, 1788 RSC.
- F. Arico, T. Chang, S. J. Cantrill, S. I. Khan and J. F. Stoddart, Chem.–Eur. J., 2005, 11, 4655 CrossRef CAS.
- (a) B. A. McNally and B. D. Smith, Abs. Pap. Am. Chem. Soc., 2005, 230, U543 Search PubMed; (b) B. A. McNally, M. Yamashita, A. Engh and M. Prakriya, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 22516 CrossRef CAS; (c) V. Sidorov, F. W. Kotch, J. L. Kuebler, Y. F. Lam and J. T. Davis, J. Am. Chem. Soc., 2003, 125, 2840 CrossRef CAS.
- V. Sidorov, F. W. Kotch, G. Abdrakhmanova, R. Mizani, J. C. Fettinger and J. T. Davis, J. Am. Chem. Soc., 2002, 124, 2267 CrossRef CAS.
- J. J. P. Stewart, Stewart Computational Chemistry, Version 7.213W Search PubMed.
- M. Yoshii, M. Yamamura, A. Satake and Y. Kobuke, Org. Biomol. Chem., 2004, 2, 2619 CAS.
- R. E. Dawson, A. Hennig, D. P. Weimann, D. Emery, V. Ravikumar, J. Montenegro, T. Takeuchi, S. Gabutti, M. Mayor, J. Mareda, C. A. Schalley and S. Matile, Nat. Chem., 2010, 2, 533 CrossRef CAS.
Footnotes |
| † We are grateful to the Natural Sciences and Engineering Research Council of Canada, the Fonds Québécois de la Recherche sur la Nature et les Technologies, the Canada Foundation for Innovation and Université de Montréal for financial support. |
| ‡ Electronic supplementary information (ESI) available: Synthetic procedures, NMR spectra, liposome preparation and anion transport studies. procedures and characterization data. See DOI: 10.1039/c1md00128k |
| This journal is © The Royal Society of Chemistry 2011 |
