N-Substituted salicylamides as selective malaria parasite dihydroorotate dehydrogenase inhibitors†
Ingela
Fritzson
*ab,
Paul T. P.
Bedingfield
c,
Anders P.
Sundin
b,
Glenn
McConkey
c and
Ulf J.
Nilsson
b
aActive Biotech AB, PO Box 724, 220 07, Lund, Sweden. E-mail: ingela.fritzson@activebiotech.com.; Tel: +46 46 191233
bOrganic Chemistry, Lund University, PO Box 124, 221 00, Lund, Sweden
cFaculty of Biological Sciences, University of Leeds, Leeds, LS2 9JT, UK
First published on 21st July 2011
Abstract
In our continuing program to develop Plasmodium falciparum dihydroorotate dehydrogenase (PfDHODH) inhibitors, a series of N-substituted salicylamides were synthesized and their ability to selectively inhibit PfDHODH was examined. The synthetic program was based on 2-hydroxy-N-(2-phenylethyl)benzamide (1) that weakly inhibits both PfDHODH and human DHODH (hDHODH). Structure activity relationships were examined for developing derivatives. Selective PfDHODH inhibitors with improved potency were obtained by introducing a 2,2-diphenylethyl substitution on the salicylamidic nitrogen. Biological activity of the most potent compounds was confirmed on parasite infected cells in vitro.
Introduction
Malaria causes great suffering with estimated annual mortalities of over 800.000 people, principally in Africa and Asia.1 A major problem in the fight against malaria is resistance to current drug treatments. Several strategies have been employed to combat this resistance including structural modification of antimalarial drugs to regain activity on resistant parasites, combination therapy with multiple drugs with different mechanisms of action, and the use of new drug targets. Inhibitors of the newly validated target dihydroorotate dehydrogenase (DHODH) could play an important role as future single or combinatory treatment of malaria.2 The enzyme is essential for the biosynthesis of pyrimidines by oxidation of dihydroortate to orotate using ubiquinone (CoQ) as the external source of oxidant.3 The necessity of this enzyme is due to the inability of Plasmodium parasites, unlike humans, to salvage pyrimidines, thus making them dependent on de novo pyrimidine synthesis and DHODH function.4,5 In addition, a recent study indicates that the electron potential in the mitochondrial membrane of Plasmodium falciparum (Pf), the most pathogenic of the human malaria species, solely serves for reoxidation of ubiquinone.6In eukaryotes, DHODH is associated to the inner mitochondrial membrane and binds flavin mononucleotide (FMN) that passes the electrons from dihydroorotate to ubiquinone in a redox process. The first published three dimensional structure of PfDHODH was an X-ray structure with an active metabolite of the drug leflunomide as ligand.7 The crystal structure reveals that the secondary structure of human DHODH (hDHODH) has great similarities with PfDHODH but alignment shows variability in amino acid side-chains and topology of an amino-terminal cavity.7,8 There are several examples of potent human and parasitic DHODH inhibitors that bind in this cavity situated close to FMN in the enzyme.9–15 The binding pocket has been thought to bind ubiquinone although some studies suggest the contrary, but clearly, ligands with high affinity to this cavity block the electron transfer between ubiquinone and FMN.9,16–18 The high amino acid sequence variability in this region justifies the cavity as an ideal target to find inhibitors with selectivity. For example, potent inhibitors against PfDHODH have been found that are active against parasites,19 of which several show species-selectivity.10,13,14,20
Results and discussion
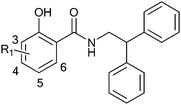
In a preliminary study, N-substituted amides of salicylic acid (salicylamides) were found to be moderately good inhibitors of hDHODH, with IC50 values near 50 μM (unpublished results). A few salicylamides were tested to elucidate their ability to inhibit PfDHODH and compound 1 (Fig. 1) was found to inhibit the Plasmodium enzyme at 53% at 100 μM concentration. The structure–activity relationship of the N-substituted salicylamides was analyzed to assess whether it would be possible to develop analogues to increase the potency and the selectivity for PfDHODH inhibition. For this purpose, a series of closely related derivatives (2–17) of compound 1 was synthesized using amide coupling of an amine and an acid or corresponding ester derivative (Scheme 1). The compounds 2–17 were tested for inhibition at 10 μM concentration against both hDHODH and PfDHODH (Table 1).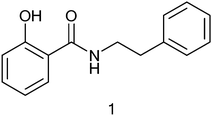 | ||
| Fig. 1 First N-substituted salicylamide found to inhibit Plasmodium falciparum DHODH. | ||
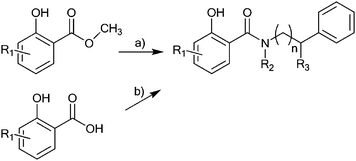 | ||
| Scheme 1 General synthetic route for the preparation of the N-substituted salicylamides 1–30. Reagents and conditions: a) salicylic acid methyl ester, primary amine, 110 °C, 20 h without solvent; b) salicylic acid, primary or secondary amine, N,N′-dicyclohexylcarbodiimide, toluene, 80 °C, 4 h. | ||
|
|
||||||
|---|---|---|---|---|---|---|
| R1 | R2 | R3 | n | %inhibition at 10 μMa | ||
| PfDHODH | hDHODH | |||||
| a Data represent mean of inhibition (%) at 10 μM ± standard error. Numbers in parentheses represent numbers of determinations. b Racemic mixture. | ||||||
| 1 | H | H | H | 1 | 21 ± 2(3) | 6 ± 0.3(3) |
| 2 | 6-OH | H | H | 1 | 17 ± 2(5) | 15 ± 3(4) |
| 3 | 3-Cl | H | H | 1 | 10 ± 1(5) | 8 ± 0.3(3) |
| 4 | 4-Cl | H | H | 1 | 16 ± 1(4) | 8 ± 0.4(3) |
| 5 | 5-Cl | H | H | 1 | 23 ± 2(4) | 6 ± 1(3) |
| 6 | 3-OMe | H | H | 1 | 7 ± 2(5) | 8 ± 1(2) |
| 7 | 4-OMe | H | H | 1 | 9 ± 1(5) | 5 ± 1(3) |
| 8 | 5-OMe | H | H | 1 | 8 ± 2(5) | 8 ± 1(3) |
| 9 | 5-Me | H | H | 1 | 16 ± 2(5) | 4 ± 2(3) |
| 10 | 5-NO2 | H | H | 1 | 5 ± 1(6) | 26 ± 2(3) |
| 11 | H | H | H | 0 | 16 ± 1(6) | 4 ± 1(3) |
| 12 | H | H | H | 2 | 39 ± 2(5) | 7 ± 1(3) |
| 13 | H | H | Me | 1 | 20 ± 2(5) | 4 ± 0.4(3) |
| 14 | H | H | Phenyl | 0 | 0 ± 0.6(6) | 6 ± 0.2(3) |
| 15 | H | H | Phenyl | 1 | 38 ± 1(5) | 4 ± 0.6(3) |
| 16 | H | H | Phenyl | 2 | 22 ± 2(5) | 21 ± 2(3) |
| 17 | H | Me | H | 1 | 3 ± 1(5) | 3 ± 1(2) |
Compounds 1–10 were evaluated to establish the structure–activity relationship of PfDHODH inhibition for the salicylic (left) part. Only compound 5, with a chlorine substituent in position 5, have maintained activity compared to the unsubstituted parent compound 1. In each position 3, 4 and 5, a chlorine substituent is better than methoxy and as these substituents have similar van der Waals volumes, it is possible that the electron withdrawing substituent has a positive influence. Conversely, when the electron withdrawing group is more polar, as for compound 10, with a nitro-group in the 5-position, the potency decreases.
The linker length (n = 0, 1 and 2) between the salicylamide part and the phenyl moiety was examined for two compound series; with R3 = H and R3 = phenyl, respectively (1, 11, 12 and 14, 15, 16). When R3 = H, a three carbon chain has the highest potency but the original two carbon chain is beneficial when choosing a phenyl group as R3. By introduction of a third phenyl group to form a diphenyl ethyl amide as in compound 15 (R3 = phenyl) the inhibition at 10 μM is almost doubled compared to parent salicylamide 1. In addition it also decreases the affinity for human DHODH and thereby increasing the selectivity.
In order to further explore compounds with a diphenyl ethyl moiety and analyze the influence of pKa of the salicylic hydroxyl proton, derivatives of compound 15 with various substituents in the salicylic phenyl ring were synthesized (Table 2). The theory behind the pKa analysis is that the electro-negativity of the substituent could cause a change in pKa and thereby influence the enzyme binding affinity of the N-substituted salicylamides. The 3,5-dichloro substituted compound 21 had the lowest calculated pKa (6.9) and is also the most potent N-substituted salicylamide found (IC50 = 7.0 μM). Compounds 19, 20, 22, 24, 26 and 28 with a substituent in position 4 or 5 and pKa's varying from 7.0 to 9.4 all have IC50 values below 15 micromolar, a gain in potency compared to parent compound 15 (IC50 = 21.2 μM, pKa = 9.0). In contrast, the 3-chloro, 3-fluoro, 6-fluoro and 3-methoxy compounds 18, 23, 25 and 27, with pKa as low as 7.5, 7.7, 8.6 and 8.4, respectively, lose potency compared to parent compound. Introduction of functional groups in position 3 may disturb binding due to steric interference with the binding site, thus counteracting possible benefits of lowering the pKa. Compounds substituted in the 6 position (25, 30) have high energy conformers upon rotation of the salicylic aromatic ring which may explain the lack of activity. With small functional groups in position 4 or 5, the substituted compounds are more potent than the unsubstituted compounds with a slight advantage in those with electron withdrawing groups. Additionally, compounds 26, 28 and 29 with higher pKa have higher potencies than the unsubstituted compounds and filling the binding pocket using small substituents in position 4 or 5 appears to be advantageous irrespective of pKa.
|
|
||||
|---|---|---|---|---|
| R1 | pKab | IC50 (μM) a | ||
| PfDHODH | hDHODH | |||
| a Data represent mean of IC50 ± standard error. Numbers in parentheses represent numbers of determinations. b pKa was calculated using SPARC Online Calculator. c Not determined. | ||||
| 15 | H | 9.0 | 21.2 ± 1.3(6) | >200(3) |
| 18 | 3-Cl | 7.5 | 44.1 ± 1.9(6) | >200(3) |
| 19 | 4-Cl | 8.2 | 10.3 ± 0.6(6) | >200(3) |
| 20 | 5-Cl | 8.4 | 9.1 ± 0.5(6) | >200(3) |
| 21 | 3,5-diCl | 6.9 | 7.0 ± 0.4(6) | >200(3) |
| 22 | 5-Br | 8.4 | 10.8 ± 0.8(6) | >200(3) |
| 23 | 3-F | 7.7 | 26.1 ± 1.9(6) | >200(3) |
| 24 | 3,4-diF | 7.0 | 9.9 ± 0.5(5) | 130(6) |
| 25 | 6-F | 8.6 | >125(6) | n.d.c |
| 26 | 5-Me | 9.4 | 14.4 ± 0.6(6) | >200(3) |
| 27 | 3-OMe | 8.4 | >500(6) | >200(3) |
| 28 | 4-OMe | 8.8 | 14.0 ± 0.6(6) | >200(3) |
| 29 | 5-OMe | 9.3 | 18.1 ± 1.0(6) | >200(3) |
| 30 | 6-OMe | 9.1 | >125(6) | n.d.c |
The structure–activity relationship (SAR) of the diphenyl ethyl compounds in Table 2 is inconsistent with the SAR of compounds presented in Table 1 in that it is favorable for this newer series to have substitutions with methyl and methoxy groups in position 4 and 5. This suggests that there is some difference in binding mode for the two compound series.
The compounds in Table 2 all show good selectivity between P. falciparum and human DHODH. The best inhibitor found is compound 21 that has an IC50 that is higher than 200 μM in hDHODH and IC50 of 7.0 μM in PfDHODH, giving a selectivity factor that is greater than 20 times.
Computational modeling of PfDHODH-salicylamide complexes, based on a PfDHODH crystal structure (pdb3I6521), was used to explore diphenyl ethyl salicylamide interactions with the ligand-binding site of PfDHODH (Fig. 2). A conformational search utilizing the Monte Carlo multiple minima method, was performed on the ligand. Low energy conformations of the ligand were manually placed in the ligand-binding site, with at least one of the ligand oxygens close to arginine 265, and the diphenyl group in the hydrophobic pocket. Phenylalanine 188, which is flexible,21 did initially overlap with the diphenyl group, but could be rotated 120 degrees to a free volume. Each complex was energy minimized with Macromodel, utilizing the OPLS-2005 force field and the GB/SA model for water solvation.
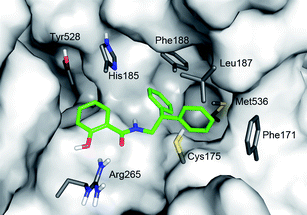 | ||
| Fig. 2 Proposed binding mode for N-substituted salicylamide 15 (green). The salicylic hydroxy group of the ligand is placed near Arg265 and the diphenyl group in the hydrophobic part of the pocket. Van der Waals surfaces and residues derive from PfDHODH crystal structure pdb3I65.21 The image was generated with the PyMOL software package (The PyMOL Molecular Graphics System, DeLano Scientific, San Carlos, CA (USA), 2002). | ||
After analyzing the results the pose presented in Fig. 2 for compound 15 was considered as the most probable. This binding mode places both oxygen atoms of the salicylamide close to arginine 265 in the binding pocket and a single hydrogen bond is formed between the amide oxygen and arginine 265. Phenylalanine 188 makes a face to face π-stacking with an aromatic ring in the biphenyl part of the ligand. The resulting complex allows substitution in the 4 and 5 position of the salicylic ring but the 3 position is highly restricted, which is consistent with structure activity relationship according to Table 2.
In a variation of this binding mode, arginine 265 was rotated to make a double hydrogen bond to both oxygens of the ligand. The energy minimum for this complex was lower but this more rigid binding will probably suffer from considerable entropic costs.
The protonation state of the ligand at binding is unclear. A lowering of pKa seem to have a slight beneficial affect on the inhibition, and the explanation could be that an increased fraction of the ligand gets deprotonated in the binding site, thus strengthening the interaction between the inhibitor oxygens and arginine 265. However, deprotonation was found to not significantly alter the geometry of the binding mode in the model.
In the PfDHODH X-ray diffraction data, a crystal water (W15) is situated near the hydroxy group of tyrosine 528. Replacing this water with a functional group on the salicylamide, may improve potency. Compounds 32 and 33 (Table 3) were modeled to explore water replacement, and possible hydrogen bond interactions with tyrosine 528. These compounds were synthesized, and the best ligand in this series had an IC50 of 8.0 μM, which is similar to the best results presented in Table 2. The result suggests that water replacement may be relevant for compounds 32 and 33, however, with non-optimal interaction between the hydroxymethyl group and tyrosine 528. Compound 31 was synthesized as negative control and no inhibition was seen at 100 μM. It can be noticed that the compound 34 (Table 3), an intermediate in the synthesis of compound 32, has a 3-fold lower IC50 than the target compound 32. This indicates that the substituent in the 3-position preferably acts as a hydrogen bond acceptor in the interaction with tyrosine 528. This observation opens up future optimization possibilities to increase the potency of the salicylamides by introduction of other hydrogen bond acceptors in the 3-position.
|
|
||||
|---|---|---|---|---|
| R1 | pKab | IC50 (μM) a | ||
| PfDHODH | hDHODH | |||
| a Data represent mean of IC50 ± standard error. Numbers in parentheses represent numbers of determinations. b pKa was calculated using SPARC Online Calculator. | ||||
| 31 | 3-CH2OH | 9.5 | >100(6) | >200(3) |
| 32 | 4-CH2OH | 9.0 | 46.2 ± 2.4(6) | >200(3) |
| 33 | 5-CH2OH | 9.2 | 8.0 ± 0.4(6) | >200(3) |
| 34 | 4-CHO | 8.4 | 16.5 ± 0.6(6) | >200(3) |
The four most potent compounds 20, 21, 24, and 33 from the PfDHODH enzyme assays were tested for activity against cultured P. falciparum 3D7 parasites. The parasites were synchronized before incubation with decreasing concentrations of compounds for 48 h following standard assay procedures. The incorporation of [3H] hypoxanthine by the parasites over an 18 h period was then established by scintillation counting and the concentration at which growth was reduced to 50% (EC50) relative to untreated controls (Table 4). Compound 20 was the best inhibitor of parasite growth in cells; the EC50 was measured to 23 ± 4 μM.
Conclusion
In summary, the most potent N-substituted salicylamides 20, 21, 24 and 33 were found to have affinity for Plasmodium falciparum DHODH enzyme in the low micromolar range with at least 18 times selectivity over human DHODH. These results may be a positive harbinger for development of selective antimalarial DHODH inhibitors that do not invoke immune suppression via the human enzyme. Although the PfDHODH inhibition is moderate compared to two recently discovered inhibitor classes with inhibition in the nanomolar range (IC50 of 22 nM at best),21,22 further optimization of the salicylamides could yield higher potency inhibitors.The N-substituted salicylamides are a new class of PfDHODH inhibitors that are promising lead structures for further optimization into antimalarial drugs. Particularly, the N-diphenylethyl substituent and the substituent in the 4- or 5-position of the developed compounds have potential to be structurally modified to gain improved potency. The proposed binding mode may provide valuable information about protein–ligand interaction to use in the drug design of next generation salicylamide inhibitors.
Acknowledgements
We thank Ann-Charlotte Johansson and Tomas Fristedt for analytical data. This work was supported in part by the Swedish Research Council.References
- WHO, World Malaria Report, 2009 Search PubMed.
- L. McRobert and G. A. McConkey, Mol. Biochem. Parasitol., 2002, 119, 273–278 CrossRef CAS.
- W. E. Gutteridge, D. Dave and W. H. G. Richards, Biochim. Biophys. Acta, Gen. Subj., 1979, 582, 390–401 CrossRef CAS.
- P. Reyes, P. K. Rathod, D. J. Sanchez, J. E. K. Mrema, K. H. Rieckmann and H.-G. Heidrich, Mol. Biochem. Parasitol., 1982, 5, 275–290 CrossRef CAS.
- W. E. Gutteridge and P. I. Trigc, J. Protozool., 1970, 17, 89–96 CAS.
- H. J. Painter, J. M. Morrisey, M. W. Mather and A. B. Vaidya, Nature, 2007, 446, 88–91 CrossRef CAS.
- D. E. Hurt, J. Widom and J. Clardy, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2006, 62, 312–323 CrossRef.
- S. Liu, E. A. Neidhardt, T. H. Grossman, T. Ocain and J. Clardy, Structure, 2000, 8, 25–33 CrossRef CAS.
- J. Baldwin, C. H. Michnoff, N. A. Malmquist, J. White, M. G. Roth, P. K. Rathod and M. A. Phillips, J. Biol. Chem., 2005, 280, 21847–21853 CrossRef CAS.
- T. Heikkilä, C. Ramsey, M. Davies, C. Galtier, A. M. W. Stead, P. A. Johnson, C. W. G. Fishwick, A. N. Boa and G. A. McConkey, J. Med. Chem., 2007, 50, 186–191 CrossRef.
- D. E. Hurt, A. E. Sutton and J. Clardy, Bioorg. Med. Chem. Lett., 2006, 16, 1610–1615 CrossRef CAS.
- J. Leban, W. Saeb, G. Garcia, R. Baumgartner and B. Kramer, Bioorg. Med. Chem. Lett., 2004, 14, 55–58 CrossRef CAS.
- M. A. Phillips, R. Gujjar, N. A. Malmquist, J. White, F. El Mazouni, J. Baldwin and P. K. Rathod, J. Med. Chem., 2008, 51, 3649–3653 CrossRef CAS.
- V. Patel, M. Booker, M. Kramer, L. Ross, C. A. Celatka, L. M. Kennedy, J. D. Dvorin, M. T. Duraisingh, P. Sliz, D. F. Wirth and J. Clardy, J. Biol. Chem., 2008, 283, 35078–35085 CrossRef CAS.
- I. Fritzson, B. Svensson, S. Al-Karadaghi, B. Walse, U. Wellmar, U. J. Nilsson, D. da Graça Thrige and S. Jönsson, ChemMedChem, 2010, 5, 608–617 CrossRef CAS.
- J. E. McLean, E. A. Neidhardt, T. H. Grossman and L. Hedstrom, Biochemistry, 2001, 40, 2194–2200 CrossRef CAS.
- W. Knecht and M. Löffler, Biochem. Pharmacol., 1998, 56, 1259–1264 CrossRef CAS.
- N. A. Malmquist, R. Gujjar, P. K. Rathod and M. A. Phillips, Biochemistry, 2008, 47, 2466–2475 CrossRef CAS.
- A. N. Boa, S. P. Canavan, P. R. Hirst, C. Ramsey, A. M. W. Stead and G. A. McConkey, Bioorg. Med. Chem., 2005, 13, 1945–1967 CrossRef CAS.
- R. Gujjar, A. Marwaha, F. El Mazouni, J. White, K. L. White, S. Creason, D. M. Shackleford, J. Baldwin, W. N. Charman, F. S. Buckner, S. Charman, P. K. Rathod and M. A. Phillips, J. Med. Chem., 2009, 52, 1864–1872 CrossRef CAS.
- X. Deng, G. Ramesh, F. El Mazouni, W. Kaminsky, N. A. Malmquist, E. J. Goldsmith, P. K. Rathod and M. A. Phillips, J. Biol. Chem., 2009, 284, 26999–27009 CrossRef CAS.
- M. L. Booker, C. M. Bastos, M. L. Kramer, R. H. Barker Jr, R. Skerlj, A. B. Sidhu, X. Deng, C. Celatka, J. F. Corteses, J. E. Guerrero Bravo, K. N. Crespo Llado, A. E. Serrano, I. Angulo-Barturen, M. B. Jiménez-Díaz, S. Viera, H. Garuti, S. Wittlin, P. Papastogiannidis, J. Lin, C. J. Janse, S. M. Khan, M. Duraisingh, B. Coleman, E. J. Goldsmith, M. A. Phillips, B. Munoz, D. F. Wirth, J. D. Klinger, R. Wiegand and E. Sybertz, J. Biol. Chem., 2010, 285, 33054–33064 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details for enzyme inhibition assay, in vitro parasite growth assay and the synthesis and characterization of chemical compounds 1–34. See DOI: 10.1039/c1md00118c |
| This journal is © The Royal Society of Chemistry 2011 |