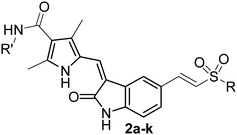
Synthesis and evaluation of novel 5-sulfonyl-indolin-2-ones as potent cytotoxic agents†
Yu
Luo‡
a,
Feng
Xiao‡
a,
Shijing
Qian
c,
Qiaojun
He
c,
Wei
Lu
*ab and
Bo
Yang
*c
aDepartment of Chemistry, East China Normal University, Shanghai, 200062, China
bInstitute of Drug Discovery and Development, iAIR, East China Normal University, Shanghai, 200062, China. E-mail: wlu@chem.ecnu.edu.cn; Fax: +86(21)62602475; Tel: +86(21)62602475
cCollege of Pharmaceutical Science, Zhejiang University, Hangzhou, 310058, China. E-mail: yang924@zju.edu.cn; Fax: +86(571)88208400; Tel: +86(571)88208400
First published on 7th September 2011
Abstract
A series of novel 5-sulfonyl-indolin-2-ones were designed, synthesized, and screened for their antitumor activity on SGC-7901, A549, HCT116, and ECA-109 cell lines. Four compounds with the most potent antitumor activity were further determined as fibroblast growth factor receptor 2 (FGFR2) inhibitors. Among them, compound 2b was further identified to inhibit HUVECs tube formation.
Angiogenesis plays a central role in the growth and metastasis of solid tumors.1 In addition, angiogenesis has been considered the key step in the transformation of some tumor cells from the dormant state to the malignant state. Thus, inhibition of tumor angiogenesis is an attractive target for the development of new antitumor agents.2–4
Among the members of the receptortyrosine kinase (RTK) superfamily, fibroblast growth factor receptor 2 (FGFR2) plays important roles in the regulation of cancer cell functions, such as growth, differentiation and apoptosis.5–7 Thus, it is worthwhile to develop antitumor agents with potent inhibitory activities of antiangiogenesis as well as FGFR2 kinase.8,9
Sunitinib , a multi-targeted RTK inhibitor, has been approved for the treatment of advanced renal cell carcinoma and imatinib-resistant gastrointestinal stromal tumor.10 Recently, many efforts have been devoted to develop novel Sunitinib analogs.11–14 Moreover, some investigations have confirmed substitutents at position 5 of indolin-2-one scaffold can locate into a hydrophobic pocket in the receptor tyrosine kinases.12–14 Thus, added substituents, such as phenyl group, might enhance the binding capacity via the hydrophobic interaction.
On the basis of these results, we devised and synthesized two series of indolin-2-one derivatives (compounds 1 and 2, Fig. 1), containing α,β-unsaturated sulfone moieties at position 5 of indolin-2-one scaffold. These vinyl sulfonyl moieties might locate into this pocket, which could further enhance the binding capacity. The antitumor effect of all the newly synthesized compounds on the in vitrogrowth of four cell lines was evaluated. Some compounds were further investigated for their inhibitory activity of the fibroblast growth factor receptor 2 (FGFR2) and the human umbilical vein endothelial cell (HUVEC) tube formation. Herein, we report the details of our investigation of these series of indolin-2-one compounds.
 | ||
| Fig. 1 The design of the novel 5-sulfonyl-indolin-2-ones. | ||
Four 2-carbaldehyde-pyrroles 4a–d were first prepared via the method reported by Manley and co-workers (Scheme 1).15 Subsequently, the first subseries of indolin-2-one derivatives (compound 1) was synthesized as illustrated in Scheme 1. Indolin-2-one compound 5, prepared according to the reported method,16 was condensed with aldehyde 4b to give compound 6. Knoevenagel reaction of 6 with two aromatic aldehydes afforded compounds 1a and 1b with completely trans-configuration.
 | ||
| Scheme 1 Synthesis of the first subseries of compounds. Conditions and reagents: (a) NaOH, H2O/ethanol, 60%; (b) aryl aldehydes, pyrrolidine, ethanol, reflux, 28–33%. | ||
The second subseries of indolin-2-one derivatives (compound 2) were synthesized from compound 7, which was prepared according to the published method.13 Thus, thioetherfication of 7 with different sulfhydryl compounds afforded the sulfoethers 8a–h. Reduction of 8a–h with KBH4 in DMSO gave the alcohol 9a–h, which was further oxidized with Oxone to give the corresponding β-hydroxyl sulfone 10a-h. These β-hydroxyl sulfones 10a-h were allowed to undergo dehydration17 to afford the key intermediate 11a–h. Finally, compounds 2a–k were synthesized via Knoevenagel reactions of intermediates 11a–h with 4a–d (Scheme 2). The structures of this subseries of compounds were illustrated in Table 1.
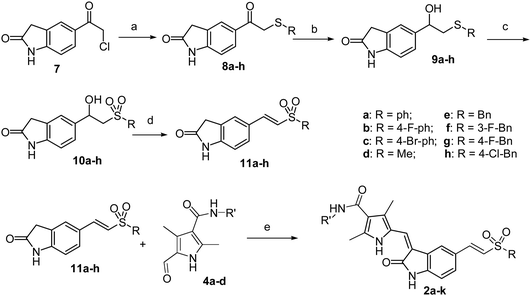 | ||
| Scheme 2 Synthesis of the second subseries of compounds. Conditions and reagents: (a) thiol compounds, K2CO3, DMF, 52–96%; (b) DMSO, KBH4, 43–70%; (c) CH3OH, Oxone, 47–73%; (d) CH3SO2Cl, DMAP, CH2Cl2, 34–57%; (e) pyrrolidine, ethanol, reflux, 67–88%. | ||
|
|
||
|---|---|---|
| Compound | R′ | R |
| 2a | –CH2CH2N(CH2CH3)2 | C6H5– |
| 2b | –CH2CH2N(CH2CH3)2 | 4-F–C6H5– |
| 2c | –CH2CH2N(CH2CH3)2 | 4-Br–C6H5– |
| 2d | –CH2CH2N(CH2CH3)2 | CH3– |
| 2e | –CH2CH2N(CH2CH3)2 | C6H5CH2– |
| 2f | –CH2CH2N(CH2CH3)2 | 3-F–C6H5CH2– |
| 2g | –CH2CH2N(CH2CH3)2 | 4-F–C6H5CH2– |
| 2h | –CH2CH2N(CH2CH3)2 | 4-Cl–C6H5CH2– |
| 2i | –CH2CH2N(CH3)2 | 4-F–C6H5– |
| 2j | –CH2CH2CH2N(CH3)2 | 4-F–C6H5– |
| 2k | –CH2CH2CH2N(CH2CH3)2 | 4-F–C6H5– |
All targeted compounds were investigated for cytotoxic activity against four human cancer cell lines. Sunitinib was used as the reference compound. The results are illustrated in Table 2.
| Compound | IC50 (μM) | |||
|---|---|---|---|---|
| A549 | SGC-7901 | ECA-109 | HCT116 | |
| a Each value was reproduced in three experiments. | ||||
| 2a | 6.86 | 5.82 | 2.7 | 6.44 |
| 2b | 4.34 | 6.87 | 5.74 | 1.82 |
| 2c | 5.21 | 2.28 | 6.23 | 3.35 |
| 2d | 29.05 | 16.66 | 12.5 | 2.64 |
| 2e | 8.05 | 14.46 | 10.6 | 13.73 |
| 2f | 8.41 | 11.06 | 7.33 | 12.68 |
| 2g | >12.5 | >12.5 | >12.5 | >12.5 |
| 2h | >25.0 | >25.0 | >25.0 | >25.0 |
| 2i | 6.62 | 6.25 | 2.99 | 7.2 |
| 2j | >12.5 | 6.35 | 2.87 | 9.84 |
| 2k | >12.5 | 3.45 | 4.75 | 5.77 |
| 1a | 2.87 | 4.99 | 1.37 | 2.41 |
| 1b | 2.85 | 2.58 | 4.84 | 1.95 |
| Sunitinib | 18.58 | 18.60 | 16.86 | 2.11 |
The data showed that many of the compounds exhibited more potent anticancer activity than Sunitinib. In all assays, compounds 2a–c were generally more active than 2e–h, suggesting that insertion of a methylene group between the phenyl group and the sulfonyl group resulted in reduced activity. In addition, compound 2d exhibited much less antitumor activity than those with aromatic rings, indicating that aromatic substituents could enhance the binding capacity via the hydrophobic interaction at this pocket. However, exchange of sulfonyl group and the unsaturated double bond of the sulfonyl moiety had limited influence on the antitumor potency. Besides, the side chain at the pyrrole ring also affected the cytotoxic potency (compare 2b with 2i, 2j and 2k). Among all the compounds, 2b was the most active, with an IC50 of 4.34, 6.87, 5.74 and 1.82 μM against human lung adenocarcinoma A549, human gastric cancer SGC-7901, human esophageal cancer ECA-109 and colorectal carcinoma HCT116 cell lines, respectively.
Compounds 2b, 2c and 1b were selected to study the FGFR2 inhibitory activity. The standard compound, quercetin dehydrate, was used as a control. In addition, the FDA approved receptor tyrosine kinase inhibitor Sunitinib was also incorporated for a comparison with the in vitro study against FGFR2. As shown in Table 3, all the compounds exhibited more potent kinase inhibitory activity than Quercetin dehydrate and Sunitinib. These results suggested that the α,β-unsaturated sulfone could exert a significant effect on FGFR2 inhibitory activity, as well as the cytotoxic activity.
| DMSO | 2b | 2c | 1b | Sunitinib | Quercetin dihydrate | |
|---|---|---|---|---|---|---|
| a The concentration of these compounds was 50 uM. Each value was reproduced in three experiments. | ||||||
| Inhibition ratio (%) | — | 76.6 | 67.8 | 72.0 | 61.8 | 67.3 |
Our results showed that 2b exhibited strong anti-proliferative activity on all of the tested cell lines. Meanwhile, based on Flow Cytometric Analysis, the DNA fragmentation revealed that cytotoxicity of 2b was related to the apoptotic process. As indicated in Fig. 2, the percentage of hypodiploid DNA contents increased up to 72 h after adding 5.0 μM 2b to A549 cells, at which time the percentage of sub-G0/G1 cells reached up to 68.21%, while the cells treated with 5.0 μM Sunitinib showed an apoptosis rate of 15.81%. This data confirms that 2b induced apoptosis in A549 cells, as expected for an inhibitor of RTK assembly.
 | ||
| Fig. 2 2b Triggered A549 cells to apoptosis in concentration-dependent manner. The cells were treated with 2b 1.25 μM, 2.5 μM and 5.0 μM for 72 h. DNA content of 20,000 events was analyzed by flow cytometry. | ||
The production of tubular structures is an important step in angiogenesis. We therefore, investigated the effects of 2b and Sunitinib on HUVECs tube formation. As shown in Fig. 3, control HUVECs, plated on Matrigel and incubated with control medium, aligned to form lumen-like structures and anastomosing tubes with multicentric junctions. HUVECs cultures, treated with various concentrations of 2b or Sunitinib, formed fewer tubes as well as fewer and weaker anastomoses in a dose-dependent manner.
 | ||
| Fig. 3 HUVECs tube formation.HUVECs (2 × 104cells) were re-suspended for 1 h in standard medium (A), or treated with 0.5 μM 2b (B), 1.0 μM 2b (C), 2.0 μM 2b (D), or 2.0 μM Sunitinib (E). | ||
We determined that 2b (2.0 uM) significantly reduced HUVEC migration just like the angiogenesis inhibitor Sunitinib. Meanwhile, the ability of endothelial cells to form capillary tubes is a specialized function of this cell type. Vascular tube formation results from a finely tuned balance between proliferation, migration and differentiation. This study indicated that antiangiogenesis process of 2b plays an essential role in its antitumour activity.
In summary, two series of 5-α,β-unsaturated sulfonyl-indolin-2-ones were synthesized and identified as potent inhibitor of human cancer in cell proliferation and angiogenesis. The compound 2b exhibited high inhibitory activity against FGFR2 protein kinase, as well as HUVECs tube formation.
Acknowledgements
This study was financially supported by the Shanghai Municipal Natural Science Foundation (10ZR1409600) and the Fundamental Research Funds for the Central Universities. And we also thank Lab of Organic Functional Molecules, the Sino-French Institute of ECNU for supports.References and notes
- J. Folkman, N. Engl. J. Med., 1971, 285, 1182–1186 CAS.
- J. M. Cherrington, L. M. Strawn and L. K. Shawver, Adv. Cancer Res., 2000, 79, 1–38 CrossRef CAS.
- L. Sun and G. McMahon, Drug Discovery Today, 2000, 5, 344–353 CrossRef CAS.
- B. I. Rini, Clin. Cancer Res., 2007, 13, 1098–1106 CrossRef CAS.
- Y. Zhang, H. Wang, S. Toratani, J. D. Sato, M. Kan, W. L. McKeehan and T. Okamoto, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 11336–11340 CrossRef CAS.
- A. U. Dignass, S. Tsunekawa and D. K. Podolsky, Gastroenterology, 1994, 106, 1254–1262 CAS.
- J. H. Jang, K. H. Shin and J. G. Park, Cancer Res., 2001, 61, 3541–3543 CAS.
- S. A. Byron and P. M. Pollock, Future Oncol., 2009, 5, 27–32 Search PubMed.
- A. Yoshinari, I. Tsuneo and K. Kazuhiko, Expert Opin. Invest. Drugs, 2011, 20, 595–604 CrossRef.
- R. J. Motzer, T. E. Hutson, P. Tomczak, M. D. Michaelson, R. M. Bukowski, O. Rixe, S. Oudard, S. Negrier, C. Szczylik, S. T. Kim, I. Chen, P. W. Bycott, C. M. Baum and R. A. Figlin, N. Engl. J. Med., 2007, 356, 115–124 CrossRef.
- F. Xiao, Y. Luo, W. Lu and J. Tang, Chin. J. Org. Chim., 2009, 29, 459–461 Search PubMed.
- K. Lackey, M. Cory, R. Davis, S. V. Frye, P. A. Harris, R. N. Hunter, D. K. Jung, B. McDonald, R. W. McNutt, M. R. Peel, R. D. Rutkowske, J. M. Veal and E. R. Wood, Bioorg. Med. Chem. Lett., 2000, 10, 223–226 CrossRef CAS.
- N. Kammasud, C. Boonyarat, K. Sanphanya, M. Utsintong, S. Tsunoda, H. Sakurai, I. Saiki, I. Andre, D. S. Grierson and O. Vajragupta, Bioorg. Med. Chem. Lett., 2009, 19, 745–750 CrossRef CAS.
- N. Kammasud, C. Boonyarat, S. Tsunoda, H. Sakurai, I. Saiki, D. S. Grierson and O. Vajragupta, Bioorg. Med. Chem. Lett., 2007, 17, 4812–4818 CrossRef CAS.
- J. M. Manley, M. J. Kalman, B. G. Conway, C. C. Ball, J. L. Havens and R. Vaidyanathan, J. Org. Chem., 2003, 68, 6447–6450 CrossRef CAS.
- A. Andreani, S. Burnelli, M. Granaiola, A. Leoni, A. Locatelli, R. Morigi, M. Rambaldi, L. Varoli, L. Landi, C. Prata, M. V. Berridge, C. Grasso, H.-H. Fiebig, G. Kelter, A. M. Burger and M. W. Kunkel, J. Med. Chem., 2008, 51, 4563–4570 CrossRef CAS.
- M. C. Clasby, D. Craig, A. A. Jaxa-Chamiec, J. Y. Q. Lai, A. Marsh, A. M. Z. Slawin, A. J. P. White and D. J. Williams, Tetrahedron, 1996, 52, 4769–4802 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c1md00105a |
| ‡ The two first authors contributed equally to the article. |
| This journal is © The Royal Society of Chemistry 2011 |