Enantioselective preparation of ferrocenyl amino phosphines and their cytotoxic activities
Stanton Hon-lung
Kok
a,
Wing-sze
Lam
b,
Albert Sun-chi
Chan
c,
Wai-yeung
Wong
c,
Roberto
Gambari
d,
Raymond Siu-ming
Wong
e,
Kenneth Ka-ho
Lee
a,
Johnny Cheuk-on
Tang
*b,
Kim-hung
Lam
*b and
Chung-hin
Chui
*ef
aStem Cell and Regeneration Thematic Research Programme, School of Biomedical Sciences, The Chinese University of Hong Kong, China
bDepartment of Applied Biology and Chemical Technology, The Hong Kong Polytechnic University, China. E-mail: bcjoelam@inet.polyu.edu.hk; Fax: +86 852 2364 9932; Tel: +86 852 3400 8705; bccotang@inet.polyu.edu.hk; Fax: +86 852 2364 9932; Tel: +86 852 3400 8727
cDepartment of Chemistry, Baptist University of Hong Kong, China
dBio-Pharm Net, Department of Biochemistry and Molecular Biology, University of Ferrara, Italy
eDepartment of Medicine and Therapeutics, Prince of Wales Hospital, The Chinese University of Hong Kong, China. E-mail: tcechui@polyu.edu.hk; Fax: +86 852 2773 1432; Tel: + 86 852 2766 6456
fInstitute of Textiles and Clothing, The Hong Kong Polytechnic University, China
First published on 21st July 2011
Abstract
In connection to our previously reported phosphine compounds as antitumor agents, this article describes the preparation of ferrocenyl amino-phosphine and their possible cytotoxic potential towards the human hepatocellular carcinoma cell line Hep3B. The preliminary results suggested that ferrocenyl amino-phosphines can be potential anti-tumour agents which might be considered as novel cancer chemotherapeutic agents. In particular, compounds 4b, 4d and 4f exhibit strong cytotoxicity with IC50 < 5 μg ml−1. These compounds possess more promising cytotoxic activities than their precursor non-substituted ferrocene as well as the reference metallodrug cisplatin.
Introduction
Cisplatin is a classical metal coordination complex showing antitumor activity.1 After successful application, researchers continued to explore new classes of organometallic agents with significant biological activities such as metallocene dihalides2 and ferrocene derivatives.3 In particular, the synthesis and pharmacological studies of organometallic ferrocenyl units also show significant biological activities and draw scientists' attention. Many ferrocenyl compounds can be applied for organometallic catalysis and they also demonstrate cytotoxic, antitumor, antimalarial, antifungal and DNA-cleaving activities.4 The unexpected biological activity of ferrocenyl derived organics may be due to their special membrane permeation properties and metabolism; hence, these agents offer good pharmaceutical treatment properties such as non-toxicity, good stability and compatibility.5 The integration of ferrocenyl scaffolds into heteroaromatic compounds was recognized as an attractive way to produce new and effective molecules.6 It is reported that ferrocenyl Schiff-base esters of aniline,7fluconazole8 and aspirin9 demonstrated biological activities. The modification of the tamoxifen skeleton by a ferrocenyl unit can give antiestrogens with unique antiproliferative effects.10 Unsaturated conjugated ketones containing ferrocenyl pyrazole moieties were synthesized and their in vitro cytotoxic activities against melanoma Fem-x, cervix adenocarcinoma HeLa, and myelogenous leukemia K562 cell lines were reported through the use of a MTT method.11Ferrocenyl chalcones had been reported to possess antimalarial activity.12 The incorporation of glycoside scaffolds into ferrocenyl chalocones shows cytotoxic activity on HL-60 human leukemia cells.13 The retinoyl ferrocene derivatives were prepared by using monoarylferrocenyl alcohols and retinoic acid, and they exhibited higher antiproliferative activities than their mother compounds.14 The “unnatural” ferrocenyl amino acid, Na-Ne-(ferrocene-1-acetyl)-L-lysine, can work like nuclease for DNA cleavage.15 Romao and coworkers reported that 1,2-disubstituted ferrocenes and cyclodextrin inclusion complexes show in vitro activities against Ehrlich ascites tumor cell.16Ferrocene–indole hybrids can be applied for cancer and malarial therapy.17 Shin et al. reported that 5-alkyl-2-ferrocenyl-6,7-dihydropyrazolo[1,5-a]pyrazin-4(5H)-one derivatives demonstrated inhibitory effects on the growth of A549 cells.18 The ferrocenyl diphenol compound, such as 1,1-di(4-hydroxyphenyl)-2-ferrocenyl-but-1-ene, exhibited strong in vitro anti-proliferative effects on MCF7 and MDA-MB231 breast cancer cells.19 In addition to mononuclear organometallic agents, bimetallic complexes such as ethylene-linked binuclear ferrocene/ruthenium complexes were studied for their anti-estrogenic effects.20Thiolato gold(I) complexes of 1,1′-bis(diphenylphosphino)ferrocene show cytotoxicity,21 and the bis(phosphinecatecholato)platinum(II) moiety tethered to ferrocene or ruthenocene demonstrates activity against P388 antileukemia cells.22Following our previously reported pyridinyl and bifunctional organophosphine compounds23 as antitumor agents, many scientists reported that ferrocene derivatives exhibited unexpected biological activities. In this work, we tried to extend the idea through the incorporation of phosphinyl and amino bifunctionality into a ferrocene core structure and test for their possible cytotoxic potential towards human hepatocellular carcinoma cell line Hep3B.
Results and discussion
The preparation of 4a–l is described in Scheme 1.24 The enantioselective pure ferrocenyl alcohol (S)-1 was prepared using Friedel–Crafts acylation of ferrocene and asymmetric hydrogenation of ferrocenyl ketones catalyzed by freshly prepared [{(R)-XylylP-Phos}RuCl2{(R,R)-DPEN}] or purchased from a commercial supplier. The optically pure 1-ferrocenylethanol (S)-1 was quantitatively converted to the corresponding acetate by treatment with acetic anhydride in pyridine. Removal of the volatiles by vacuum provided pure materials without the need for further purification. In the second step, nucleophilic substitution of the acetate was accomplished in methanol using an excess of dimethylamine.25 Optically pure Ugi's amine (S)-2 was easily obtained without any crystallization process due to retention of configuration in nucleophilic substitution.26 The planar chirality was introduced by diastereoselective ortho-lithiation of ferrocenyl amine (S)-2 using t-BuLi leading to (S,Rp)-3 which has its α-alkyl group in a position of less steric repulsion. The diastereomerically pure (S,Rp)-3 was obtained in moderate to good yield after electrophilic substitution. The dimethylamino group of (S,Rp)-3 underwent nucleophilic substitution with retention of configuration. A series of ferrocenyl amino-phosphine ligands (S,Rp)-4a–l were obtained without any change in chirality. The percentage yields obtained for compound (S, Rp)-4 were moderate to good, from 63% to 99%.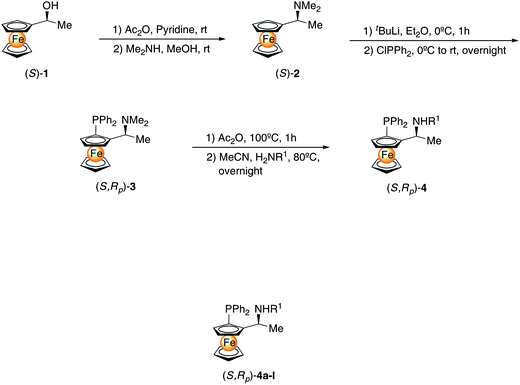 | ||
| Scheme 1 Synthesis of ferrocenyl amino phosphines. Reagents and conditions: (a) (i) Ac2O, pyridine, rt; (ii) Me2NH, MeOH, rt. (b) (i) t-BuLi, Et2O, 0 °C, rt; (ii) ClPPh2, 0 °C to rt. (c) (i) Ac2O, 100 °C, 1 h; (ii) MeCN, H2NR1, 80 °C, overnight. | ||
Here, we reported the synthesis and biological activities of a series of ferrocenyl amino-phosphine compounds 3 and 4 as antitumor agents. The biological activities of 3 and 4 are significant as compared to that of the bare ferrocene scaffold (which does not show any biological activity even at a concentration of over 100 μg ml−1) as control. In connection with this idea, we tried using various substitutions for 4, and we found that the chain length and bulkiness are important to account for the biological activity (Table 1). From the IC50 results obtained, the bulky aromatic groups, such as phenyl, naphthyl and tetrahydronaphthyl substitutions (compounds 4g, 4h and 4j), do not exhibit biological activities even at a high dosage of over 100 μg ml−1. Bulky groups, such as 2-naphthyl, diphenyl and adamantyl bulky groups, show only little biological activities (compounds 4i, 4k and 4l). Aliphatic substitutions, such as methyl, propyl, cyclopropyl, isopropyl, isobutyl and tert-butyl groups (compounds 4a–f), exhibited more significant biological activities than aromatic bulky groups in general (compounds 4g–l). Among the compounds 4f, 4b and 4d show significant high biological activities with IC50 of 0.75, 1.5 and 3 μg ml−1, respectively. tert-Butyl substitution on compound 4 seems to be the best candidate from this study for further biological investigation.
| Compound | R | IC50/μg ml−1 |
|---|---|---|
| a No significant biological activities shown even at over 100 μg mL−1. | ||
| 4a | Methyl | 12 |
| 4b | Propyl | 1.5 |
| 4c | Cyclopropyl | 25 |
| 4d | Isopropyl | 3 |
| 4e | Isobutyl | 6 |
| 4f | tert-Butyl | 0.75 |
| 4g | Phenyl | a |
| 4h | Naphthyl | a |
| 4i | 2-Naphthyl | 70 |
| 4j | 1,2,3,4-Tetrahydronaphthyl | a |
| 4k | Diphenyl | 80 |
| 4l | Adamantyl | 40 |
| 3 | 6 | |
| Ferrocene | a | |
| Cisplatin | 12.5 |
Conclusion
Here we reported the examination of ferrocenyl amino phosphines and their possible cytotoxic potential towards the human Hep3B hepatocellular carcinoma cell. Compounds 4b, 4d and 4f exhibit strong cytotoxicity with IC50 < 5 μg ml−1.Experimental section
1. General procedures
Unless otherwise indicated, all reactions were carried out under nitrogen atmosphere. Melting points were measured using an electrothermal 9100 apparatus in capillaries and the data were uncorrected. NMR spectra were recorded on a Varian 500 MHz Fourier transform spectrometer. 1H and 13C NMR spectra were recorded relative to residual protiated solvent; a positive value of the chemical shift denotes a resonance downfield from TMS. 31P NMR spectra were recorded using H3PO4 as an external standard (0 ppm). Mass analyses were performed on a Finnigan model Mat 95 ST mass spectrometer. High-performance liquid chromatography (HPLC) analyses were performed using a Hewlett-Packard model HP 1050/1100 LC interfaced to a HP 1050 series computer workstation using a variety of optically active columns (Daicel Chiracel AS-H, Chiracel AS, Chiracel AD, or Chiracel AD-H). Optical rotations were measured on a Perkin-Elmer model 341 polarimeter at 20 °C. The solid prochiral ferrocenyl ketones were re-crystallized before use and all other chemicals were purchased from commercial suppliers and used without further purification. Diethyl ether was freshly distilled from Na/benzophenone ketyl under nitrogen. Dichloromethane was freshly distilled from calcium hydride under nitrogen. All reactions were monitored by analytical thin-layer chromatography (TLC) on Merck aluminium-precoated plates of silica gel 60 F254 with detection by spraying with 5% (w/v) dodecamolybdophosphoric acid in ethanol or 5% (w/v) ninhydrin in ethanol and subsequent heating. E. Merck silica gel 60 (230–400 mesh) was used for flash chromatography. Hydrogenation reactions were carried out using a Parr 4714 bomb and a Parr 4520 series stirred reactor.2. Synthesis of ferrocenyl amino phosphines 4a–l
The enantioselective pure (S)-1 was prepared using Friedel–Crafts acylation of ferrocene and asymmetric hydrogenation of ferrocenyl ketones catalyzed by freshly prepared [{(R)-XylylP-Phos}RuCl2{(R,R)-DPEN}] (ref. 24) or purchased from a commercial supplier. The ferrocenyl alcohol, (S)-1, was dissolved in a mixture of pyridine and acetic anhydride (4.3 equiv.) and stirred for 12 h at room temperature. Solvents were removed under vacuum. The solid acetate was treated with dimethylamine (1.8 equiv., 33% solution in water) in MeOH. After stirring for 12 h at room temperature, solvents were removed under reduced pressure and the crude product was extracted with 10% H3PO4 after being layered with Et2O. The aqueous layer was then basified with 10% NaOH and was extracted with Et2O. The combined organic phase was washed with water and brine, and dried over MgSO4. The crude product was purified by column chromatography in alumina to give the corresponding ferrocenyl amine (S)-2. The amine (S)-2 was dissolved in diethyl ether and cooled to 0 °C. t-BuLi (1.2 equiv., 1.5 M) was added, the mixture was stirred for 1 h at 0 °C and diphenylchlorophosphine (1.2 equiv.) was added. After the reaction mixture was warmed to rt and stirred overnight, water was added slowly to hydrolyze the unreacted t-BuLi. The crude product was extracted with 10% H3PO4 after being layered with Et2O. The aqueous layer was then basified with 10% NaOH and was extracted with Et2O. The combined organic phase was washed with water and brine, and dried over MgSO4. The crude product was purified by column chromatography to give the corresponding (S,Rp)-3. The 1,2-disubstituted ferrocenyl amine was sealed in an air-free tube with acetic anhydride. After the reaction mixture was heated to 100 °C for 1 h, the acetic anhydride was removed under reduced pressure. The residue was dissolved in CH3CN and a primary amine (5 equiv.) was added. The reaction mixture was stirred at 80 °C overnight. The reaction mixture was washed with water and extracted with diethyl ether. The crude product was purified by column chromatography to give the corresponding ferrocenyl amino-phosphine (S,Rp)-4.3. In vitro cytotoxicity assay
Human carcinoma cell line Hep3B hepatocellular carcinoma was used in order to perform preliminary anti-cancer screening for the selected ferrocenyl compounds. Cancer cells (1 × 104 per well) seeded in the 96-well microtitre plates for 24 h were prepared for the ferrocenyl compound screening. The selected compounds were prepared as a stock concentration of 10 mg mL−1 in dimethyl sulfoxide (DMSO) and were added at a concentration of 100 μg mL−1 and incubated for a further 48 h (Fig. 1). Untreated controls received either total complete medium or 1% of DMSO. Cisplatin was used as the positive cytotoxic reference and dissolved in cell culture medium. Afterwards, the evaluation of possible antiproliferative effect or cytotoxicity of those ferrocenyl compounds was examined by the sulforhodamine B protein staining methods. Briefly, cancer cells were fixed with trichloroacetic acid, washed with distilled water and stained with sulforhodamine B. Afterwards, cells were washed again with acetic acid and stained cells were dissolved in unbuffered Tris-base. Finally, optical absorptions were measured at 575 nm using a microplate reader (Victor V form Perkin Elmer, Life Sciences). The 50% inhibitory concentrations of compounds and cisplatin were calculated from these experimental results.![Hep3B
cells treated with (A) vehicle (1% DMSO) for 48 hours and (B) 4f [3 μg mL−1] for 48 hours. Significant cell death could be observed.](/image/article/2011/MD/c1md00005e/c1md00005e-f1.gif) | ||
| Fig. 1 Hep3B cells treated with (A) vehicle (1% DMSO) for 48 hours and (B) 4f [3 μg mL−1] for 48 hours. Significant cell death could be observed. | ||
Acknowledgements
The authors acknowledge the research grants 1ZV-5L and APK-08 offered by HKPU. Dr C. H. Chui is supported by the Assistant Professorship kindly offered by Professor X. M. Tao. Professor R. Gambari is sponsored by AIRC (Italian Association for Cancer Research). The authors wish to dedicate to Professor Albert Sun-Chi Chan on his occasion of 60th birthday.Notes and references
- (a) A. Harstrick, H. J. Schmoll, H. Poliwoda, G. Sass and Y. Rustum, Eur. J. Cancer, 1997, 7, 1000 Search PubMed; (b) A. H. Calvert, D. R. Newell and M. J. Tilby, Oxford Textbook of Oncology, 1995, p. 552 Search PubMed; (c) E. R. T. Tiekink, Crit. Rev. Oncol. Hematol., 2002, 42, 225 CrossRef.
- (a) B. Rosenberg, L. Vancamp, J. E. Trosco and V. H. Mansour, Nature, 1969, 222, 385 CrossRef CAS; (b) R. J. Boyles, M. C. Baird, B. G. Campling and N. Jain, J. Inorg. Biochem., 2001, 84, 159 CrossRef; (c) J. B. Waem and M. M. Harding, J. Organomet. Chem., 1990, 393, 205 CrossRef; (d) G. Mokdsi and M. M. Harding, J. Inorg. Biochem., 2001, 83, 205 CrossRef CAS.
- (a) R. J. Boyles, M. C. Baird, B. G. Campling and N. Jain, J. Inorg. Biochem., 2001, 84, 159 CrossRef; (b) N. Motohashi, R. Meyer, S. R. Gollapudi and K. R. Bhattiprolu, J. Organomet. Chem., 1990, 393, 205 CrossRef.
- (a) P. N. Kelly, A. Pretre, S. Devoy, O. Rielly, R. Devery, A. Goel and J. F. Gallagher, J. Organomet. Chem., 2007, 692, 1327 CrossRef CAS; (b) D. Osella, M. Ferrali, P. Zanello, F. Laschi, M. Fontani, C. Nervi and G. Cavigiolio, Inorg. Chim. Acta, 2000, 306, 42 CrossRef CAS.
- C. Biot, L. Delhaes, L. A. Macjejewaski, M. Mortuaire, D. Camus, D. Dive and J. S. Brocard, Eur. J. Med. Chem., 2000, 35, 707 CrossRef CAS.
- (a) H. Sun, Q. Wang, R. Huang, H. Li and Y. J. Li, J. Organomet. Chem., 2002, 655, 182 CrossRef CAS; (b) R. Huang and Q. Wang, J. Organomet. Chem., 2001, 94, 637 Search PubMed; (c) B. Cottineau, P. Toto, C. Marot, A. Pipaud and J. Chenault, Bioorg. Med. Chem. Lett., 2002, 12, 2105 CrossRef CAS; (d) H. Yu, L. Shao and J. Fang, J. Organomet. Chem., 2007, 692, 991 CrossRef CAS; (e) M. Zora and M. Gormen, J. Organomet. Chem., 2007, 692, 5026 CrossRef CAS.
- H. Nawaz, Z. Akhter, S. Yameen, H. M. Siddiqi, B. Mirza and A. Rifat, J. Organomet. Chem., 2009, 694, 2198 CrossRef CAS.
- C. Biot, N. Francois, L. Maciejewski, J. Brocard and D. Poulain, Bioorg. Med. Chem. Lett., 2000, 10, 839 CrossRef CAS.
- R. Epton, G. Marr and G. K. Rogers, J. Organomet. Chem., 1978, 150, 93 CrossRef CAS.
- G. Jaouen, S. Top, A. Vessieres, G. Leclercq, J. Quivy, L. Jin and A. Croisy, Organ. & Organomet. Syn., 2000, 3, 89 CAS.
- M. D. Joksovic, V. Markovic, Z. D. Juranic, T. Stanojkovic, L. S. Jovanovic, I. S. Damljanovic, K. M. Szecsenyi, L. S. Jovanovic, I. S. Damljanovic, K. M. Szecsenyi, N. Todorovic, S. Trifunovic and R. D. Vukicevic, J. Organomet. Chem., 2009, 694, 3935 CrossRef CAS.
- Z. Ratkovic, Z. D. Juranic, T. Stanojkovic, D. Manojlovic, R. D. Vukicevic, N. Radulovic and M. D. Joksovic, Bioorg. Chem., 2010, 38, 26 CrossRef CAS.
- V. Zsoldos-Mady, A. Csampai, R. Szabo, E. Meszaros-Alapi, J. Pasztor, F. Hudecz and P. Sohar, ChemMedChem, 2006, 1, 1119 CrossRef CAS.
- B. Long, S. Liang, D. Xin, Y. Yang and J. Xiang, Eur. J. Med. Chem., 2009, 44, 2572 CrossRef CAS.
- A. M. Gellett, P. W. Huber and P. J. Higgins, J. Organomet. Chem., 2008, 693, 2959 CrossRef CAS.
- Z. Petrovski, M. R. P. Norton de Matos, S. S. Braga, C. C. L. Pereira, M. L. Matos, I. S. Goncalves, M. Pillinger, P. M. Alves and C. C. Romao, J. Organomet. Chem., 2008, 693, 675 CrossRef CAS.
- J. Quirante, F. Dubar, A. González, C. Lopez, M. Cascante, R. Cortés, I. Forfar, B. Pradines and C. Biot, J. Organomet. Chem., 2011, 696, 1011 CrossRef CAS.
- X. H. Pan, X. Liu, B. X. Zhao, Y. S. Xie, D. S. Shin, S. L. Zhang, J. Zhao and J. Y. Miao, Bioorg. Med. Chem., 2008, 16, 9133 CrossRef.
- E. A. Hillard, A. Vessie'res, S. Top, P. Pigeon, K. Kowalski, M. Huche and G. Jaouen, J. Organomet. Chem., 2007, 692, 1315 CrossRef CAS.
- I. Ott, K. Kowalski, R. Gust, J. Maurer, P. Mucke and R. F. Winter, Bioorg. Med. Chem. Lett., 2010, 20, 866 CrossRef CAS.
- M. Viotte, B. Gautheron, I. Nifant'ev and L. G. Kuz'mina, Inorg. Chim. Acta, 1996, 253, 71 CrossRef CAS.
- A. Rosenfeld, J. Blum, D. Gibson and A. Ramu, Inorg. Chim. Acta, 1992, 201, 219 CrossRef CAS.
- (a) K. H. Lam, C. H. Chui, R. Gambari, R. S. M. Wong, G. Y. M. Cheng, F. Y. Lau, P. B. S. Lai, S. W. Tong, K. W. Chan, W. Y. Wong, A. S. C. Chan and J. C. O. Tang, Eur. J. Med. Chem., 2010, 45, 5527 CrossRef CAS; (b) K. H. Lam, R. Gambari, M. C. W. Yuen, C. W. Kan, P. Chan, L. J. Xu, W. J. Tang, C. H. Chui, Y. M. Cheng, S. M. Wong, F. Y. Lau, S. W. Tong, K. W. Chan, B. S. Lai, S. H. L. Kok, C. H. Cheng, A. S. C. Chan and J. C. O. Tang, Bioorg. Med. Chem. Lett., 2009, 2266 CrossRef CAS.
- (a) W. S. Lam, S. H. L. Kok, T. T. L. Au-Yeung, J. Wu, H. Y. Cheung, F. L. Lam, C. H. Yeung and A. S. C. Chan, Adv. Synth. Catal., 2006, 348, 370 CrossRef CAS; (b) W. S. Lam, MPhil thesis, The Hong Kong Polytechnic University, 2006.
- (a) T. Hayashi, T. Mise, M. Fukushima, M. Kagotani, N. Nagashima, Y. Hamada, A. Matsumoto, S. Kawakami, M. Konishi, K. Yamamoto and M. Kumada, Bull. Chem. Soc. Jpn., 1980, 53, 1138 CrossRef CAS; (b) T. Hayashi, M. Konishi, M. fukushima, T. Mise, M. Kagotani, M. Tajika and M. Kumada, J. Am. Chem. Soc., 1982, 104, 180 CrossRef CAS.
- G. W. Gokel, D. Marquarding and I. K. Ugi, J. Am. Chem. Soc., 1972, 37, 3052 CAS.
| This journal is © The Royal Society of Chemistry 2011 |
