Pharmacological properties of indolone-N-oxides controlled by a bioreductive transformation in red blood cells?
Hany
Ibrahim
ab,
Antonella
Pantaleo
c,
Francesco
Turrini
c,
Paolo
Arese
c,
Jean-Pierre
Nallet
d and
Françoise
Nepveu
*ab
aUniversité de Toulouse, UPS; UMR 152 (PHARMA-DEV), F-31062, Toulouse Cédex 9, France. E-mail: nepveu@cict.fr; Fax: +33 5 62 25 98 02; Tel: +33 5 62 25 6869
bIRD, UMR 152, F-31062, Toulouse Cédex 9, France
cUniversity of Turin Medical School (Dipartimento di Genetica, Biologia e Biochimica), Via Santena 5 bis, 10126, Torino, Italy
dIDEALP-PHARMA, Bâtiment CEI, 66 Bd Niels Bohr, BP 2132, 69603, Villeurbanne Cédex, France
First published on 18th July 2011
Abstract
Indolone-N-oxides, long known for their biological activities, possess remarkable anti-infectious properties. With the aim of improving the pharmacological and antimalarial properties of indolone-N-oxide derivatives (INODs), 6-(4-chlorophenyl)-7H-[1,3]dioxolo[4,5-f]indol-7-one-5-oxide, compound 1, was selected to study its penetration and biotransformation in red blood cells (RBC) in vitro. Compound 1 accumulated inside RBCs and was rapidly bio-transformed giving a major fluorescent metabolite, the dihydroanalogue, 1-HH, identified after extraction, through LC-MS and NMR analyses. This bioreductive transformation was (i) observed with other INOD derivatives (ii: 1–7); (ii) observed in normal, β-thalassemic and Plasmodium falciparum infected RBCs; (iii) temperature and thiol-dependent; (iv) not observed with heat-denatured RBCs, suggesting an enzyme-dependent biotransformation. The dihydro form, 1-HH, has antiplasmodial activity but lower than the parent compound. Since the RBCs represent 99% of the total cellular space of blood in humans, this leads to extensive metabolism of indolone-N-oxide type compounds. Given the redox events occurring in Plasmodium infected RBCs, this bioreductive transformation may be pivotal for parasite redox balance and antiplasmodial activity. However, it may be a drawback when other pharmacological properties of INODs are investigated. These results show the importance of RBCs as an in vitro model to study the biotransformation of drugs, especially antimalarial drugs in the early discovery stages.
Introduction
Indolone-N-oxides have biological activities that have been regularly reported. In mammalian systems they inhibit the synthesis of adenosine triphosphate (ATP) in mitochondria.1In vitro, indolone-N-oxides show antibacterial2,3 and antifungal activities,4 elicit smooth muscle relaxation,5,6 and are neuroprotective.7 These compounds, containing nitrone functional group, are capable of trapping hydroxyl and superoxide radicals.8 Despite these pharmacological properties, few investigations in vivo have been reported and no pharmaceuticals have been launched.As a part of our search for new lead compounds for the treatment of malaria, we recently showed that indolone-N-oxide derivatives (INODs) have antimalarial activity.9 This new pharmacological activity gave rise to chemical optimization to develop new hits and leads (Fig. 1).9 In parallel we explored some ADMET properties with the binding affinity of INODs to human serum albumin10 and cell-based metabolism studies. To study the cellular biotransformation of INODs, human red blood cells (RBCs) were selected, as the erythrocytic stages of the Plasmodium parasites are determinant in the pathology of malaria in humans. It is worth noting that human RBCs are not a common cell model for metabolic studies as they are devoid of endoplasmic reticulum, cytochrome P450 isozymes, nuclei and mitochondria.11,12 For metabolite identification, the chromatographic and spectroscopic data obtained for compound 1 were compared with those of other chlorinated and carbon-13 labelled derivatives of the series. In addition, compound 1 was selected because its metabolite, 1-HH, had significant fluorescent properties that were helpful for its identification and quantification at trace levels using LC-Fluorescence, as fluorescence detection has the advantages of higher selectivity and sensitivity, notably in complex media.13
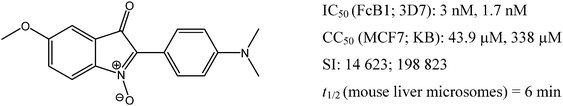 | ||
| Fig. 1 Structure and biological properties of the “hit” compound 6; IC50 is the drug concentration needed to obtain 50% parasite growth-inhibition, evaluated against Pf strains (FcB1, 3D7); CC50 is the drug concentration which causes a 50% decrease in cell viability, evaluated against two cell lines (MCF7, KB); SI (selectivity index) is the ratio CC50/IC50; t1/2 is the half-life with mouse hepatic microsomes, expressed in min. | ||
The interaction between INODs and RBCs has never been reported, in spite of the large number of in vitro biological studies reported in the literature for these compounds. We present here the first results on the interaction between indolone-N-oxides and human erythrocytes and analyze the factors affecting this interaction, as well as characterizing the major metabolite and evaluating its biological activity. We also demonstrate the relevance of RBCs as an in vitro model to study the early biotransformation of xenobiotics, especially antimalarial drugs.
Results and discussion
Identification of the major metabolite by LC-MS in normal human RBCs
When we were studying the penetration and the partition coefficient of compound 1 by determining the ratio of its concentration in RBCs to that in the equilibrating plasma,12,14 it was difficult to reach a steady state equilibrium between the plasma and RBC due to the rapid metabolism of the compound. These experiments led to the discovery of a major metabolite resulting from the intracellular biotransformation of compound 1, a finding that oriented the work towards the identification of the metabolites and the factors affecting the rate of the biotransformation. The identification of the metabolites made it possible to study the kinetics of the penetration of the compounds into the RBCs. Fig. 2 shows the structure of compound 1 and its major metabolite, the corresponding dihydro-compound, 1-HH.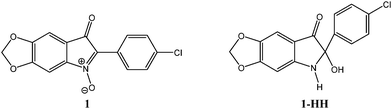 | ||
| Fig. 2 Chemical structure of compound 1 and its metabolite 1-HH. | ||
Metabolites of compounds 1–7 (Table 1) were obtained after incubation in normal RBCs as described in the Experimental section. The use of structurally related analogues either chlorinated (compounds 1, 2, 3) (natural relative abundance of 35Cl/37Cl = 75.8/24.2) or cold-labelled with 13C (compound 5) was a convenient way to follow up their analysis by MS. Table 1 gives the retention times, detected molecular mass and UV absorption maxima of the parent compounds and the major metabolites.
| Compound ii | Chemical structure | Molecular mass | UV λmax/nm | t R a/min | Metabolite ii-HH | UV λmax/nm | t r a/min | |
|---|---|---|---|---|---|---|---|---|
| a Chromatographic retention time in min. | ||||||||
| Chlorinated compounds | 1 |

|
301 | 296 | 18.2 | 303 | 252, 414 | 9.6 |
| 2 |

|
291 | 290, 440 | 20.9 | 293 | 230, 409 | 11.2 | |
| 3 |

|
301 | 291, 487 | 22.6 | 303 | ND | 10.4 | |
| Labelled compounds | 4 |

|
345 | 290, 498 | 23.5 | 347 | 433, 340 | 11.3 |
| 5 |

|
357 | 359 | |||||
| Non-chlorinated, non-labeled compounds | 6 |

|
296 | 334, 254, 586 | 14.0 | 298 | 271 | 8.2 |
| 7 |

|
303 | 271, 303, 485 | 17.4 | 305 | 333 | 9.9 |
The metabolites are less retained on reversed phase chromatography (C-18), with a retention time two times less than the parent compound (Fig. 3), indicating that the metabolite was more polar than the parent compound. This observation is very important for the analysis of the differences in the partition of the parent compounds and metabolites between plasma and RBCs.
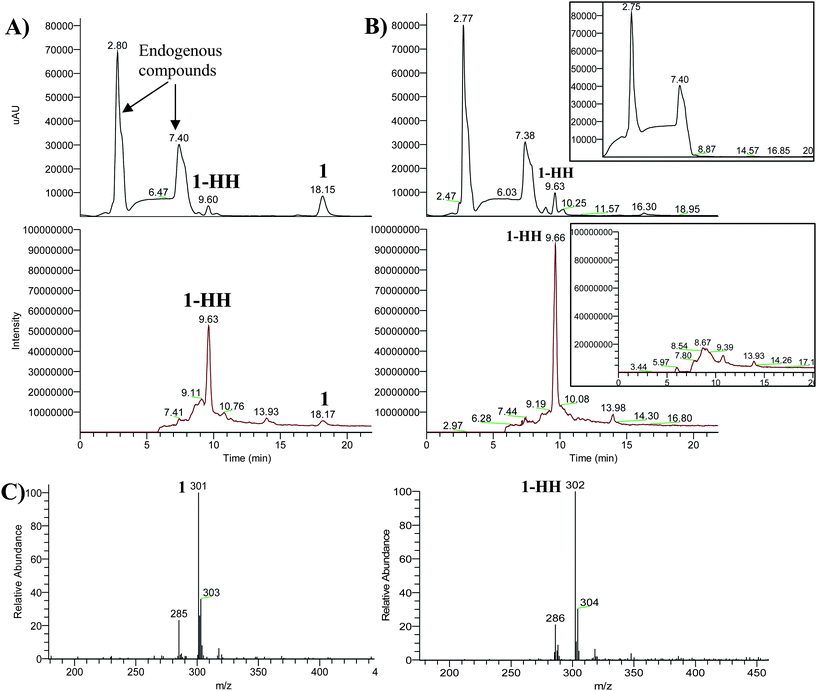 | ||
| Fig. 3 Chromatograms obtained after incubation of compound 1 in healthy RBCs, (A) at the beginning (t = 0), (B) after 4 h; top chromatograms: photodiode array detector total scan; the middle chromatograms: mass detector total ion current (−)APCI/MS. The inset in each window shows the chromatogram of the blank without compound 1. (C) Mass spectra of compound 1 and of its reduced form 1-HH. | ||
The parent compounds and metabolites had different UV absorption maxima (Table 1) that may indicate that the conjugated system of the molecule was changed. X-Ray crystallography of a similar compound (2-phenylisatogen) shows that the isatogen ring and the phenyl ring are almost fully co-planar.15,16 Therefore, the observed color change from deep pink for compound 1 to pale greenish yellow for compound 1-HH was certainly due to a loss of conjugation, which implies a loss of planarity. Moreover, compound 1-HH was fluorescent while compound 1 was not, which could be attributed to the disappearance of the nitrone function (electron withdrawing group with high dipole moment, quencher). The fluorescent properties of the metabolites will be helpful for the forthcoming experiments as fluorescence detection has the advantages of higher selectivity and sensitivity.
All the compounds tested (ii: 1–7) were metabolised in the same manner, giving one major reduced metabolite (ii-HH) (Table 1). Compound 1 was then chosen as a representative example of the indolone-N-oxide series for further investigations, full structural identification, kinetics of penetration into RBCs and parameters affecting the bioreductive transformation.
Tentative structural MS analysis was done using a deuterated protic solvent and it revealed that the major metabolite contained two labile hydrogen atoms (Table 2). The final structure was confirmed with NMR and HR-MS after bio-extraction of the metabolite 1-HH from human RBCs.
| Compound/solvent | Base peak ion (m/z) | Possible corresponding ion species structure | Corresponding molecular mass |
|---|---|---|---|
(1-HH)/(CH3OH/0.1% aq. formic acid, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v) 50, v/v) |
302 | [–N(H)–C(OH)–H]− | 303 |
(1-HH)/(CD3OD/deuterated 0.1% aq. formic acid, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v) 50, v/v) |
303 | [–N(D)–C(OD)–D]− | 305 |
| [–N(D)–C(OH)–H]− | 304 | ||
| [–N(H)–C(OD)–H]− | 304 |
From these results a metabolic chemical pathway is proposed (Fig. 4) for the bio-reduction of compound 1 to compound 1-HH. This may occur via either deoxygenation followed by hydration, or epoxide formation followed by hydrogenation. However, the two intermediates could not be isolated from the culture media, which may be the result of a rapid intracellular reduction.
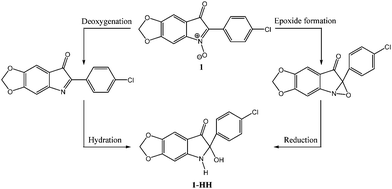 | ||
| Fig. 4 Proposed intracellular chemical pathway of the metabolite 1-HH formation. | ||
Kinetics of the uptake of compound 1 by RBC
The kinetic profile of the biotransformation of compound 1 in human RBCs is shown in Fig. 5. Peak areas were used to represent levels of compound 1 and its metabolite 1-HH. The results indicate that compound 1 is rapidly taken up by RBCs, as at t = 0 min the intracellular compound 1 level was about ten times higher than that of the extracellular one. The compound 1 level decreased with time at the tested concentration, and its extracellular level was depleted rather rapidly compared with its intracellular level. The increase in extracellular metabolite level was time-dependent, indicating that compound 1-HH was formed intracellularly and then diffused through the cell membrane to the extracellular compartments as shown in our proposed model (Fig. 6). To confirm that the biotransformation of compound 1 occurred within the cellular compartment (intracellularly), a control experiment (without the addition of RBC) was carried out in parallel. No formation of the metabolite, 1-HH, was observed. The latter experiment confirmed that the metabolite was formed only in the intracellular space and then expelled to the outside of the cell.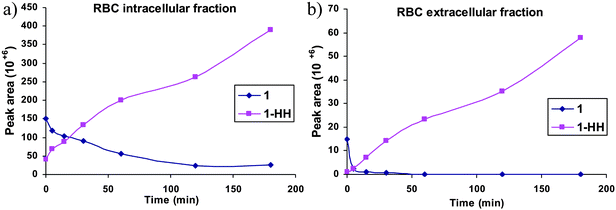 | ||
| Fig. 5 Kinetic profile of the biotransformation of compound 1 and its metabolite 1-HH: (a) in the intracellular (RBC) and (b) in the extracellular medium; quantitative data obtained by LC-PDA-MS. Each point represents the mean of three independent experiments with a relative SD less than 10%. | ||
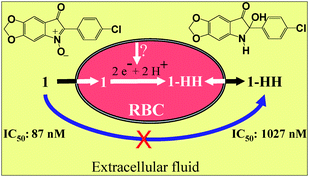 | ||
| Fig. 6 Proposed model: compound 1 is transported across the RBC membrane and accumulated in the intracellular space. In the cytosol, compound 1 is reduced rapidly and irreversibly to its dihydroanalogue, 1-HH, which is expelled to the extracellular space. The reduced form, less hydrophobic, shows lower antiplasmodial activity in vitro against P. falciparum strain (FcB1). | ||
Antiplasmodial activity in vitro of the major metabolite 1-HH extracted from RBCs and purified
The metabolite 1-HH has been isolated from RBCs by extraction and tested on Pf infected RBCs. At this point, it is worth noting that this family of compounds show antiplasmodial activity in the low nanomolar range.9 The purpose of this work was to show that this series of compounds is rapidly reduced upon contact with human RBCs and that RBCs are not inert towards drugs. In addition, the in vitro evaluation of antiplasmodial activities needs to be done with RBCs because they are the cellular host for Plasmodium parasites. During the parasite culture, the compounds are reduced rapidly and totally within 4 hours while the parasitic cycle (incubation time) is 48 h (Pf). Moreover, these compounds are reduced in the RBC cytoplasm before reaching the parasitic membrane. As the intracellular reduction is very fast, the isolated reduced form should exhibit comparable biological activity, but the results (Table 3) show that the IC50 was about 10 times higher than that of the parent compound. These results deserve some comments. Firstly, both oxidized and reduced forms are active against Pf. Secondly, the difference in the IC50 is certainly related to the partitioning of the two forms between the intra- and extracellular media of the assay in vitro. The indolone-N-oxide form has a strong dipolar moment (N+–O−), is very lipophilic with a high affinity for RBCs, and concentrates inside the cells (unidirectional passage) (see Fig. 6). On the other hand, the bio-reduced form is more hydrophilic, lacks the strong N-oxide dipole, and therefore distributes inside and outside the cell (bidirectional passage). Thus the real level of the reduced form never reaches the level of the parent form inside the RBC leading to a higher IC50 in the assay used here in vitro. Compound 1 has cytotoxicity against MCF-7 slightly higher than its metabolite 1-HH (Table 3), which may be attributed to its hydrophobicity.Factors affecting the bioreductive transformation
Fig. 7 shows the rate of compound 1-HH formation in normal, NEM-treated and CDNB-treated RBCs. The rate of compound 1 biotransformation in either NEM-treated or CDNB-treated RBCs was much slower than the non-treated ones. Therefore, intracellular thiols, especially GSH, play an important role in the erythrocytic bioreduction.
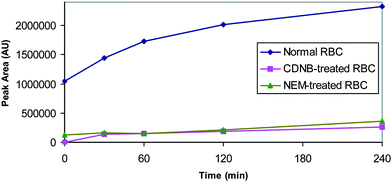 | ||
| Fig. 7 The rate of the metabolite (1-HH) formation in normal, NEM-treated and CDNB-treated RBCs; quantitative data obtained by HPLC-fluorescence. Each point represents the mean of two independent experiments with a relative SD less than 5%. AU = arbitrary units. | ||
Haemoglobin types
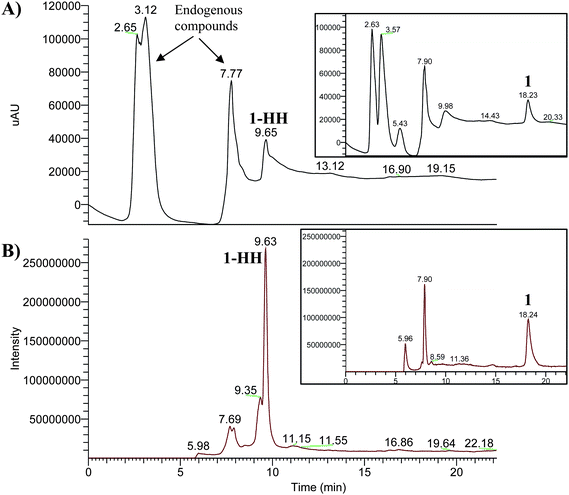 | ||
| Fig. 8 Chromatograms obtained after incubation in parasitized RBC for 4 h, (A) photodiode array detector, total scan, (B) mass detector, total ion current (−)APCI/MS. The inset in each window shows the corresponding chromatogram of compound 1 after its incubation with the culture medium without RBCs. | ||
In addition, the bioreductive metabolite 1-HH could not be detected with commercial haemoglobin or its equivalents such as lyophilized human haemoglobin, haemin, haematin, or ferrous iron. Therefore, neither haemoglobin nor the heme-iron influenced the RBC reductive metabolism.
From a biochemical point of view, the bioreduction of compound 1 to compound 1-HH is in agreement with the biochemical composition of healthy RBCs (glutathione, reducing agents, antioxidant proteins), which is a reductive medium to control any oxidative stress in RBCs. From a chemical point of view, the indolone-N-oxides, containing a nitrone functionality, may trap free radicals (generated during the catabolism of haemoglobin in parasitized RBCs) giving radical adducts. However, stable radical adducts could not be detected from parasitized RBCs under our experimental conditions. Moreover, neither compound 1 nor compound 1-HH can undergo an oxidation, except a hydroxylation on the aromatic ring that generally occurs at the para-position. This para-position is blocked in compound 1 by the presence of a chlorine atom. Another possible chemical transformation is the addition of a water molecule across the nitrone functional group of compound 1, but this hydrated form was not detected.
Metabolism of compound 1-HH by liver microsomes
When compound 1 was incubated with microsomes, compound 1-HH was never detected, which confirms that RBCs are metabolically different from microsomes. When tested separately in microsomes, the half-lives9 were <1 and 10 min for compounds 1 and 1-HH, respectively. Thus the reduced form was relatively more stable than its parent compound in the presence of mouse liver microsomes.Conclusion
We have described the bio-reduction of indolone-N-oxides in RBCsin vitro in relation to the antimalarial properties of the series. The RBC cytosol is the sub-cellular compartment where the bio-reduction occurs which is an enzyme-dependent process. The metabolism of compound 1 in RBCs is very rapid and is a thiol-dependent process. The lower antiplasmodial activity of metabolite 1-HH compared to the parent compound may be due to its hydrophilicity and its partition between the intra- and extra-cellular compartments. The bioreduction of this family of compounds in RBCs requires further studies aimed at characterizing the enzyme(s) possibly involved and how these molecules cross the cell membrane (either via specific channels, passive diffusion along a concentration gradient or active transport by a protein carrier), also how the metabolite (1-HH) permeates across the cell membrane. The identification of this bio-reduction should be included in any subsequent studies aimed at identifying the target, and the site and mechanism of action in the parasite.These results are important for the forthcoming development of indolone-N-oxide structure-based drug design. Actually, there is extensive metabolism of these compounds when they reach the systemic circulation, because RBCs represent 99% of the total cellular space of blood in humans. The intra-erythrocytic reduction may represent the key point into the antiplasmodial activity of this family. Given the redox events occurring in Plasmodium infected RBCs, this bioreductive transformation may be pivotal for the parasite redox balance and antiplasmodial activity which may be very different from other pharmacological targeting. While the intra-erythrocytic reduction may be considered positive with respect to the antimalarial pharmacological activity (the RBC is the pharmacological (cellular) target site in this case), the effect of rapid bioreduction may be negative regarding other pharmacological properties, due to the extensive drug metabolism when the drug reaches the systemic circulation (whatever the route of administration: p.o, i.m, i.p., s.c., i.v.). This may prevent the drug from attaining its pharmacological target. These results confirm that RBCs are not inert towards drugs and that interaction with RBCs should be studied in the early drug discovery stages.
Experimental section
Reagents, solutions and cells
INODs were prepared as previously reported.9 Reagents were purchased from Sigma (St Quentin, France) unless otherwise indicated in the text. Methanol and acetonitrile (ACN) were of HPLC grade (Fischer, Illkirch, France). All aqueous solutions were prepared using high-purity water obtained from a Milli Q® water purification system (Millipore, St Quentin, France). A glass vacuum-filtration apparatus was employed for the filtration of the aqueous solutions, using 0.20 μm membrane filters (Millipore). Dissolution of compounds was enhanced by sonication in an ultrasonic bath (Elma®, Germany). A small vortex mixer and a centrifugation system Eppendorf AG® 5804R (Hamburg, Germany) were used for sample pre-treatment. RPMI 1640 with L-glutamine was purchased from Cambrex Bio Science Verviers, and the LD column from MACS®, France. Human RBC and serum were obtained from “Etablissement Français du Sang”, Purpan, Toulouse. Human RBCs were washed twice with RPMI and centrifuged at 2000 rpm for 15 min and kept at 50% haematocrit in RPMI at 4 °C until use (for a maximum period of 21 days).LC-MS analysis
A LC-PDA-MSn system was used (Thermo electron Corporation and Spectra system (SS)) including an automatic injector with an oven (SS-AS3000), a degasser (SS-SCM1000), and a quaternary pump (SS-P1000 XR) coupled to a photodiode array detector PDA (SS-UV6000LP), and an ion trap mass spectrometer (Finnigan LCQ Deca XP Max). Nitrogen was used as a nebulizing and drying gas. Data acquisition was carried out using Finnigan Xcalibur software (version 1.4). The atmospheric pressure chemical ionization (APCI) source was used in the negative ion mode that generates less chemical noise than the positive mode and therefore offers a better selectivity and sensitivity, notably for this family of compounds. Mass scans were performed in the range m/z 50–650. The chromatographic separation was done on an analytical column Luna® C-18 (5 μm, 250 mm × 4.6 mm) using a C-18 pre-column (5 μm, 4.6 mm × 3 mm) (Phenomenex, France). The separation was done in gradient mode using solvent A (water) and solvent B (CH3OH). The gradient program was the following: at t = 0–3 min; solvents (95% A/5% B, v/v); at t = 4–20 min, solvents (15% A/85% B, v/v); at t = 22 min, solvents (95% A/5% B, v/v); the column regeneration time was 10 min and the mobile phase flow rate was 1 ml min−1. The analysis was performed at room temperature (RT), with 20 μl of sample injected. The gradient elution was started with 95% water to eliminate the polar compounds naturally present in the biological matrix (to prevent interference and contamination with the MS system and analysis), while the MS was started 5 min after the beginning of the gradient program. As the major metabolite was fluorescent, another chromatographic system with a fluorescence detector (FL) was also used. The HPLC-FL system (Waters Corp. (W), Milford, MA) consisted of an automatic injector (W-717 plus), a degasser, a quaternary pump (W-600), and an oven coupled to a fluorescence detector (W-474). Data acquisition was carried out using Waters Millenium32 software, version 3.2.Metabolism and kinetics of compound 1 uptake by normal RBCs
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm at 4 °C for 10 min to stop drug uptake and to separate the RBC pellet from the culture medium. The RBC pellet was lysed by two cycles of freezing/thawing.
000 rpm at 4 °C for 10 min to stop drug uptake and to separate the RBC pellet from the culture medium. The RBC pellet was lysed by two cycles of freezing/thawing.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min, to separate the precipitated protein. The supernatants were directly injected into the LC-MS.
000 rpm for 5 min, to separate the precipitated protein. The supernatants were directly injected into the LC-MS.
Structural identification of the metabolite, 1-HH
1H and 13C NMR spectra were recorded on an AC Bruker spectrometer at 500 MHz (1H) and 125 MHz (13C) using CD3CN as solvent. Chemical shifts (δ) are reported in parts per million (ppm) relative to tetramethylsilane (0 ppm) and the following multiplicity abbreviations were used: s, singlet; br, broad; d, doublet; dd, double of doublet. For high resolution mass spectrometry, an electrospray ionization-quadrupole-time of flight analysis (ESI-Q-TOF MS) was carried out in the negative ion mode, using a Q-TOF Ultima Instrument (Waters, Milford, MA, USA). IR spectra were recorded on a Perkin-Elmer PARAGON 1000 FT-IR spectrometer. Structural features of compound 1-HH: 6-(4-Chlorophenyl)-6-hydroxy-5,6-dihydro-[1,3]dioxolo[4,5-f]indol-7-one. UV (CH3OH/H2O, 85/15, v/v) λmax nm: 252. IR (CH2Cl2) cm−1: 3354, 2928, 2854, 1682, 1628, 1398, 1291, 1143. 1H NMR (CD3CN, 500 MHz): δ ppm 6.51 (s, 1H), 6.84 (s, 1H), 7.49 (dd, 2H), 7.38 (dd, J = 2 Hz, J = 9 Hz; 2H), 6.04 (dd, J = 2 Hz, J = 9 Hz; 2H), 6.32 (br s, 1H, NH). 13C NMR (CD3CN, 125 MHz): δ ppm 88.68 (C), 93.0 (CH), 102.0 (CH), 103.0 (CH2), 109.47 (C), 128.74 (CH× 2), 129.10 (CH× 2), 133.7 (C), 138.24 (C), 142.68 (C), 157.49 (C), 160.19 (C), 196.73 (C). MS-(−)APCI, m/z: 302 [M–H]−. HR-MS-ESI: m/z [M–H]− calcd for C15H9NO4Cl: 302.0220, found: 302.0218.Antiplasmodial activity of 1-HHmetabolite
The isolated and purified metabolite (1-HH) was screened for in vitro antiplasmodial activity against the Pf strain (FcB1), and cytotoxicity against MCF-7 cells as previously reported.9Factors affecting the biotransformation in normal human RBCs
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min at 20 °C. The supernatant solutions were subjected to HPLC-FL analysis using the same chromatographic conditions as described under LC-MS analysis. The blank experiments were done without addition of compound 1.
000 rpm for 10 min at 20 °C. The supernatant solutions were subjected to HPLC-FL analysis using the same chromatographic conditions as described under LC-MS analysis. The blank experiments were done without addition of compound 1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm (21
000 rpm (21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g) for 10 min at 4 °C. The supernatant (cytosol) was either used directly to assess drug metabolites or separated on a Sepharose column, pre-conditioned with MilliQ water, to obtain the purified haemoglobin fraction for haemoglobin–drug interaction studies. The residue containing RBC membranes (RBC ghosts) was washed five times with haemolysing buffer (5 mM sodium phosphate and 1 mM EDTA, pH 8.0) solution until the residue was almost white indicating extensive haemoglobin removal. The centrifugation steps were carried out at 14
000g) for 10 min at 4 °C. The supernatant (cytosol) was either used directly to assess drug metabolites or separated on a Sepharose column, pre-conditioned with MilliQ water, to obtain the purified haemoglobin fraction for haemoglobin–drug interaction studies. The residue containing RBC membranes (RBC ghosts) was washed five times with haemolysing buffer (5 mM sodium phosphate and 1 mM EDTA, pH 8.0) solution until the residue was almost white indicating extensive haemoglobin removal. The centrifugation steps were carried out at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min at 4 °C. Compound 1 was added separately to each fraction, the cytoplasmic, the purified haemoglobin and the ghost fractions and incubated for 3 h at 37 °C. A qualitative analysis was done to detect the metabolite formation. For each fraction, 100 μl of the sample were treated with 200 μl of ACN, vortexed for 30 s and centrifuged at 14
000 rpm for 10 min at 4 °C. Compound 1 was added separately to each fraction, the cytoplasmic, the purified haemoglobin and the ghost fractions and incubated for 3 h at 37 °C. A qualitative analysis was done to detect the metabolite formation. For each fraction, 100 μl of the sample were treated with 200 μl of ACN, vortexed for 30 s and centrifuged at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm (21
000 rpm (21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g) for 10 min at 4 °C. The supernatant fractions were subjected to LC-MS analysis.
000g) for 10 min at 4 °C. The supernatant fractions were subjected to LC-MS analysis.
Metabolism of compounds by liver microsomes
Liver microsomes (pooled from male mice (CD-1) 20 mg protein per ml in 250 mM sucrose, stored at −80 °C) were quickly thawed at 37 °C using a water bath and kept on ice until use. NADPH (50 mM) was prepared in PBS buffer solution. Due to the poor aqueous solubility, DMSO stock solutions of the test compounds were prepared at 2.2 mM concentration. The final concentration of DMSO was 1%. 12.5 μl of microsomal suspension were added to 464 μl PBS solution followed by 3.5 μl of test solution. The mixture was pre-incubated at 37 °C for 5 min before the addition of the cofactor NADPH (20 μl) and then the solution was incubated at 37 °C for 10 min with gentle shaking. The reaction was quenched by adding 500 μl of cold acetonitrile followed by centrifugation at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min at 20 °C. The supernatants were then analysed by LC-FL.
000 rpm for 10 min at 20 °C. The supernatants were then analysed by LC-FL.
Acknowledgements
This work was supported by the European Union (Redox antimalarial drug discovery, Read-Up, FP6-2004-LSH-2004-2.3.0-7, Strep no. 018602). We thank E. Augugliaro, P. Perio, S. Maurel-Chevalley and E. Pelissou for technical assistance.References
- A. J. Sweetman, A. P. Green and M. Hooper, Biochem. Biophys. Res. Commun., 1974, 58, 337 CrossRef CAS.
- M. Hooper, D. A. Patterson and D. G. Wibberley, J. Pharm. Pharmacol., 1965, 17, 734 CrossRef CAS.
- J. Slatt and J. Bergman, Tetrahedron, 2002, 58, 9187 CrossRef CAS.
- H. Hagen, R. D. Kohler and E. H. Pommer, EP. Pat., 54147, Chem. Abstr., 1982, 97, 216185.
- H. E. Foster, M. Hooper, M. Spedding, A. J. Sweetman and D. F. Weetman, Br. J. Pharmacol., 1978, 63, 309 CAS.
- H. Foster, M. Hooper, S. H. Imam, G. S. Lovett, J. Nicholson, C. J. Swain, A. J. Sweetman and D. F. Weetman, Br. J. Pharmacol., 1983, 79, 273 CAS.
- K. Menton, M. Spedding, P. Gressens, P. Villa, T. Williamson and A. Markham, Eur. J. Pharmacol., 2002, 444, 53 CrossRef CAS.
- F. Nepveu, J.-P. Souchard, Y. Rolland, G. Dorey and M. Spedding, Biochem. Biophys. Res. Commun., 1998, 242, 272 CrossRef CAS.
- F. Nepveu, S. Kim, J. Boyer, O. Chatriant, H. Ibrahim, K. Reybier, M.-C. Monje, S. Chevalley, P. Perio, B. H. Lajoie, E. Deharo, M. Sauvain, R. Tahar, L. Basco, A. Pantaleo, F. Turini, P. Arese, A. Valentin, E. Thompson, L. Vivas, S. Petit and J.-P. Nallet, J. Med. Chem., 2010, 53, 699 CrossRef CAS.
- N. Ibrahim, H. Ibrahim, S. Kim, J.-P. Nallet and F. Nepveu, Biomacromolecules, 2010, 11, 3341 CrossRef CAS.
- P. A. Cossum, Biopharm. Drug Dispos., 1988, 9, 321 CrossRef CAS.
- P. H. Hinderling, Pharmacol. Rev., 1997, 49, 279 CAS.
- H. Ibrahim, J. Bouajila, N. Siri, G. Rozing, F. Nepveu and F. Couderc, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2007, 850, 481 CrossRef CAS.
- S. Yu, S. Li, H. Yang, F. Lee, J.-T. Wu and M. G. Qian, Rapid Commun. Mass Spectrom., 2005, 19, 250 CrossRef CAS.
- D. B. Adams, M. Hooper, J. S. Christopher, S. E. Raper and B. Stoddart, J. Chem. Soc., Perkin Trans. 1, 1986, 1005 RSC.
- C. C. Bond and M. Hooper, J. Chem. Soc. C, 1969, 2453 RSC.
- Y. C. Awasthi, H. S. Garg, D. D. Dao, C. A. Partridge and S. K. Srivastava, Blood, 1981, 58, 733 CAS.
- S. Casella, M. Pace, P. Romano, O. Romano, A. Geraci and M. Crupi, Cell Biochem. Funct., 2008, 26, 297 CrossRef CAS.
- D. Teti, M. Crupi, M. Busa, A. Valenti, S. Loddo, M. Mondello and L. Romano, Cell. Physiol. Biochem., 2005, 16, 77 CrossRef CAS.
- E. C. Smriti, Nemergut and D. L. Daleke, Biochemistry, 2007, 46, 2249 CrossRef CAS.
- H. Ibrahim, PhD thesis, Toulouse 3 University, France, 2008.
- F. Paul, S. Roath, D. Melville, D. C. Warhurst and J. O. S. Osisanya, Lancet, 1981, 318, 70 CrossRef.
- D. T. X. Trang, N. T. Huy, T. Kariu, K. Tajima and K. Kamei, Malar. J., 2004, 3, 7 CrossRef.
| This journal is © The Royal Society of Chemistry 2011 |
