A novel strategy for the synthesis of enzymatically stable biotin–DOTA conjugates for in vivo use†
Petri A.
Turhanen
*ab,
Janne
Weisell
ab,
Pauliina
Lehtolainen-Dalkilic
c,
Ann-Marie
Määttä
c,
Jouko
Vepsäläinen
ab and
Ale
Närvänen
ab
aDepartment of Biosciences, University of Eastern Finland, Kuopio, Finland. E-mail: Petri.Turhanen@uef.fi; Tel: +358 40 3553857
bBiocenter Kuopio, University of Eastern Finland, Kuopio, Finland
cArk Therapeutics Oy, Kuopio, Finland
First published on 25th July 2011
Abstract
Two 111In radiolabelled biotin–DOTA conjugates were prepared containing an enzymatically stable amine bond (–CH2–NH–CH2–). Syntheses are straightforward and molecules were labelled with high radiochemical purity. They were also found to be stable in human plasma. This strategy provides a method to produce a simple biotin derivative not requiring any additional protection groups against biotinidase.
Introduction
Monoclonal antibodies have been used in radioimmunotherapy as the targeting moiety for the radioisotopes. Several tumors over-express receptors and thus they are good targets for radiolabelled antibodies. Therapy with radioactive long circulating antibodies is impaired by their poor tumour to normal tissue distribution ratio and furthermore the dosage of radioisotope is restricted by bone marrow toxicity.1 Thus, successful radioimmunotherapy is limited mainly to radiosensitive lymphomas. However, pretargeted radioimmunotherapy can overcome this problem since it dissociates the delivery of a targeting moiety from the delivery of radionuclide in order to clear circulating antibodies before the administration of a radionuclide.2–4 An alternative to conventional pre-targeted radioimmunotherapy is virus-mediated gene therapy, where an artificial receptor is transduced into the target tissue. Local virus mediated avidin gene transfer to target tissues represents a novel tool for the delivery of biotinylated radioligands.5Ligands with biotin are widely used in both in vitro diagnostics and in vivo therapy due to its high affinity to avidin or streptavidin, for example, several biotinylated radioligands containing both iodine and chelate conjugates have been described in the literature.6 Unfortunately, their poor stability and bioavailability may decrease the usefulness of the biotinylated radioligands for in vivo use. Biotin contains a carboxylic acid functionality providing a conjugation position with simple chemistry via the amide bond. However, these amide type conjugates between the acid moiety in biotin and the chelating moiety tend to be sensitive to serum biotinidase. Biotinidase is a human enzyme that allows the body to use and recycle biotin. There are significant amounts of biotinidase in the human circulation and tissues and this enzyme can release biotin before the radioligand reaches its target i.e. avidin or streptavidin.7Biotinidase removes biotin from biocytin, its form in biotin carriers, and makes it available to be reused by other enzymes. Biotinidase is not specific for biocytin but also cleaves structurally similar structures like desthiobitinyl-p-aminobenzoic acid8 and lipoic acid—lysine adducts.9,10 Due to the non-specificity of biotinidase, it has proved challenging to block its activity against different types of biotin conjugates. Several structures protecting the amide bonds against biotinidase have been devised mainly by using α-amino methylation or sterical shielding of the amino bond with additional large groups near to the bond. This approach complicates the synthesis route and the additional chemical groups may lower the affinity of biotin for avidin or streptavidin.11,12
Tetraazacyclododecane tetraacetic acid (DOTA) is widely used as a chelating agent in radiotherapy and in vivo imaging.13 When conjugated to biotin, it provides a high affinity radioligand for avidin or streptavidin mediated therapy. In order to stabilize the structure and to simplify the synthesis we have prepared two novel biotinylated DOTA conjugates with stable amine bonds (Fig. 1) and tested their labelling properties using 111indium as the radionuclide and evaluated their stability in human plasma in vitro.
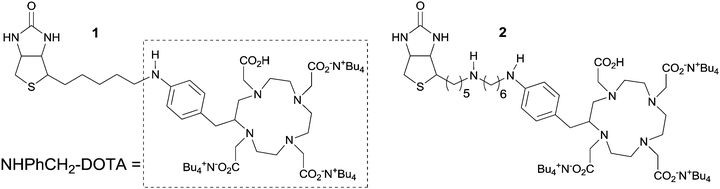 | ||
| Fig. 1 Chemical structures of the prepared compounds 1 and 2. | ||
Results and discussion
Chemistry
In practice, there are two main strategies, which can be used to prepare amines from carboxylic acids: (1) CO2R (R![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) H, alkyl) ester group is first converted to an amide (–CONR2), which is then reduced to amine functionality (–CH2NR2)14 or (2) carboxylic group is first reduced to alcohol (–CH2OH) moiety and the poor OH leaving group is then transformed into a good leaving group like –CH2O–SO2R or halogen (Br or I), which is then treated with required amine.15 In this case, we prepared the studied compounds adhering to the latter method, since DOTA itself contains also carboxylic fragments, which are sensitive to strong reducing agents like LiAlH4.
H, alkyl) ester group is first converted to an amide (–CONR2), which is then reduced to amine functionality (–CH2NR2)14 or (2) carboxylic group is first reduced to alcohol (–CH2OH) moiety and the poor OH leaving group is then transformed into a good leaving group like –CH2O–SO2R or halogen (Br or I), which is then treated with required amine.15 In this case, we prepared the studied compounds adhering to the latter method, since DOTA itself contains also carboxylic fragments, which are sensitive to strong reducing agents like LiAlH4.
Based on our long experience in preparing various polyamine derivatives from alcoholsviamesyl intermediates, we successfully used this strategy to prepare the target compounds 1 and 2. The prepared mesyl intermediates are rather stable, and readily obtained from methanesulfonyl chloride and the corresponding alcohol under basic conditions. Moreover, mesyl intermediates can be purified by chromatographic methods, if necessary.
Compounds 1 and 2 were the starting points and they were prepared from commercially available biotin (3), which was first reduced to biotinol 4 (see Scheme 1) following slight modification of the method described by Shoup et al.16 A chromatographic method was developed to purify crude biotinol 4, since the literature method based on re-crystallization from methanol16 or a mixture of methanol/water17 was not successful in our hands. Recently, biotinol 4 has also been prepared from its ethyl ester with an almost quantitative yield.18
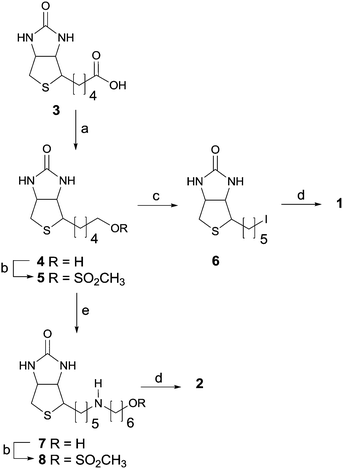 | ||
| Scheme 1 Synthetic routes to 1 and 2. (a) LiAlH4; (b) MsCl; (c) NaI; (d) H2NPhCH2–DOTA, DMF; (e) H2N(CH2)6OH. | ||
Two alternative strategies to utilize the mesyl intermediates were tested: DOTA derivative 1 was prepared from mesylate 5viaiodine derivative 618 and DOTA derivative 2 from its mesylate 815 directly after chain elongation with 6-amino-1-hexanol. In our hands, both methods led successfully to target DOTA derivatives after purification by a semi-preparative HPLC system, but in the route with iodines there was an extra synthetic step and competitive reactions might have occurred due to nucleophilic sulfur in the biotin ring, especially during long-term storage of iodines.
Bioassays
The relative binding affinity of the biotin–DOTA molecules 1 and 2 was compared to free biotin using a modified EIA method. The IC50 values for biotin, biotin–DOTA 1 and 2 were 0.026, 0.032 and 0.061 nM, respectively. The small differences indicate that DOTA conjugation does not interfere with avidin–biotin interaction. The biotin–DOTA ligands 1 and 2 were labelled with 111indium in 10 mM acetate buffer at pH 4.5. According to ITL-chromatography, the radiochemical purity was 99% for (1) and 96% for (2), respectively. The stability of both radiolabelled ligands was tested using 5 μg per 100 μl of human plasma19 at four different time points. On average, 5% (1) and 7% (2) of the indium was released from the biotinyl-DOTA radioligands immediately after incubation in plasma (Table 1). No additional release occurred during the next 60 minutes of incubation indicating that ligands had not been degraded. On the basis of our results, both molecules were easily labelled in aqueous solutions using methods, which would be acceptable for in vivo use. Furthermore, the simple synthetic method and the high stability against plasma biotinidase indicate that our molecules are potential new agents for advanced radiotherapy. Biotin–streptavidin based radiotherapy is a complicated procedure containing several steps. Since naturally occurring free biotin is circulating, it should be removed by streptavidin injection before the therapy can be started. Typically the targeting unit is biotinylated or streptavidin conjugated tumor specific antibody, which is first injected to the patient. After the clearance of the extra antibody, streptavidin or radioactive biotin–DOTA is injected. If streptavidin has been used as a bridge, radioactive biotin–DOTA is administered. The treatment takes days with several injections and may increase the nonspecific distribution of the components. Furthermore, the immunoresponse against streptavidin may limit the number of treatments in the same patient.20 The next generation pretargeted therapy when overcoming the problems holds great possibilities for more specific cancer treatment.| Time/min | Compound 1 | Compound 2 |
|---|---|---|
| 0 | 95% | 93% |
| 15 | 96% | 92% |
| 40 | 95% | 93% |
| 60 | 96% | 92% |
Conclusions
The biotin–DOTA conjugates were determined to be stable, water soluble ligands with high affinity to avidin and are thus promising chelates for avidin–biotin mediated radiotherapy. Due to the simple synthesis protocol, production is both repeatable and cost effective.Acknowledgements
The authors would like to thank Mrs Maritta Salminkoski for her skilful technical assistance. This work was supported by Ark Therapeutics Oy, Kuopio, Finland, Academy of Finland and by the strategic funding of the University of Eastern Finland.Notes and references
- R. F. Meredith and D. J. Buchsbaum, Int. J. Radiat. Oncol., Biol., Phys., 2006, 66, S57–S59 CrossRef CAS.
- G. Paganelli, C. Grana, M. Chinol, M. Cremonesi, C. De Cicco, F. De Braud, C. Robertson, S. Zurrida, C. Casadio, S. Zoboli, A. G. Siccardi and U. Veronesi, Eur. J. Nucl. Med., 1999, 26, 348–357 CrossRef CAS.
- M. Cremonesi, M. Ferrari, C. M. Grana, A. Vanazzi, M. Stabin, M. Bartolomei, S. Papi, G. Prisco, P. F. Ferrucci, G. Martinelli and G. Paganelli, J. Nucl. Med., 2007, 48, 1871–1879 CrossRef.
- N. Urbano and S. Modoni, Nucl. Med. Commun., 2007, 28, 943–950 CrossRef.
- P. Lehtolainen, T. Wirth, A. K. Taskinen, P. Lehenkari, O. Leppänen, M. Lappalainen, K. Pulkkanen, A. Marttila, V. Marjomäki, K. J. Airenne, M. Horton, M. S. Kulomaa and S. Ylä-Herttuala, Gene Ther., 2003, 10, 2090–2097 CrossRef CAS.
- R. E. Weiner and M. L. Thakur, BioDrugs, 2005, 19, 145–163 CrossRef CAS.
- J. Pispa, Ann. Med. Exp. Biol. Fenn., 1965, 43(suppl. 5), 1–39 Search PubMed.
- J. Chauhan and K. Dakshinamurti, J. Biol. Chem., 1986, 261, 4268–4275 CAS.
- L. Nilsson and B. Kagedal, Biochem. J., 1993, 291, 545–551 CAS.
- L. Nilsson and B. Kagedal, Eur. J. Clin. Chem. Clin. Biochem., 1994, 32, 501–509 CAS.
- D. S. Wilbur, P. M. Pathare, D. K. Hamlin and S. A. Weerawarna, Bioconjugate Chem., 1997, 8, 819–832 CrossRef CAS.
- D. S. Wilbur, D. K. Hamlin and M. K. Chyan, Bioconjugate Chem., 2006, 17, 1514–1522 CrossRef CAS.
- C. Ansquer, F. Kraeber-Bodere and J. F. Chatal, Curr. Pharm. Des., 2009, 15, 2453–2462 CrossRef CAS.
- V. M. Micovic and M. L. J. Mihailovic, J. Org. Chem., 1953, 18, 1190 CrossRef CAS.
- A. J. Järvinen, M. Cerrada-Gimenez, N. A. Grigorenko, A. R. Khomutov, J. J. Vepsäläinen, R. M. Sinervirta, T. A. Keinänen, L. I. Alhonen and J. E. Jänne, J. Med. Chem., 2006, 49, 399–406 CrossRef.
- T. M. Shoup, A. J. Fischman, S. Jaywook, J. W. Babich, H. W. Strauss and D. R. Elmaleh, J. Nucl. Med., 1994, 35, 1685–1690 CAS.
- H. Flaster and H. Kohn, J. Heterocycl. Chem., 1981, 18, 1425 CrossRef CAS.
- C. Corona, B. K. Bryant and J. B. Arterburn, Org. Lett., 2006, 8, 1883–1886 CrossRef CAS.
- M. Pakkala, C. Hekim, P. Soininen, J. Leinonen, H. Koistinen, J. Weisell, U. H. Stenman, J. Vepsäläinen and A. Närvänen, J. Pept. Sci., 2007, 13, 348–353 CrossRef CAS.
- H. P. Lesch, M. U. Kaikkonen, J. T. Pikkarainen and S. Ylä-Herttuala, Expert Opin. Drug Delivery, 2010, 7, 551–564 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: General experimental procedures, synthesis of the compounds, NMR assignments, labelling studies and plasma stability. See DOI: 10.1039/c1md00111f |
| This journal is © The Royal Society of Chemistry 2011 |
