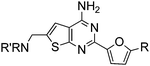
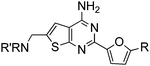
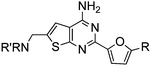
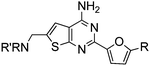
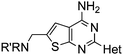
Aminomethyl substituted thieno[2,3-d]pyrimidines as adenosine A2A receptor antagonists
Brian C.
Shook
*a,
Devraj
Charavarty
a,
J. Kent
Barbay
a,
Aihua
Wang
a,
Kristi
Leonard
a,
Vernon
Alford
a,
Mark
Powell
a,
Derek A.
Beauchamp
a,
Stefanie
Rassnick
b,
Robert
Scannevin
b,
Karen
Carroll
b,
Nathaniel
Wallace
b,
Jeffrey
Crooke
b,
Mark
Ault
b,
Lisa
Lampron
b,
Lori
Westover
b,
Kenneth
Rhodes
b and
Paul F.
Jackson
a
aMedicinal Chemistry, Johnson & Johnson PRD, Welsh and McKean Roads, P.O. Box 776, Spring House, PA 19477, USA. E-mail: bshook@its.jnj.com; Fax: +1 215-540-4612; Tel: +1 215-628-7047
bCNS Biology, Johnson & Johnson PRD, Welsh and McKean Roads, P.O. Box 776, Spring House, PA 19477, USA
First published on 19th May 2011
Abstract
A novel series of aminomethyl substituted thieno[2,3-d]pyrimidines have been identified as adenosine A2A receptor antagonists. Analogues show excellent in vitro activities and have excellent activity in vivo in mouse models of Parkinson's disease.
Introduction
Parkinson's disease (PD) is a chronic, progressive neurological disease that affects ∼1% of the population over the age of 65.1 It is characterized by progressive impairment in motor function caused by gradual loss of dopamine (DA) producing neurons in ventral midbrain and concomitant loss of DA input into the striatum.2,3 The loss of DA input leads to dysregulation of striatal function and the classic motor symptoms of PD, such as resting tremor, muscular rigidity and bradykinesia. The impairment of motor function is often accompanied by anxiety, depression and cognitive impairment.Most treatments for PD focus on restoring dopamine signaling to reduce the severity of the motor symptoms.4Dopamine replacement therapy using L-DOPA, the precursor to dopamine, remains the gold-standard treatment for PD. Although the dopamine targeted therapies work well to address the motor impairments of PD, they all produce undesirable side effects (dyskinesia, hallucinations, on–off effects) that become more severe with continued treatment. This type of therapy has reduced efficacy as the disease progresses and does not address the co-morbidities associated with PD like mood disturbances, postural instability and cognitive impairment.
As a result of these limitations many drug companies have sought non-dopamine based therapies for PD. One approach that has received considerable attention is modulation of adenosine receptors.5Adenosine is a neuromodulator that coordinates responses to dopamine and other neurotransmitters in areas of the brain that are responsible for motor function, learning and memory.6Adenosine is comprised of four distinct sub-types designated A1, A2A, A2B, and A3.7 Both A2A and A1 receptors are highly expressed in the brain, particularly striatum, while A2B and A3 receptors are not.8 In the striatum A2A receptors co-localize and physically associate with dopamine D2 receptors.2,3A2A and D2 receptors have opposing effects on adenylate cyclase and cAMP production in cells such that A2A receptor antagonists enhance D2 dependent signaling. Of importance to PD, pharmacological blockade of A2A receptors had shown dramatic beneficial effects in preclinical animal models of PD. In fact, several selective A2A antagonists have advanced into clinical development.9
There have been several reports published suggesting that adenosine A1antagonists may improve learning and memory.10 This would suggest that a dual A2A/A1antagonist may offer improved benefit to PD patients as it is known that cognitive deficiencies increase as the disease progresses.11 Unfortunately, it is unknown what balance of A1vs.A2A antagonism would be ideal for PD patient benefit. We would like to report herein a novel series of thieno[2,3-d]pyrimidines as selective A2A and dual A2A/A1receptor antagonists for the potential treatment of PD.
Results and discussion
Initial lead generation, through a de novo approach, led to a series of aminomethyl thieno[2,3-d]pyrimidines that were potent adenosine A2A receptor antagonist with varying degrees of selectivity against adenosine A1 receptors and are represented generically by structure 4 (Scheme 1). The compounds were synthesized starting from the commercially available aminonitrile 1 and reacting with various aryl and heteroaryl nitriles by heating with a catalytic amount of t-BuOK in dioxane.12 The resulting aminopyrimidine 2 was oxidized to the corresponding aldehyde 3 using SeO2 (Path 1), that underwent reductive amination with a variety of amines to give the target compound 4. An alternative route (Path 2) was developed to prepare the target compounds where the aminopyrimidine 2 was protected using excess (Boc)2O in the presence of 4-dimethylamino pyridine (DMAP) to give compound 5. Radical bromination of the methyl group using N-bromosuccinimide (NBS) gave the corresponding bromide 6. The Boc protecting groups were removed with TFA and the resulting bromide was alkylated with a variety of amines to provide the target compounds. This route proved to be helpful for incorporating hindered secondary amines.![Synthesis of aminomethyl substituted thieno[2,3-d]pyrimidines.](/image/article/2011/MD/c1md00082a/c1md00082a-s1.gif) | ||
| Scheme 1 Synthesis of aminomethyl substituted thieno[2,3-d]pyrimidines. | ||
A third synthetic route was also used to install aryl and heteroaryl substituents that contained methyl groups to avoid mixtures of oxidized or brominated products that would be obtained from the paths outlined in Scheme 1. The aminonitrile 7 was condensed with a variety of heteroaryl nitriles to afford an intermediate aminopyrimidine that was brominated using NBS to afford 8 (Scheme 2). Bromide 8 was converted to the corresponding vinyl intermediate 9 under standard Suzuki conditions using the corresponding vinyl boronic ester. Dihydroxylation using AD-mix followed by oxidation with periodic acid (HIO4) afforded the aldehyde 3. Reductive amination with a variety of amines affords the target compounds 4.
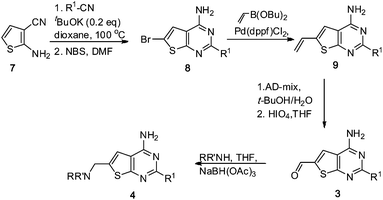 | ||
| Scheme 2 Synthesis of compounds where R1 contains a methyl group. | ||
We started exploring a variety of amines by keeping the right hand portion of the molecule as 2-substituted furan or 2-substituted-5-methylfuran, two heterocycles that were identified that gave good in vitro potency (Table 1). The acyclic amines (10–13) showed modest functional activity against A2A but weak activity for A1 receptors. Cyclopropyl amine 12 did not show any activity in reversing haloperidol induced catalepsy in mice, which was our primary in vivo screening model.13 Greater than 50% reversal of catalepsy indicates a positive result in the catalepsy model (see Experimental section for more details). The very basic pyrrolidines and piperidine analogues (14–16) showed slightly increased activity for A2Ain vitro, but showed no effect in vivo reversing haloperidol induced catalepsy in mice at 10 mg kg−1, p.o. Interestingly, the less basic morpholine analogues 18 and 19 did show good in vivo activity with 18 having an ED50 = 1.3 mg kg−1. The calculated pKa's14 for the pyrrolidine 14 and piperidine 16 were 8.2 and 8.5, respectively, while the less basic morpholine analogue 18 has a calculated pKa of 6.5. With a possible trend being identified our focus was to examine the in vitro and in vivo activity of analogues containing less basic amino substituents. We thought that this approach would increase log P values and potentially increase blood brain barrier (BBB) penetration which would increase in vivo activity.
|
|
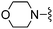
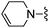
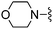
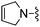
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | NRR′ | R | A2A Ki/nM | A1Ki/nM | Mouse catalepsy activitya,b | Compound | NRR′ | R | A2A Ki/nM | A1Ki/nM | Mouse catalepsy activitya,b |
| a Results are reported as an ED50, active/not active at a single dose or (—) for not tested. b Active is at least 50% reversal of cataleptic activity. | |||||||||||
| 10 |
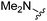
|
H | 140 ± 5 | 3550 ± 409 | — | 21 |
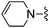
|
H | 6.5 ± 0.9 | 150 ± 9 | ED50 < 3.0 mg kg−1 |
| 11 |
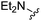
|
H | 55.5 ± 7.4 | 2130 ± 176 | — | 22 |

|
H | 19.7 ± 2.9 | 740 ± 69 | ED50 < 1.0 mg kg−1 |
| 12 |
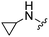
|
H | 36.0 ± 3.4 | 1890 ± 96 | Not active at 10 mg kg−1 | 23 |
|
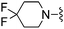
H | 49.6 ± 4.3 | 2230 ± 208 | Active at 10 mg kg−1 |
| 13 |
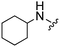
|
H | 170 ± 13 | 2480 ± 100 | — | 24 |
|
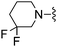
H | 6.6 ± 1.0 | 290 ± 42 | ED50 < 1.0 mg kg−1 |
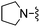
| 14 |
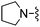
|
H | 28.1 ± 3.7 | 6430 ± 860 | Not active at 10 mg kg−1 | 25 |
|
H | 5.3 ± 0.7 | 100 ± 16 | Active at 10 mg kg−1 |
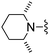
| 15 |
|
Me | 26.1 ± 2.2 | 620 ± 96 | Not active at 10 mg kg−1 | 26 |
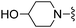
|
H | 300 ± 8 | 4000 ± 365 | — |
| 16 |
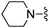
|
Me | 28.8 ± 8.1 | 610 ± 123 | Not active at 10 mg kg−1 | 27 |
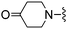
|
H | 230 ± 17 | 2720 ± 188 | — |
| 17 |
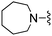
|
H | 27.6 ± 1.8 | 1170 ± 304 | — | 28 |
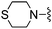
|
H | 9.2 ± 2.1 | 369 ± 66 | ED50 = 3.2 mg kg−1 |
| 18 |
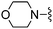
|
H | 29.0 ± 2.0 | 1680 ± 177 | ED50 = 1.3 mg kg−1 | 29 |
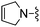
|
H | 7.2 ± 1.0 | 290 ± 10 | ED50 < 0.1 mg kg−1 |
| 19 |
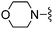
|
Me | 32.8 ± 3.9 | 1050 ± 88 | Active at 10 mg kg−1 | 30 |
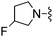
|
H | 10.3 ± 2.6 | 1340 ± 102 | Not active at 10 mg kg−1 |
| 20 |
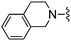
|
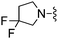
Me | 200 ± 21 | 3320 ± 131 | — | 31 |
|
H | 8.7 ± 1.3 | 1240 ± 307 | ED50 = 10.0 mg kg−1 |
The unsaturated piperidine 21 (calc. pKa = 7.2) was significantly less basic than the saturated compound 16 (calc. pKa = 8.5) and resulted in a 4-fold increase in A2A activity, but more importantly was very potent in vivo having an ED50 < 3.0 mg kg−1, p.o (Table 1). The fluorinated piperidines 22–24 also showed excellent in vitro and in vivo activity. The calculated pKa's for the fluorinated piperidines 22–24 are 7.0, 5.5, and 4.7, respectively, and are also significantly lower than the pKa's for the corresponding pyrrolidine and piperidine analogues. The cis-2,6-dimethylpiperidine 25 (calc. pKa = 8.5)15 proved to be the most potent group for both A2A and A1 activity while maintaining activity in vivo at 10 mg kg−1. The thiomorpholine analog 28 (calc. pKa = 6.7) also showed good in vitro potency and had an in vivoED50 = 3.2 mg kg−1. The pyrroline 29 (calc. pKa = 6.8) was the most potent compound in vivo having an ED50 < 0.1 mg kg−1. The fluorinated pyrrolidines 30 (calc. pKa = 5.9) and 31 (calc. pKa = 3.3) maintained good activity in vitro against A2A but were less effective in vivo compared to the fluorinated piperidines 22 and 24 despite having comparable pKa values.
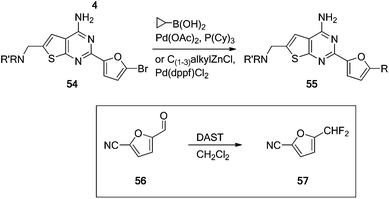
With a good handle on the amino substituents we wanted to further explore the scope of the furan substituent. Although the mono-substituted furans showed excellent in vitro and in vivo activity, mono-substituted furans are potential metabolic liabilities. The furan replacement strategy has also been reported in other chemical scaffolds of A2A receptor antagonists.16 In addition, several of the compounds from Table 1 had low metabolic stability in human liver microsomes (HLM) and short in vivo half-lives in rodent. Therefore, the alkyl substituent on the furan was extended from methyl to ethyl, isopropyl, cyclopropyl and CHF2 (32–42), and chloro- and bromo-substituted furans (43–53) were also prepared (Table 2). The majority of aryl and heteroaryl nitriles were commercially available except propyl, cyclopropyl and CHF2 substituted 2-furanonitriles. The substituted furan nitriles that were not available were synthesized via the corresponding bromide 51 as indicated in Scheme 3.12,17 The bromide 54, prepared viaScheme 1, was converted to the alkyl substituent, shown in 55, via a Suzuki or Negishi reaction using the appropriate boronic acid or alkyl zinc reagents. The aldehyde 56 was converted to the corresponding difluoride 57 using (diethylamino)sulfur trifluoride (DAST) which was incorporated into the thienopyrimidines as outlined in Scheme 1.12
|
|
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | NRR′ | R | A2A Ki/nM | A1Ki/nM | Mouse catalepsy activitya,b | Compound | NRR′ | R | A2A Ki/nM | A1Ki/nM | Mouse catalepsy activitya,b |
| a Results are reported as an ED50, active/not active at a single dose or (—) for not tested. b Active is at least 50% reversal of cataleptic activity. | |||||||||||
| 32 |
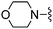
|
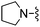
Et | 78.9 ± 10.1 | >610 | — | 43 |
|
Cl | 22.2 ±.3.5 | 710 ± 55 | Not active at 10 mg kg−1 |
| 33 |
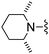
|
Et | 25.2 ± 4.4 | 190 ± 17 | — | 44 |
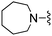
|
Cl | 33.4 ± 3.7 | 1420 ± 175 | Active at 10 mg kg−1 |
| 34 |
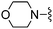
|
i-Pr | >1360 | >600 | — | 45 |
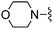
|
Cl | 45.4 ± 7.4 | 640 ± 34 | — |
| 35 |
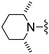
|
i-Pr | 17.0 ± 2.4 | 140 ± 17 | Not active at 10 mg kg−1 | 46 |
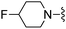
|
Cl | 36.7.± 3.5 | 1080 ± 293 | Active at 10 mg kg−1 |
| 36 |
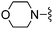
|
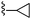
|
960 ± 78 | >1100 | — | 47 |
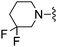
|
Cl | 9.1 ± 1.2 | 188 ± 27 | Active at 10 mg kg−1 |
| 37 |
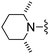
|
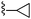
|
110 ± 10 | 330 ± 36 | — | 48 |
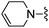
|
Cl | 11.0 ± 0.8 | 190 ± 18 | active at 10 mg kg−1 |
| 38 |
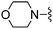
|
CHF2 | 30.8 ± 3.7 | 2700 ± 220 | Not active at 10 mg kg−1 | 49 |
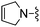
|
Cl | 10.0 ± 0.5 | 780 ± 100 | Not active at 10 mg kg−1 |
| 39 |
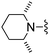
|
CHF2 | 28.6 ± 2.0 | 730 ± 105 | Not active at 10 mg kg−1 | 50 |
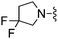
|
Cl | 15.9 ± 2.9 | 245 ± 14 | — |
| 40 |
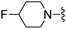
|
CHF2 | 42.2 ± 6.7 | 1670 ± 354 | — | 51 |
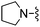
|
Br | 22.5 ± 4.5 | 710 ± 76 | Not active at 10 mg kg−1 |
| 41 |
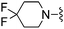
|
CHF2 | 610 ± 33 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 820 ± 798 820 ± 798 |
— | 52 |
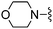
|
Br | 30.0 ± 4.0 | 725 ± 102 | Active at 10 mg kg−1 |
| 42 |
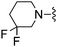
|
CHF2 | 43.2 ± 6.2 | 2100 ± 99 | — | 53 |
|
Br | 12.1 ± 3.1 | 40.2 ± 20.2 | — |
 | ||
| Scheme 3 Synthesis of substituted furans. | ||
We chose morpholine and cis-2,6-dimethylpiperidine as the amines to explore the new substitutions and it was clear that the cis-2,6-dimethylpiperidine analogues are generally more potent than their corresponding morpholine analogues (comparing 32–39), but they are still less potent than the corresponding unsubstituted furan 25 (Table 1). Unfortunately, this was also true when comparing the in vivo activity of 25 and 35, or comparing 18 and 38, where 18 is much more active in reversing catalepsy in mice. This decrease in activity in vivo for 35 and 39 could possibly be explained by the 3–5 fold decrease in A2A functional activity compared to the corresponding unsubstituted analogue 25. However, the inactivity of 38 and the potent activity of the unsubstituted analogue 18 cannot be explained similarly and is surprising considering the comparable in vitro activity, 30.8 and 29.0 nM, respectively.
We also explored chloro- and bromo-substituted furans in compounds 43–53. In general they all maintained good in vitro activity for A2A, but also had decreased activity in vivo for those tested against their corresponding unsubstituted furan analogs. The pyrrolidine 43 had no activity in vivo at 10 mg kg−1 which was consistent with the results obtained from 14 and 15. The piperidines 46–48 and morpholine 52 were all active in vivo at 10 mg kg−1, p.o. Interestingly, the pyrroline 49 was not active at 10 mg kg−1 which was in stark contrast to the des-Cl analogue 29 which had an ED50 < 0.1 mg kg−1. The general decrease in potency in vivo for the alkyl, chloro- and bromo-substituted furan compounds is surprising given the similar in vitro potencies, but was consistent for all compounds.
Next we explored replacement of the furan with various 5-membered heterocycles (Table 3). The 2-substituted oxazoles 58–60 were significantly less potent (12–40 fold) in vitro against A2A than their corresponding 2-substituted furan analogs 18, 29, and 21, respectively. This was much to our surprise as the replacement of just one atom resulted in such a dramatic loss in activity. Likewise the thiazole 61, methyl thiazoles 62 and 63, isoxazoles 64 and 65, thiophenes 66 and 67, and oxazoles 68 and 69 all had similar decreases in A2A activity compared to their corresponding furan analogues. Even the benzofuran 70 had significantly decreased in vitro activity. This was somewhat discouraging that the furan was an ideal substituent and could not be readily replaced with other 5-membered heterocycles.
|
|
||||
|---|---|---|---|---|
| Compound | NRR′ | Het | A2A Ki/nM | A1Ki/nM |
| 58 |
|
|
360 ± 28 | 3250 ± 310 |
| 59 |
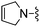
|
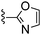
|
240 ± 22 | 3890 ± 267 |
| 60 |
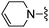
|
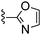
|
240 ± 13 | 2960 ± 403 |
| 61 |
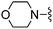
|
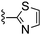
|
250 ± 17 | 2730 ± 167 |
| 62 |
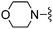
|
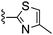
|
210 ± 12 | >930 |
| 63 |
|
|
89.1 ± 8.6 | 300 ± 24 |
| 64 |
|
|
1010 ± 30 | 680 ± 30 |
| 65 |
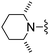
|
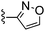
|
130 ± 7 | 500 ± 19 |
| 66 |
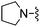
|
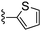
|
110 ± 7 | >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| 67 |
|
|
125 ± 5 | 1020 ± 220 |
| 68 |
|
|
238 ± 12 | 3895 ± 352 |
| 69 |
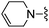
|
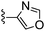
|
240 ± 10 | 2964 ± 311 |
| 70 |
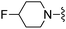
|
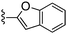
|
350 ± 17 | 630 ± 79 |
After exploring various heterocyclic replacements for the furan, we decided to explore aryl substituents (Table 4). The phenyl substituted analog 71 was a starting point for us despite the modest A2A activity. Interestingly, fluorine or chlorine substituted in the meta-position gave nearly a 10-fold increase in A2A activity. Further exploration at the meta-position revealed that a cyano group in that position was ideal and gave the most potent compounds (76–84) against A2A. The less basic amines gave the best activity for A2A similar to compounds having the furan substituent. Very basic groups like pyrrolidine 83 were not well tolerated and resulted in no activity against A2A although it had decent A1 activity at 130 nM. The less basic compounds 77, 79 and 80 all had very potent activity in vivo ED50's < 1 mg kg−1. Moving the cyano group to the ortho (85) or para (86–88) positions resulted in decreased in vitro activity against A2A. Other electron withdrawing groups at the meta position like CF3, SO2Me, NO2, and CONMe2 decreased A2A activity significantly. A methoxy group (96–98) in the meta position had modest activity for A2A but still less than that obtained from the cyano group.
|
|
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | NR1R2 | R3 | A2AKi/nM | A1Ki/nM | Mouse catalepsy activitya,b | Compound | NR1R2 | R3 | A2A Ki/nM | A1Ki/nM | Mouse catalepsy activitya,b |
| a Results are reported as an ED50, active/not active at a single dose or (—) for not tested. b Active is at least 50% reversal of cataleptic activity. | |||||||||||
| 71 |
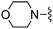
|
H | 995 ± 57 | >5000 | — | 85 |
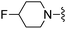
|
2–CN | 1030 ± 77 | 5050 ± 333 | — |
| 72 |
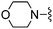
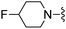
|
3-F | 110 ± 8 | 620 ± 29 | — | 86 |
|
4–CN | 390 ± 25 | 8430 ± 664 | — |
| 73 |
|
3-Cl | 120 ± 8 | 790 ± 50 | — | 87 |
|
4–CN | 180 ± 20 | 1630 ± 219 | — |
| 74 |
|
3,5-diF | 350 ± 29 | 2300 ± 235 | — | 88 |
|
4–CN | 79.2 ± 10.2 | 2350 ± 165 | — |
| 75 |
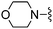
|
3,4-diF | 360 ± 45 | 2890 ± 314 | — | 89 |
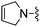
|
3–CF3 | 1070 ± 86 | 2960 ± 344 | — |
| 76 |
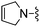
|
3–CN | 83.5 ± 8.8 | 2070 ± 310 | — | 90 |
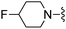
|
3–CF3 | >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
3030 ± 277 | — |
| 77 |
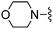
|
3–CN | 36.1 ± 5.4 | 1010 ± 87 | ED50 < 1.0 mg kg−1 | 91 |
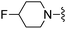
|
4–CF3 | >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
3560 ± 423 | — |
| 78 |
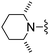
|
3–CN | 64.6 ± 4.4 | 460 ± 67 | — | 92 |
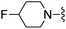
|
2–CF3 | >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
>10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— |
| 79 |
|
3–CN | 54.4 ± 6.1 | 2950 ± 355 | ED50 < 1.0 mg kg−1 | 93 |
|
3–SO2Me | >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
>10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— |
| 80 |
|
3–CN | 39.0 ± 4.0 | 840 ± 36 | ED50 < 1.0 mg kg−1 | 94 |
|
3–NO2 | 3760 ± 309 | >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— |
| 81 |
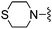
|
3–CN | 50.9 ± 2.3 | 980 ± 80 | Active at 10 mg kg−1 | 95 |
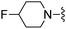
|
3-CONMe2 | 2250 ± 297 | > 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— |
| 82 |
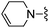
|
3–CN | 13.2 ± 3.0 | 210 ± 52 | Active at 10 mg kg−1 | 96 |
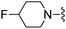
|
3-OMe | 300 ±.31 | 3220 ± 200 | — |
| 83 |
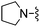
|
3–CN | >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
130 ± 18 | — | 97 |
|
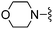
3-OMe | 370 ± 23 | 2990 ± 189 | — |
| 84 |
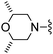
|
3–CN | 150 ± 13 | 1070 ± 60 | — | 98 |
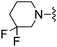
|
3-OMe | 97.7 ± 12.6 | 1810 ± 323 | — |
Conclusions
A novel series of aminomethyl substituted thieno[2,3-d]pyrimidines was developed as potent adenosine A2A receptor antagonists. The 2-substituted furans and 2-substituted-5-methyl furans were ideal groups for optimal potency in vitro and in vivo. Investigation of a wide variety of cyclic and acyclic amines revealed some interesting SAR in vivo. The less basic amino substituents in general gave slightly more potent in vitro activity, but proved to be essential for potent in vivo activity. To expand the SAR we chose a few of the better amino substituents to keep constant while we tried to modify the furan portion of the molecule. Replacement of furan with various 5-membered heterocycles significantly decreased A2A activity. Surprisingly the furan could be replaced with meta-substituted phenyl groups and maintain good in vitro activity against A2A. The 3-cyanophenyl substituent proved to be the ideal group for in vitro A2A activity as well as in vivo activity.Experimental
Reagent grade chemicals and solvents were purchased from commercial suppliers and used without further purification. All chromatography was performed on silica gel and were carried out on a combi-flash system equipped with an automated fraction collector. High resolution MS was performed on a JEOL Accutof JMS-T100 LC with a DART CE ionization source operating in the positive mode. All proton nuclear magnetic resonance spectra were determined using a 300 or 400 MHz Bruker NMR with the appropriate internal standards, and chemical shifts are reported in ppm relative to TMS. Defined J values are reported in Hz. All final compounds were purified to ≥95% purity as determined by Agilent 1100 series high performance liquid chromatography (HPLC) with UV detection at 254 nm using the following method: Supelcosil ABZ + PLUS, 3.3 cm × 2.1 cm, 11 min; 1.2 mL min−1 flow rate; 5–95% 0.1% TFA in CH3CN/0.1% TFA in H2O.Compound 18: 2-furan-2-yl-6-morpholin-4-ylmethyl-thieno[2,3-d]pyrimidin-4-ylamine
Solid potassium-tert-butoxide (325 mg, 2.9 mmol) was added to a dioxane solution (7 mL) of 2-amino-5-methyl-thiophene-3-carbonitrile (2.0 g, 14.5 mmol) and furan-2-carbonitrile (1.3 g, 14.5 mmol). The resulting mixture was heated in an oil bath at 130 °C for 10 minutes. The dark slurry was cooled to room temperature, diluted with THF, and dry packed onto silica gel. The material was then purified viacolumn chromatography to give 1.6 g of 2-furan-2-yl-6-methyl-thieno[2,3-d]pyrimidin-4-ylamine.Solid SeO2 (4.3 g, 39.0 mmol) was added to a dioxane (50 mL)/water (0.5 mL) suspension of 2-furan-2-yl-6-methyl-thieno[2,3-d]pyrimidin-4-ylamine (3.0 g, 13.0 mmol) and the mixture was heated to 100 °C. After 20 h the mixture was filtered hot and diluted with EtOAc. The organic phase was washed with water and brine, dried (Na2SO4) and dry packed onto silica gel. Column chromatography gave 1.7 g of 4-amino-2-(furan-2-yl)thieno[2,3-d]pyrimidine-6-carbaldehyde.
Solid NaBH(OAc)3 (173 mg, 0.82 mmol) was added to a THF solution (4 mL) of 4-amino-2-(furan-2-yl)thieno[2,3-d]pyrimidine-6-carbaldehyde (100 mg, 0.41 mmol) and morpholine (72 μL, 0.82 mmol) and the mixture was heated to 45 °C. After 16 h the mixture was cooled, diluted with EtOAc, washed with saturated aqueous NaHCO3, water and brine, dried (Na2SO4), and dry packed onto silica gel. Column chromatography gave 90 mg of 2-furan-2-yl-6-morpholin-4-ylmethyl-thieno[2,3-d]pyrimidin-4-ylamine 18. 1H NMR (DMSO-d6, 300 MHz): δ = 7.81 (1H, s), 7.50 (2H, s), 7.41 (1H, s), 7.11 (1H, d, J 3.4), 6.51–6.72 (1H, m), 3.71 (2H, s), 3.60 (4H, t, J 4.3), 2.44 (4H, br. s); MSm/z 317 (M + H). HRMS calcd for C15H16N4O2S 316.0994, found 317.1051 (M+ + H)
The procedure used to prepare compound 18 was also used to prepare the following compounds with the appropriate amine substituent. Also, the corresponding nitrile replacement for the furan-2-carbonitrile is specified where appropriate.
Compound 10: 6-dimethylaminomethyl-2-furan-2-yl-thieno[2,3-d]pyrimidin-4-ylamine
1H NMR (300 MHz, chloroform-d): δH 7.59 (1H, s), 7.20–7.33 (1H, m), 7.12 (1H, s), 6.55 (1H, dd, J 3.4, 1.9), 5.82 (2H, br. s), 3.78 (2H, s), 2.38 (6H, s); MSm/z 275 (M + H).Compound 11: 6-diethylaminomethyl-2-furan-2-yl-thieno[2,3-d]pyrimidin-4-ylamine
1H NMR (300 MHz, chloroform-d): δH 7.59 (1H, dd, J 1.7, 0.9), 7.25 (1H, dd, J 3.5, 0.8), 6.96 (1H, s), 6.54 (1H, dd, J 3.4, 1.7), 5.28 (2H, br. s), 3.83 (2H, s), 2.61 (4H, q, J 7.2), 1.08 (6H, t, J 7.2); MSm/z 303 (M + H).Compound 12: 6-cyclopropylaminomethyl-2-furan-2-yl-thieno[2,3-d]pyrimidin-4-ylamine
1H NMR (300 MHz, chloroform-d): δH 7.60 (1H, d, J 0.8), 7.25 (1H, s), 6.99 (1H, s), 6.55 (1H, dd, J 3.5, 1.8), 5.42 (2H, br. s), 4.09 (2H, s), 2.15–2.32 (1H, m), 0.33–0.56 (4H, m); MSm/z 287 (M + H).Compound 13: 6-cyclohexylaminomethyl-2-furan-2-yl-thieno[2,3-d]pyrimidin-4-ylamine
1H NMR (300 MHz, chloroform-d): δH 7.59 (1H, d, J 0.8), 7.24 (1H, d, J 3.4), 7.06 (1H, s), 6.54 (1H, dd, J 3.4, 1.7), 5.44 (2H, br. s), 4.05–4.13 (2H, m), 3.49 (1H, s), 2.52–2.66 (1H, m), 2.06 (2H, s), 1.73 (2H, br. s), 1.06–1.35 (6H, m); MSm/z 329 (M + H).Compound 14: 2-furan-2-yl-6-pyrrolidin-1-ylmethyl-thieno[2,3-d]pyrimidin-4-ylamine
1H NMR (400 MHz, chloroform-d): δH 7.59 (1H, s), 7.25 (1H, d, J 3.3), 7.06 (1H, s), 6.54 (1H, dd, J 3.3, 1.8), 5.44 (2H, br. s), 3.92 (2H, s), 2.61–2.74 (4H, m), 1.85 (4H, dt, J 6.8, 3.3); MSm/z 301 (M + H).Compound 17: 6-(azepan-1-ylmethyl)-2-(furan-2-yl)thieno[2,3-d]pyrimidin-4-amine
MS m/z 329 (M + H).Compound 20: 6-(3,4-dihydro-1H-isoquinolin-2-ylmethyl)-2-(5-methyl-furan-2-yl)-thieno[2,3-d]pyrimidin-4-ylamine
1H NMR (300 MHz, chloroform-d): δH 7.06–7.23 (4H, m), 6.95–7.04 (2H, m), 6.14 (1H, dd, J 3.3, 0.8), 5.35 (2H, br. s), 3.91 (2H, s), 3.74 (2H, s), 2.91 (3H, t, J 5.3), 2.82 (2H, t, J 5.5); MSm/z 377 (M + H).Compound 21: 6-(3,6-dihydro-2H-pyridin-1-ylmethyl)-2-furan-2-yl-thieno[2,3-d]pyrimidin-4-ylamine
1H NMR (300 MHz, chloroform-d): δH 7.60 (1H, s), 7.25 (1H, s), 7.00 (1H, s), 6.55 (1H, dd, J 3.4, 1.9), 5.77 (1H, br. s), 5.69 (1H, br. s), 5.25 (2H, br. s), 3.83 (2H, s), 2.99–3.16 (2H, m), 2.64 (2H, t, J 5.7), 2.12–2.26 (2H, m); MSm/z 313 (M + H).Compound 22: 6-(4-fluoro-piperidin-1-ylmethyl)-2-furan-2-yl-thieno[2,3-d]pyrimidin-4-ylamine
1H NMR (300 MHz, DMSO-d6): δH 7.93 (1H, br. s), 7.72 (1H, s), 7.24 (1H, d, J 3.4), 6.70 (1H, d, J 3.4), 4.64 (2H, br. s), 3.35 (1H, m), 3.16 (4H, br. s), 2.08 (4H, br. s); MSm/z 333 (M + H).Compound 23: 6-(4,4-difluoro-piperidin-1-ylmethyl)-2-furan-2-yl-thieno[2,3-d]pyrimidin-4-ylamine
1H NMR (300 MHz, chloroform-d): δH 7.59 (1H, s), 7.26 (1H, d, J 3.4), 6.96 (1H, s), 6.55 (1H, dd, J 3.4, 1.5), 5.46 (2H, s), 3.78 (2H, s), 2.63 (4H, t, J 5.5), 1.91–2.11 (4H, m); MSm/z 351 (M + H).Compound 24: 6-(3,3-difluoro-piperidin-1-ylmethyl)-2-furan-2-yl-thieno[2,3-d]pyrimidin-4-ylamine
1H NMR (300 MHz, acetone-d6): δH 7.70 (1H, s), 7.36 (1H, s), 7.16 (1H, d, J 3.4), 6.79 (2H, br. s), 6.59 (1H, dd, J 3.4, 1.9), 3.74 (2H, s), 3.63 (2H, br. s), 2.13–2.31 (2H, m), 1.83 (2H, dd, J 12.6, 3.6), 1.46–1.65 (2H, m); MSm/z 351 (M + H). HRMS calcd for C16H16F2N4OS 350.1013, found 350.1066 (M+ + H).Compound 25: 6-(2,6-dimethyl-piperidin-1-ylmethyl)-2-furan-2-yl-thieno[2,3-d]pyrimidin-4-ylamine
1H NMR (300 MHz, chloroform-d): δH 7.59 (1H, s), 7.25 (1H, d, J 3.4), 6.95 (1H, s), 6.55 (1H, dd, J 3.4, 1.5), 5.32 (2H, br. s) 4.11 (2H, s), 2.56 (2H, br. s), 1.76 (2H, br. s), 1.52–1.70 (4H, m), 1.21 (3H, s), 1.19 (3H, s); MSm/z 343 (M + H). HRMS calcd for C18H22N4OS 342.1514, found 343.1621 (M+ + H).Compound 26: 1-(4-amino-2-furan-2-yl-thieno[2,3-d]pyrimidin-6-ylmethyl)-piperidin-4-ol
1H NMR (300 MHz, chloroform-d): δH 7.60 (1H, s), 7.25 (1H, s), 6.99 (1H, s), 6.55 (1H, dd, J 3.4, 1.9), 5.33 (2H, br. s), 3.85 (2H, s), 2.75 (2H, t, J 11.1), 2.56 (2H, t, J 5.3), 1.70–1.99 (5H, m); MSm/z 331 (M + H).Compound 27: 1-(4-amino-2-furan-2-yl-thieno[2,3-d]pyrimidin-6-ylmethyl)-piperidin-4-one
1H NMR (300 MHz, chloroform-d): δH 7.60 (1H, s), 7.26 (1H, s), 7.00 (1H, s), 6.55 (1H, dd, J 3.4, 1.5), 5.47 (2H, s), 3.86 (2H, s), 2.84 (4H, t, J 6.0), 2.49 (4H, t, J 6.0); MSm/z 329 (M + H).Compound 28: 2-(furan-2-yl)-6-(thiomorpholinomethyl)thieno[2,3-d]pyrimidin-4-amine
1H NMR (300 MHz, chloroform-d): δH 7.58 (1H, s), 7.21–7.32 (1H, m), 7.12 (1H, s), 6.55 (1H, dd, J 3.4, 1.9), 5.79 (2H, br. s), 3.80 (2H, s), 2.75–2.84 (4H, m), 2.67–2.73 (4H, m); MSm/z 333 (M + H).Compound 29: 6-((2,5-dihydro-1H-pyrrol-1-yl)methyl)-2-(furan-2-yl)thieno[2,3-d]pyrimidin-4-amine
1H NMR (300 MHz, chloroform-d): δH 7.59 (1H, s), 7.20–7.33 (1H, m), 7.12 (1H, s), 6.55 (1H, dd, J 3.4, 1.9), 5.82 (2H, s), 5.77 (2H, br. s), 3.78 (2H, s), 3.62 (4H, m); MSm/z 299 (M + H).Compound 30: 6-(3-fluoro-pyrrolidin-1-ylmethyl)-2-furan-2-yl-thieno[2,3-d]pyrimidin-4-ylamine
1H NMR (300 MHz, chloroform-d): δH 7.60 (1H, s), 7.25 (1H, d, J 3.4), 6.99 (1H, s), 6.55 (1H, dd, J 3.4, 1.9), 5.34 (2H, br. s), 5.20–5.32 (1H, m), 3.92 (2H, s), 2.93–2.98 (1H, m), 2.82–2.94 (2H, m), 2.57–2.68 (1H, m), 2.06–2.27 (2H, m); MSm/z 319 (M + H).Compound 31: 6-(3,3-difluoro-pyrrolidin-1-ylmethyl)-2-furan-2-yl-thieno[2,3-d]pyrimidin-4-ylamine
1H NMR (300 MHz, DMSO-d6): δH 7.82 (1H, s), 7.54 (2H, s), 7.42 (1H, s), 7.12 (1H, d, J 3.4 Hz), 6.63 (1H, dd, J 3.4, 1.9), 3.88 (2H, s), 2.97 (2H, t, J 13.4), 2.80 (2H, t, J 7.0), 2.13–2.40 (2H, m); MSm/z 337 (M + H).Compound 43: 2-(5-chloro-furan-2-yl)-6-pyrrolidin-1-ylmethyl-thieno[2,3-d]pyrimidin-4-ylamine
5-Chlorofuran-2-carbonitrile was used in place of furan-2-carbonitrile. 1H NMR (300 MHz, DMSO-d6): δH 7.65 (1H br. s), 7.18 (1H, d, J 3.8), 6.67 (1H, d, J 3.4), 3.99 (2H, br. s), 2.68 (4H, m), 1.66–1.89 (4H, m); MSm/z 335 (M + H).Compound 44: 6-azepan-1-ylmethyl-2-(5-chloro-furan-2-yl)-thieno[2,3-d]pyrimidin-4-ylamine
5-Chlorofuran-2-carbonitrile was used in place of furan-2-carbonitrile. 1H NMR (300 MHz, DMSO-d6): δH 7.74 (1H, s), 7.24 (1H, br. s), 6.79 (1H, s), 4.61 (2H, br. s), 4.50 (2H, br. s), 3.38 (2H, br. s), 3.13 (4H, br. s), 1.84 (4H, br. s), 1.63 (4H, br. s); MSm/z 363 (M + H).Compound 46: 2-(5-chloro-furan-2-yl)-6-(4-fluoro-piperidin-1-ylmethyl)-thieno[2,3-d]pyrimidin-4-ylamine
5-Chlorofuran-2-carbonitrile was used in place of furan-2-carbonitrile. 1H NMR (300 MHz, DMSO-d6): δH 7.72 (1H, s), 7.24 (1H, d, J 3.4), 6.70 (1H, d, J 3.4), 4.64 (2H, br. s), 3.35 (1H, br. s), 3.16 (4H, br. s), 2.08 ppm (4H, br. s); MSm/z 367 (M + H).Compound 48: 2-(5-chloro-furan-2-yl)-6-(3,6-dihydro-2H-pyridin-1-ylmethyl)-thieno[2,3-d]pyrimidin-4-ylamine
5-Chlorofuran-2-carbonitrile was used in place of furan-2-carbonitrile. 1H NMR (300 MHz, chloroform-d): δH 7.22 (1H, d, J 3.4), 7.00 (1H, s), 6.33 (1H, d, J 3.4 Hz), 5.73–5.85 (1H, m), 5.60–5.73 (1H, m), 5.29 (2H, br. s), 3.83 (2H, s), 3.04–3.15 (2H, m), 2.64 (2H, t, J 5.8), 2.14–2.25 (2H, m); MSm/z 347 (M + H).Compound 49: 2-(5-chloro-furan-2-yl)-6-(2,5-dihydro-pyrrol-1-ylmethyl)-thieno[2,3-d]pyrimidin-4-ylamine
5-Chlorofuran-2-carbonitrile was used in place of furan-2-carbonitrile. 1H NMR (300 MHz, DMSO-d6): δH 7.45 (1H, s), 7.19 (1H, d, J 3.0), 6.85 (2H, s), 6.67 (1H, d, J 3.0), 5.37 (2H, s), 4.28 (6H, br. s); MSm/z 333 (M + H).Compound 58: 6-morpholin-4-ylmethyl-2-oxazol-2-yl-thieno[2,3-d]pyrimidin-4-ylamine
Oxazole-2-carbonitrile was used in place of furan-2-carbonitrile. 1H NMR (300 MHz, methanol-d4) δH 8.10 (1H, s), 7.41 (1H, s), 7.35 (1H, s), 3.79 (2H, s), 3.68–3.73 (4H, m), 2.49–2.59 (4H, m); MSm/z 318 (M + H).Compound 59: 6-((2,5-dihydro-1H-pyrrol-1-yl)methyl)-2-(oxazol-2-yl)thieno[2,3-d]pyrimidin-4-amine
Oxazole-2-carbonitrile was used in place of furan-2-carbonitrile. MSm/z 300 (M + H).Compound 60: 6-((5,6-dihydropyridin-1(2H)-yl)methyl)-2-(oxazol-2-yl)thieno[2,3-d]pyrimidin-4-amine
Oxazole-2-carbonitrile was used in place of furan-2-carbonitrile. MSm/z 314 (M + H).Compound 61: 6-morpholin-4-ylmethyl-2-thiazol-2-yl-thieno[2,3-d]pyrimidin-4-ylamine
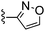
Thiazole-2-carbonitrile was used in place of furan-2-carbonitrile. 1H NMR (400 MHz, chloroform-d): δH 7.99 (1H, d, J 3.2), 7.48 (1H, d, J 3.2), 7.03 (1H, s), 5.49 (2H, br. s), 3.66–3.80 (6H, m), 2.46–2.63 (6H, m); MSm/z 334 (M + H).Compound 64: 2-isoxazol-3-yl-6-morpholin-4-ylmethyl-thieno[2,3-d]pyrimidin-4-ylamine
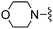
Isoxazole-3-carbonitrile was used in place of furan-2-carbonitrile. 1H NMR (300 MHz, acetone-d6): δ = 8.68 (1H, d, J 1.5), 7.33 (1H, s), 6.83–6.91 (3H, m), 3.66 (2H, s), 3.46–3.56 (4H, m), 2.31–2.43 (4H, m); MSm/z 318 (M + H).Compound 65: 6-(2,6-dimethyl-piperidin-1-ylmethyl)-2-isoxazol-3-yl-thieno[2,3-d]pyrimidin-4-ylamine
Isoxazole-3-carbonitrile was used in place of furan-2-carbonitrile. 1H NMR (300 MHz, acetone-d6): δH 8.67 (1H, d, J 1.9), 7.30 (1H, s), 6.88 (1H, d, J 1.5), 6.82 (2H, br. s), 3.97 (2H, s), 2.33–2.51 (2H, m), 1.42–1.56 (2H, m), 1.10–1.26 (4H, m), 1.03 (6H, d, J 6.0); MSm/z 344 (M + H).Compound 66: 6-pyrrolidin-1-ylmethyl-2-thiophen-2-yl-thieno[2,3-d]pyrimidin-4-ylamine
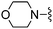
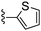
Thiophene-2-carbonitrile was used in place of furan-2-carbonitrile. 1H NMR (300 MHz, chloroform-d): δH 7.95 (1H, dd, J 3.7, 1.2), 7.41 (1H, dd, J 5.0, 1.2), 7.11 (2H, dd, J 5.0, 3.7), 5.33 (2H, br. s), 3.95 (2H, s), 2.71 (4H, br. s), 1.86 (4H, dt, J 6.7, 3.2); MSm/z 317 (M + H).Compound 67: 6-morpholin-4-ylmethyl-2-thiophen-2-yl-thieno[2,3-d]pyrimidin-4-ylamine
Thiophene-2-carbonitrile was used in place of furan-2-carbonitrile. 1H NMR (300 MHz, chloroform-d): δH 7.95 (1H, dd, J 3.7, 1.2), 7.41 (1H, dd, J 5.0, 1.2), 7.12 (1H, dd, J 5.0, 3.7), 6.96 (1H, s), 5.18 (2H, br. s), 3.65–3.85 (6H, m), 2.47–2.65 (4H, m); MSm/z 333 (M + H).Compound 68: 6-(2,5-dihydro-pyrrol-1-ylmethyl)-2-oxazol-4-yl-thieno[2,3-d]pyrimidin-4-ylamine
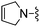
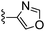
Oxazole-4-carbonitrile was used in place of furan-2-carbonitrile. 1H NMR (300 MHz, methanol-d4) δH 8.51 (1H, s), 8.28 (1H, s), 7.34 (1H, s), 5.83 (2H, s), 4.13 (2H, s), 3.63 (4H, s); MSm/z 300 (M + H).Compound 69: 6-(3,6-dihydro-2H-pyridin-1-ylmethyl)-2-oxazol-4-yl-thieno[2,3-d]pyrimidin-4-ylamine
Oxazole-4-carbonitrile was used in place of furan-2-carbonitrile. 1H NMR (300 MHz, chloroform-d) δH 8.41 (1H, s), 7.98 (1H, s), 7.04 (1H, s), 5.74–5.83 (1H, m), 5.64–5.71 (1H, m), 5.44 (2H, br s), 3.85 (2H, s), 3.06–3.12 (2H, m), 2.65 (2H, t, J 5.7), 2.15–2.24 (2H, m); MSm/z 314 (M + H).Compound 70: 2-benzofuran-2-yl-6-(4-fluoropiperdin-1-ylmethyl)thieno[2,3-d]pyrimidin-4-ylamine
Benzofuran-2-carbonitrile was used in place of furan-2-carbonitrile. 1H NMR (300 MHz, chloroform-d): δH 7.45–7.74 (3H, m), 7.30 (1H, m), 7.20 (1H, m), 6.94 (1H, br. s), 5.28 (2H, br. s), 4.66 (1H, d, JHF 48.6), 4.46–4.67 (1H, m), 3.71 (2H, s), 2.38–2.66 (4H, m), 1.72–1.94 (4H, m); MSm/z 383 (M + H).Compound 71: 6-(morpholinomethyl)-2-phenylthieno[2,3-d]pyrimidin-4-amine
Benzonitrile was used in place of furan-2-carbonitrile. MSm/z 327 (M + H).Compound 72: 2-(3-fluoro-phenyl)-6-morpholin-4-ylmethyl-thieno[2,3-d]pyrimidin-4-ylamine
3-Fluorobenzonitrile was used in place of furan-2-carbonitrile. 1H NMR (300 MHz, chloroform-d): δH 8.24 (1H, d, J 7.9 Hz), 8.15 (1H, dt, J 10.5, 2.1), 7.44 (1H, td, J 8.0, 5.8), 7.07–7.22 (1H, m), 7.03 (1H, s), 5.23 (2H, br. s), 3.69–3.84 (6H, m), 2.51–2.63 (4H, m); MSm/z 345 (M + H).Compound 73: 2-(3-chloro-phenyl)-6-morpholin-4-ylmethyl-thieno[2,3-d]pyrimidin-4-ylamine
3-Chlorobenzonitrile was used in place of furan-2-carbonitrile. 1H NMR (300 MHz, chloroform-d): δH 8.44 (1H, s), 8.31 (1H, dt, J 6.2, 2.2), 7.33–7.47 (2H, m), 6.99 (1H, s), 5.25 (2H, br. s), 3.67–3.85 (6H, m), 2.44–2.63 (4H, m); MSm/z 361 (M + H).Compound 74: 2-(3,5-difluoro-phenyl)-6-morpholin-4-ylmethyl-thieno[2,3-d]pyrimidin-4-ylamine
3,5-Difluorobenzonitrile was used in place of furan-2-carbonitrile. 1H NMR (300 MHz, chloroform-d): δH 7.87–8.05 (2H, m), 7.00 (1H, s), 6.87 (1H, tt, J 8.7, 2.4), 5.23 (2H, br. s), 3.59–3.83 (6H, m), 2.41–2.67 (4H, m); MSm/z 363 (M + H).Compound 75: 2-(3,4-difluoro-phenyl)-6-morpholin-4-ylmethyl-thieno[2,3-d]pyrimidin-4-ylamine
3,4-Difluorobenzonitrile was used in place of furan-2-carbonitrile. 1H NMR (300 MHz, chloroform-d): δH 8.28 (1H, ddd, J 11.9, 7.9, 2.1), 8.20 (1H, ddd, J 8.7, 4.5, 1.5), 7.15–7.25 (1H, m), 6.99 (1H, s), 5.19 (2H, br. s), 3.52–3.88 (6H, m), 2.46–2.62 (4H, m); MSm/z 363 (M + H).Compound 76: 3-[4-amino-6-(2,5-dihydro-pyrrol-1-ylmethyl)-thieno[2,3-d]pyrimidin-2-yl]-benzonitrile
1,3-Dicyanobenzene was used in place of furan-2-carbonitrile. 1H NMR (300 MHz, DMSO-d6) δH 8.60–8.69 (2H, m), 7.91–7.99 (1H, m), 7.70 (1H, t, J 7.7), 7.60 (2H, br. s), 7.47 (1H, s), 5.83 (2H, s), 4.03 (2H, s), 3.52 (4H, s); MSm/z 334 (M + H).Compound 77: 3-(4-amino-6-morpholin-4-ylmethyl-thieno[2,3-d]pyrimidin-2-yl)-benzonitrile
Oxazole-2-carbonitrile was used in place of furan-2-carbonitrile. 1H NMR (300 MHz, chloroform-d) δH 8.76 (1H, s), 8.67 (1H, dt, J 7.9, 1.5), 7.70 (1H, dt, J 7.8, 1.4), 7.55 (1H, t, J 7.9), 7.03 (1H, s), 5.34 (2H, br. s), 3.71–3.81 (6H, m), 2.51–2.63 (4H, m); MSm/z 352 (M + H). HRMS calcd for C18H17N5OS 351.1154, found 352.1268 (M+ + H).Compound 78: 3-[4-amino-6-(2,6-dimethyl-piperidin-1-ylmethyl)-thieno[2,3-d]pyrimidin-2-yl]-benzonitrile
1,3-Dicyanobenzene was used in place of furan-2-carbonitrile. 1H NMR (300 MHz, chloroform-d): δH 8.77 (1H, s), 8.68 (1H, d, J 7.9), 7.70 (1H, d, J 7.9), 7.56 (1H, t, J 7.7), 7.00 (1H, s), 5.25 (2H, br. s), 3.74 (2H, d, J 2.3), 2.80–3.00 (2H, m), 1.93–2.15 (2H, m), 1.57–1.78 (2H, m), 1.19–1.39 (2H, m), 0.82–0.97 (6H, m); MSm/z 378 (M + H).Compound 79: 3-[4-amino-6-(4-fluoro-piperidin-1-ylmethyl)-thieno[2,3-d]pyrimidin-2-yl]-benzonitrile
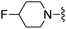
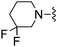
1,3-Dicyanobenzene was used in place of furan-2-carbonitrile. 1H NMR (400 MHz, chloroform-d) δH 8.77 (1H, s), 8.68 (1H, d, J 8.1), 7.70 (1H, dt, J 7.7, 1.3), 7.56 (1H, t, J 7.8), 7.00 (1H, s), 5.26 (2H, s), 4.73 (1H, d, JHF 48.7), 3.78 (2H, s), 2.59–2.69 (2H, m), 2.48–2.58 (2H, m), 1.87–1.02 (4H, m); MSm/z 368 (M + H). HRMS calcd for C19H18FN5S 367.1267, found 368.1301 (M+ + H).Compound 80: 3-[4-amino-6-(3,3-difluoro-piperidin-1-ylmethyl)-thieno[2,3-d]pyrimidin-2-yl]-benzonitrile
1,3-Dicyanobenzene was used in place of furan-2-carbonitrile. 1H NMR (300 MHz, chloroform-d) δH 8.77 (1H, t, J 1.5), 8.68 (1H, dt, J 7.9, 1.5), 7.70 (1H, dt, J 7.6, 1.5), 7.56 (1H, t, J 7.9), 7.04 (1H, s), 5.27 (2H, br. s), 3.89 (2H, s), 2.76 (2H, t, JHF 11.1), 2.55–2.63 (2H, m), 1.77–1.99 (4H, m); MSm/z 386 (M + H).Compound 81: 3-(4-amino-6-thiomorpholin-4-ylmethyl-thieno[2,3-d]pyrimidin-2-yl)-benzonitrile
1,3-Dicyanobenzene was used in place of furan-2-carbonitrile. 1H NMR (300 MHz, methanol-d4) δH 8.61–8.67 (2H, m), 7.93 (1H, ddd, J 7.7, 1.3, 1.1), 7.70 (1H, t, J 7.7), 7.60 (2H, br. s), 7.45 (1H, s), 3.78 (2H, s), 2.69–2.76 (4H, m), 2.61–2.67 (4H, m); MSm/z 368 (M + H).Compound 82: 3-[4-amino-6-(3,6-dihydro-2H-pyridin-1-ylmethyl)-thieno[2,3-d]pyrimidin-2-yl]-benzonitrile
1,3-Dicyanobenzene was used in place of furan-2-carbonitrile. 1H NMR (300 MHz, chloroform-d) δH 8.75 (1H, t, J 1.5), 8.66 (1H, ddd, J 8.1, 1.3, 1.1), 7.69 (1H, ddd, J 7.7, 1.3, 1.1), 7.55 (1H, t, J 7.7), 7.06 (1H, s), 5.75–5.82 (1H, m), 5.64–5.71 (1H, m), 5.39 (2H, br. s), 3.86 (2H, s), 3.06–3.15 (2H, m), 2.67 (2H, t, J 5.7), 2.17–2.24 (2H, m); MSm/z 348 (M + H).Compound 83: 3-(4-amino-6-pyrrolidin-1-ylmethyl-thieno[2,3-d]pyrimidin-2-yl)-benzonitrile
1,3-Dicyanobenzene was used in place of furan-2-carbonitrile. 1H NMR (300 MHz, chloroform-d) δH 8.76 (1H, s), 8.67 (1H, dt, J 7.9, 1.3), 7.69 (1H, dt, J 7.6, 1.5), 7.55 (1H, t, J 7.7), 7.03 (1H, s), 5.32 (2H, br. s), 3.90 (2H, s), 2.58–2.68 (4H, m), 1.77–1.89 (4H, m); MSm/z 336 (M + H).Compound 84: 3-[4-amino-6-(2,6-dimethyl-morpholin-4-ylmethyl)-thieno[2,3-d]pyrimidin-2-yl]-benzonitrile
1,3-Dicyanobenzene was used in place of furan-2-carbonitrile. 1H NMR (300 MHz, acetone-d6): δH 8.54–8.65 (2H, m), 7.72 (1H, d, J 7.9), 7.57 (1H, t, J 8.1), 7.30 (1H, s), 6.84 (2H, br. s), 3.64 (2H, s), 3.42–3.58 (2H, m), 2.62–2.77 (2H, m), 1.63 (2H, t, J 10.7), 0.95 (3H, s), 0.93 (3H, s); MSm/z 380 (M + H).Compound 85: 2-[4-amino-6-(4-fluoro-piperidin-1-ylmethyl)-thieno[2,3-d]pyrimidin-2-yl]-benzonitrile
1,2-Dicyanobenzene was used in place of furan-2-carbonitrile. 1H NMR (400 MHz, chloroform-d) δH 8.36 (1H, d, J 8.1), 7.81 (1H, d, J 7.6), 7.67 (1H, td, J 7.8, 1.3), 7.48–7.53 (1H, m), 7.05 (1H, s), 5.36 (2H, s), 4.74 (1H, d, JHF 48.7), 3.79 (2H, s), 2.47–2.70 (4H, m), 1.83–2.02 (4H, m); MSm/z 368 (M + H).Compound 86: 4-[4-amino-6-(4-fluoro-piperidin-1-ylmethyl)-thieno[2,3-d]pyrimidin-2-yl]-benzonitrile
1,4-Dicyanobenzene was used in place of furan-2-carbonitrile. 1H NMR (300 MHz, chloroform-d) δH 8.55 (2H, d, J 8.7), 7.74 (2H, d, J 8.7), 7.01 (1H, s), 5.25 (2H, s), 4.74 (1H, d, JHF 48.6), 3.78 (2H, s), 2.48–2.69 (4H, m), 1.85–1.99 (4H, m); MSm/z 368 (M + H).Compound 87: 4-[4-amino-6-(3,6-dihydro-2H-pyridin-1-ylmethyl)-thieno[2,3-d]pyrimidin-2-yl]-benzonitrile
1,4-Dicyanobenzene was used in place of furan-2-carbonitrile. 1H NMR (300 MHz, chloroform-d) δH 8.56 (2H, d, J 8.3), 7.74 (2H, d, J 8.3), 7.05 (1H, s), 5.76–5.84 (1H, m), 5.62–5.73 (1H, m), 5.22 (2H, br. s), 3.86 (2H, s), 3.08–3.13 (2H, m), 2.66 (2H, t, J 5.7), 2.17–2.24 (2H, m); MSm/z 348 (M + H).Compound 88: 4-[4-amino-6-(2,5-dihydro-pyrrol-1-ylmethyl)-thieno[2,3-d]pyrimidin-2-yl]-benzonitrile
1,4-Dicyanobenzene was used in place of furan-2-carbonitrile. 1H NMR (300 MHz, chloroform-d) δH 8.55 (2H, d, J 8.7), 7.74 (2H, d, J 8.7), 7.04 (1H, s), 5.81 (2H, s), 5.23 (2H, br. s), 4.09 (2H, s), 3.61 (4H, s); MSm/z 334 (M + H).Compound 89: 6-((2,5-dihydro-1H-pyrrol-1-yl)methyl)-2-(3-(trifluoromethyl)phenyl)thieno[2,3-d]pyrimidin-4-amine
3-(Trifluoromethyl)benzonitrile was used in place of furan-2-carbonitrile. 1H NMR (300 MHz, chloroform-d): δH 8.75 (1H, s), 8.65 (1H, d, J 7.9), 7.70 (1H, d, J 7.5), 7.59 (1H, t, J 7.9), 7.07 (1H, s), 5.83 (2H, s), 5.26 (2H, br. s), 4.11 (2H, s), 3.60–3.67 (4H, m). MSm/z 377 (M + H).Compound 90: 6-((4-fluoropiperidin-1-yl)methyl)-2-(3-(trifluoromethyl)phenyl)thieno[2,3-d]pyrimidin-4-amine
3-(Trifluoromethyl)benzonitrile was used in place of furan-2-carbonitrile. 1H NMR (300 MHz, chloroform-d): δH 8.75 (1H, s), 8.65 (1H, d, J 7.9), 7.70 (1H, d, J 7.9), 7.59 (1H, t, J 7.7), 7.04 (1H, s), 5.26 (2H, br. s), 3.81 (2H, s), 2.49–2.76 (4H, m), 1.88–2.04 (4H, m); MSm/z 411 (M + H).Compound 91: 6-((4-fluoropiperidin-1-yl)methyl)-2-(4-(trifluoromethyl)phenyl)thieno[2,3-d]pyrimidin-4-amine
4-(Trifluoromethyl)benzonitrile was used in place of furan-2-carbonitrile. 1H NMR (300 MHz, chloroform-d): δH 8.57 (2H, d, J 8.3), 7.72 (2H, d, J 8.3), 7.05 (1H, br. s), 5.26 (2H, br. s), 4.81 (1H, td, J 5.8, 3.0), 3.82 (2H, s), 3.37–3.67 (4H, m), 2.67 (4H, m); MSm/z 411 (M + H).Compound 92: 6-((4-fluoropiperidin-1-yl)methyl)-2-(2-(trifluoromethyl)phenyl)thieno[2,3-d]pyrimidin-4-amine
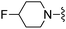
2-(Trifluoromethyl)benzonitrile was used in place of furan-2-carbonitrile. 1H NMR (300 MHz, chloroform-d): v 7.79 (1H, d, J 7.9), 7.73 (1H, d, J 7.4), 7.64 (1H, t, J 7.3), 7.54 (1H, t, J 7.6), 7.06 (1H, s), 5.29 (2H, br. s), 4.74–4.90 (1H, m), 3.81 (2H, s), 3.35–3.70 (4H, m), 2.45–2.66 (4H, m); MSm/z 411 (M + H).Compound 93: 6-((4-fluoropiperidin-1-yl)methyl)-2-(3-(methylsulfonyl)phenyl)thieno[2,3-d]pyrimidin-4-amine
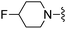
3-(Methylsulfonyl)benzonitrile was used in place of furan-2-carbonitrile. 1H NMR (300 MHz, chloroform-d): δH 9.04 (1H, s), 8.77 (1H, d, J 7.9), 8.03 (1H, d, J 7.5), 7.68 (1H, t, J 7.9), 7.07 (1H, br. s), 5.47 (2H, br. s), 4.76 (1H, d, JHF 48.6), 3.81 (2H, br. s), 3.15 (3H, s), 2.60 (4H, m), 1.95 (4H, m); MSm/z 421 (M + H).Compound 94: 6-((4-fluoropiperidin-1-yl)methyl)-2-(3-nitrophenyl)thieno[2,3-d]pyrimidin-4-amine
3-Nitrobenzonitrile was used in place of furan-2-carbonitrile. 1H NMR (400 MHz, chloroform-d) δH 9.31 (1H, t, J 2.0), 8.79 (1H, dt, J 7.8, 1.3), 8.28 (1H, ddd, J 8.2, 2.3, 1.2), 7.62 (1H, t, J 7.9), 7.01 (1H, s), 5.25 (2H, s), 4.74 (1H, d, JHF 48.7), 3.79 (2H, s), 2.48–2.69 (4H, m), 1.83–2.00 (4H, m); MSm/z 388 (M + H).Compound 95: 3-(4-amino-6-((4-fluoropiperidin-1-yl)methyl)thieno[2,3-d]pyrimidin-2-yl)-N,N-dimethylbenzamide
3-Cyano-N,N-dimethylbenzamide was used in place of furan-2-carbonitrile. 1H NMR (300 MHz, chloroform-d) δH 8.45 (1H, m), 8.41 (1H, dt, J 7.2, 1.9), 7.43–7.53 (2H, m), 7.31 (1H, s), 6.73 (2H, br. s), 4.71 (1H, d, JHF 48.6), 3.76 (2H, s), 3.17 (3H, s), 3.08 (3H, s), 2.60–2.71 (2H, m), 2.45–2.57 (2H, m), 1.84–2.02 (4H, m). MSm/z 414 (M + H).Compound 96: 6-(4-fluoro-piperidin-1-ylmethyl)-2-(3-methoxy-phenyl)-thieno[2,3-d]pyrimidin-4-ylamine
3-Methoxybenzonitrile was used in place of furan-2-carbonitrile. 1H NMR (300 MHz, chloroform-d) δH 8.02 (1H, dt, J 7.6, 1.3), 7.99 (1H, dd, J 2.6, 1.5), 7.37 (1H, t, J 7.9), 6.96–7.02 (2H, m), 5.18 (2H, br. s), 4.72 (1H, d, JHF 49.0), 3.92 (3H, s), 3.76 (2H, s), 2.59–2.69 (2H, m), 2.47–2.57 (2H, m), 1.85–1.99 (4H, m); MSm/z 373 (M + H).Compound 97: 2-(3-methoxy-phenyl)-6-morpholin-4-ylmethyl-thieno[2,3-d]pyrimidin-4-ylamine
3-Methoxybenzonitrile was used in place of furan-2-carbonitrile. 1H NMR (300 MHz, chloroform-d) δH 7.97–8.06 (2H, m), 7.37 (1H, t, J 7.9), 7.01 (1H, dd, J 2.6, 0.8), 6.98 (1H, s), 5.27 (2H, br. s), 3.91 (3H, s), 3.71–3.77 (6H, m), 2.52–2.57 (4H, m); MSm/z 357 (M + H).Compound 98: 6-(3,3-difluoro-piperidin-1-ylmethyl)-2-(3-methoxy-phenyl)-thieno[2,3-d]pyrimidin-4-ylamine
3-Methoxybenzonitrile was used in place of furan-2-carbonitrile. 1H NMR (300 MHz, chloroform-d) δH 7.97–8.05 (2H, m), 7.37 (1H, t, J 8.1), 6.96–7.03 (2H, m), 5.32 (2H, s), 3.91 (3H, s), 3.85 (2H, s), 2.74 (2H, t, JHF 11.1), 2.49–2.60 (2H, m), 1.73–1.98 (4H, m); MSm/z 391 (M + H).Compounds 38–42 were prepared according to the procedures described for compound 18 except 5-difluoro-furan-2-carbonitrile was used in place of furan-2-carbonitrile. 5-Difluoro-furan-2-carbonitrile was prepared via the following procedure: To a solution of Et2NSF3 (2.8 mL, 21.4 mmol) and CH2Cl2 (10 mL) at 4 °C was added a solution of 5-formyl-furan-2-carbonitrile (2.44 g, 20.2 mmol; W. Hoyle and G. P. Roberts, J. Med. Chem., 1973, 16, 709) in CH2Cl2 (10 mL). After 30 min at 4 °C, saturated aqueous NaHCO3 was added, the layers were separated and the aqueous layer was extracted with CH2Cl2. The combined organics were dried (Na2SO4) and concentrated to give 2.15 g of 5-difluoromethyl-furan-2-carbonitrile that was used without further purification.
Compound 38: 2-(5-difluoromethyl-furan-2-yl)-6-morpholin-4-ylmethyl-thieno[2,3-d]pyrimidin-4-ylamine
1H NMR (400 MHz, DMSO-d6) δH 7.63 (2H, br. s), 7.43 (1H, s), 7.19 (1H, d, J 3.4), 7.16 (1H, t, J 53.3), 7.02 (1H, m), 3.72 (2H, s), 3.59 (4H, t, J 4.4), 2.44 (4H, m); MSm/z 367 (M + H).Compound 39: 2-(5-difluoromethyl-furan-2-yl)-6-(2,6-dimethyl-piperidin-1-ylmethyl)-thieno[2,3-d]pyrimidin-4-ylamine
1H NMR (400 MHz, acetone-d6) δH 7.40 (1H, s), 7.19 (1H, d, J 3.4 Hz), 7.00 (1H, t, J 53.7 Hz), 6.93–6.97 (1H, m), 6.89 (1H, br. s), 4.08 (2H, s), 2.50–2.62 (2H, m), 1.53–1.67 (4H, m), 1.27–1.33 (2H, m), 1.15 (6H, d, J 6.4); MSm/z 393 (M + H).Compound 40: 2-(5-difluoromethyl-furan-2-yl)-6-(4-fluoro-piperidin-1-ylmethyl)-thieno[2,3-d]pyrimidin-4-ylamine
1H NMR (400 MHz, acetone-d6) δH 7.39 (1H, s), 7.20 (1H, d, J 3.7), 7.01 (1H, t, J 53.7), 6.94–6.98 (1H, m), 6.89 (2H, br. s), 3.78 (2H, d, J 1.2), 2.61–2.71 (2H, m), 2.43–2.52 (2H, m), 2.08–2.10 (1H, m), 1.74–1.99 (4H, m); MSm/z 383 (M + H).Compound 41: 2-(5-difluoromethyl-furan-2-yl)-6-(4,4-difluoro-piperidin-1-ylmethyl)-thieno[2,3-d]pyrimidin-4-ylamine
1H NMR (400 MHz, acetone-d6) δH 7.41 (1H, s), 7.21 (1H, d, J 3.4), 7.01 (1H, t, J 53.7), 6.94–6.99 (1H, m), 6.92 (2H, br. s), 3.87 (2H, d, J 1.0), 2.66 (4H, t, J 5.5), 1.95–2.04 (4H, m); MSm/z 401 (M + H).Compound 42: 2-(5-difluoromethyl-furan-2-yl)-6-(3,3-difluoro-piperidin-1-ylmethyl)-thieno[2,3-d]pyrimidin-4-ylamine
1H NMR (400 MHz, acetone-d6) δH 7.41 (1H, s), 7.21 (1H, d, J 3.7), 7.01 (1H, t, J 53.7), 6.89–6.98 (3H, m), 3.90 (2H, s), 2.77 (2H, t, J 11.5), 2.58 (2H, t, J 5.0), 1.85–1.98 (2H, m), 1.71–1.81 (2H, m); MSm/z 401 (M + H).Compound 63: 6-(2,6-dimethyl-piperidin-1-ylmethyl)-2-(4-methyl-thiazol-2-yl)-thieno[2,3-d]pyrimidin-4-ylamine
Solid potassium-tert-butoxide (182 mg, 1.6 mmol) was added to a dioxane solution (4 mL) of 2-amino-3-cyanothiophene (1.0 g, 8.1 mmol) and 4-methyl-thiazole-2-carbonitrile (1.0 g, 8.1 mmol). The resulting mixture was heated in an oil bath at 130 °C for 10 minutes. The dark slurry was cooled to room temperature, diluted with THF, and dry packed onto silica gel. The material was the purified viacolumn chromatography to give 1.1 g of 2-(4-methylthiazol-2-yl)thieno[2,3-d]pyrimidin-4-amine.Solid NBS (623 mg, 3.5 mmol) was added to a THF solution (30 mL) of 2-(4-methylthiazol-2-yl)thieno[2,3-d]pyrimidin-4-amine (800 mg, 3.2 mmol). After 3 h the mixture was diluted with EtOAc and washed with water and brine, dried (Na2SO4), and dry packed onto silica gel. Column chromatography gave 825 mg of 6-bromo-2-(4-methylthiazol-2-yl)thieno[2,3-d]pyrimidin-4-amine.
Neat vinylboronic acid dibutyl ester (1.0 mL, 4.7 mmol) was added to a dioxane (20 mL)/water (5 mL) solution of 6-bromo-2-(4-methyl-thiazol-2-yl)-thieno[2,3-d]pyrimidin-4-ylamine (775 mg, 2.4 mmol), Pd(dppf)Cl2 (196 mg, 0.2 mmol), and K2CO3 (650 mg, 4.7 mmol) and the mixture was heated to 80 °C. After 3 h the mixture was cooled and diluted with EtOAc. The organic phase was washed with water and brine, dried (Na2SO4) and dry packed onto silica gel. Column chromatography gave 460 mg of 2-(4-methyl-thiazol-2-yl)-6-vinyl-thieno[2,3-d]pyrimidin-4-ylamine.
Solid MeSO2NH2 (162 mg, 1.7 mmol) was added to a t-BuOH (8 mL)/water (8 mL) solution of AD mix-α (2.4 g). After 15 min the resulting mixture was added to an acetone suspension (8 mL) of 2-(4-methyl-thiazol-2-yl)-6-vinyl-thieno[2,3-d]pyrimidin-4-ylamine (460 mg, 1.7 mmol) and the mixture was stirred vigorously. After 18 h sodium sulfite (2.5 g) was added and the mixture was stirred for an additional 30 minutes. The mixture was extracted with EtOAc and the combined extracts were washed with water and brine, dried (Na2SO4), and concentrated to give 350 mg of 1-[4-amino-2-(4-methyl-thiazol-2-yl)-thieno[2,3-d]pyrimidin-6-yl]-ethane-1,2-diol that was used without further purification.
Solid HIO4 (775 mg, 3.4 mmol) was added to a THF solution (20 mL) of 1-[4-amino-2-(4-methyl-thiazol-2-yl)-thieno[2,3-d]pyrimidin-6-yl]-ethane-1,2-diol (350 mg, 1.1 mmol). After 2 h saturated aqueous NaHCO3 was added and the aqueous phase was extracted with EtOAc. The combined extracts were washed with water and brine, dried (Na2SO4), and dry packed onto silica gel. Column chromatography gave 113 mg of 4-amino-2-(4-methyl-thiazol-2-yl)-thieno[2,3-d]pyrimidine-6-carbaldehyde.
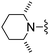
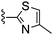
Solid NaBH(OAc)3 (45 mg, 0.21 mmol) was added to a THF solution (2 mL) of 4-amino-2-(4-methyl-thiazol-2-yl)-thieno[2,3-d]pyrimidine-6-carbaldehyde (40 mg, 0.14 mmol) and cis-2,6-dimethyl-piperidine (58 μL, 0.43 mmol) and the mixture was heated to 45 °C. After 16 h the mixture was cooled, diluted with EtOAc, washed with saturated aqueous NaHCO3, water and brine, dried (Na2SO4), and dry packed onto silica gel. Column chromatography gave 15 mg of 6-(2,6-dimethyl-piperidin-1-ylmethyl)-2-(4-methyl-thiazol-2-yl)-thieno[2,3-d]pyrimidin-4-ylamine 63. 1H NMR (Acetone, 300 MHz): δ = 7.29 (1H, s), 7.13 (1H, s), 6.87 (2H, br. s), 3.96 (2H, s), 2.43 (2H, br. s), 2.34 (3H, s), 1.38–1.58 (2H, m), 1.10–1.23 (4H, m), 1.02 (6H, d, J 6.4); MSm/z 374 (M + H).
The procedure used to prepare compound 63 was also used to prepare the following compounds with the appropriate amine substituent. Also, the corresponding nitrile replacement for the 4-methyl-thiazole-2-carbonitrile is specified where appropriate.
Compound 15: 2-(5-methyl-furan-2-yl)-6-pyrrolidin-1-ylmethyl-thieno[2,3-d]pyrimidin-4-ylamine
5-Methylfuran-2-carbonitrile was used in place of 4-methyl-thiazole-2-carbonitrile. 1H NMR (300 MHz, chloroform-d): δH 7.17 (1H, d, J 3.4), 7.11 (1H, s), 6.15 (1H, d, J 3.4), 5.50 (2H, br. s), 3.94 (2H, s), 2.66–2.80 (4H, m), 2.44 (3H, s), 1.86 (4H, dt, J 6.4, 3.3); MSm/z 315 (M + H).Compound 16: 2-(5-methyl-furan-2-yl)-6-piperidin-1-ylmethyl-thieno[2,3-d]pyrimidin-4-ylamine
5-Methylfuran-2-carbonitrile was used in place of 4-methyl-thiazole-2-carbonitrile. 1H NMR (400 MHz, chloroform-d): δH 7.09 (1H, d, J 3.0), 6.90 (1H, s), 6.08 (1H, d, J 2.3), 5.21 (2H, br. s), 3.65 (2H, s), 2.42 (4H, br. s), 2.38 (3H, s), 1.55 (4H, quin, J 5.6), 1.39 (2H, d, J 5.1); MSm/z 329 (M + H).Compound 19: 2-(5-methyl-furan-2-yl)-6-morpholin-4-ylmethyl-thieno[2,3-d]pyrimidin-4-ylamine
5-Methylfuran-2-carbonitrile was used in place of 4-methyl-thiazole-2-carbonitrile. 1H NMR (400 MHz, chloroform-d): δH 7.17 (1H, d, J 3.3), 6.95 (1H, s), 6.15 (1H, d, J 2.5), 5.27 (2H, br. s), 3.61–3.80 (6H, m), 2.49–2.58 (4H, m), 2.45 (3H, s); MSm/z 331 (M + H).Compound 20: 6-(3,4-dihydro-1H-isoquinolin-2-ylmethyl)-2-(5-methyl-furan-2-yl)-thieno[2,3-d]pyrimidin-4-ylamine
5-Methylfuran-2-carbonitrile was used in place of 4-methyl-thiazole-2-carbonitrile. 1H NMR (300 MHz, chloroform-d): δH 7.06–7.23 (4H, m), 6.95–7.04 (2H, m), 6.14 (1H, dd, J 3.3, 0.8), 5.35 (2H, br. s), 3.91 (2H, s), 3.74 (2H, s), 2.91 (3H, t, J 5.3), 2.82 (2H, t, J 5.5); MSm/z 377 (M + H).Compound 62: 2-(4-methyl-thiazol-2-yl)-6-morpholin-4-ylmethyl-thieno[2,3-d]pyrimidin-4-ylamine
1H NMR (300 MHz, acetone-d6): δH 7.31 (1H, s), 7.13 (1H, s), 6.88 (2H, br. s), 3.65 (2H, s), 3.47–3.56 (4H, m), 2.35–2.40 (4H, m), 2.34 (3H, s); MSm/z 348 (M + H).Compound 47: 2-(5-chloro-furan-2-yl)-6-(3,3-difluoro-piperidin-1-ylmethyl)-thieno[2,3-d]pyrimidin-4-ylamine
Solid potassium-tert-butoxide (162 mg, 1.4 mmol) was added to a dioxane solution (4 mL) of 2-amino-5-methyl-thiophene-3-carbonitrile (1.0 g, 7.2 mmol) and 5-chlorofuran-2-carbonitrile (920 mg, 7.2 mmol). The resulting mixture was heated in an oil bath at 130 °C for 10 minutes. The dark slurry was cooled to room temperature, diluted with THF, and dry packed onto silica gel. The material was the purified viacolumn chromatography to give 950 mg of 2-(5-chloro-furan-2-yl)-6-methyl-thieno[2,3-d]pyrimidin-4-ylamine.Solid DMAP (29 mg, 0.2 mmol) was added to a THF solution (12 mL) of (Boc)2O (1.3 g, 5.9 mmol) and 2-(5-Chloro-furan-2-yl)-6-methyl-thieno[2,3-d]pyrimidin-4-ylamine (630 mg, 2.4 mmol). After 6 h the mixture was diluted with EtOAc and the organic layer was washed with water and brine, dried (Na2SO4), concentrated and purified viacolumn chromatography to give 928 mg of [2-(5-Chloro-furan-2-yl)-6-methyl-thieno[2,3-d]pyrimidin-4-yl]-bis-carbamic acid tert-butyl ester.
Solid benzoyl peroxide (34 mg, 0.1 mmol) was added to a benzene solution (10 mL) of DBDMH (314 mg, 1.1 mmol) and [2-(5-chloro-furan-2-yl)-6-methyl-thieno[2,3-d]pyrimidin-4-yl]-bis-carbamic acid tert-butyl ester (928 mg, 2.0 mmol) and the resulting mixture was heated to reflux. After 14 h the mixture was cooled to rt, diluted with EtOAc and the organic layer was washed with water and brine, dried (Na2SO4), concentrated and purified viacolumn chromatography to give 651 mg of [6-bromomethyl-2-(5-chloro-furan-2-yl)-thieno[2,3-d]pyrimidin-4-yl]-bis-carbamic acid tert-butyl ester.
Neat TFA (2 mL) was added to a CH2Cl2 solution (8 mL) of [6-bromomethyl-2-(5-chloro-furan-2-yl)-thieno[2,3-d]pyrimidin-4-yl]-bis-carbamic acid tert-butyl ester (651 mg). After 4 h saturated aqueous NaHCO3 was added and the aqueous phase was extracted with EtOAc. The combined organics were washed with water and brine, dried (Na2SO4), and concentrated to give 369 mg of 6-bromomethyl-2-(5-chloro-furan-2-yl)-thieno[2,3-d]pyrimidin-4-ylamine that was used without further purification.
Solid 3,3-difluoro-piperidine hydrochloride (34 mg, 0.22 mmol) was added to a THF solution (1 mL) of diisopropylethyl amine (0.10 mL, 0.56 mmol) and 6-bromomethyl-2-(5-chloro-furan-2-yl)-thieno[2,3-d]pyrimidin-4-ylamine (50 mg, 0.14 mmol) and the mixture was heated to 40 °C. After 2 h the mixture was diluted with EtOAc then washed with water and brine, dried (Na2SO4), concentrated and purified viacolumn chromatography to give 31 mg of 2-(5-chloro-furan-2-yl)-6-(3,3-difluoro-piperidin-1-ylmethyl)-thieno[2,3-d]pyrimidin-4-ylamine 47. 1H NMR (chloroform-d, 300 MHz): δ = 7.23 (1H, d, J 3.4), 6.99 (1H, s), 6.34 (1H, d, J 3.4), 5.31 (2H, br. s), 3.86 (2H, s), 2.75 (2H, t, J 11.1), 2.57 (2H, t, J 5.1), 1.73–1.99 (4H, m); MSm/z 385 (M + H). HRMS calcd for C18H12ClF2N4OS 384.0623, found 385.0649 (M+ + H)
The procedure used to prepare compound 47 was also used to prepare the following compounds with the appropriate amine substituent:
Compound 45: 2-(5-chloro-furan-2-yl)-6-morpholin-4-ylmethyl-thieno[2,3-d]pyrimidin-4-ylamine
1H NMR (300 MHz, chloroform-d): δH 7.23 (1H, d, J 3.8), 6.97 (1H, s), 6.34 (1H, d, J 3.8), 5.39 (2H, br. s), 3.68–3.80 (6H, m), 2.46–2.61 (4H, m); MSm/z 351 (M + H).Compound 50: 2-(5-chloro-furan-2-yl)-6-(3,3-difluoro-pyrrolidin-1-ylmethyl)-thieno[2,3-d]pyrimidin-4-ylamine
1H NMR (300 MHz, chloroform-d): δH 7.22–7.26 (1H, m), 7.00 (1H, s), 6.34 (1H, d, J 3.4 Hz), 5.41 (2H, br. s), 3.90 (2H, s), 3.01 (2H, t, J 13.2), 2.86 (1H, t, J 7.0), 2.33 (2H, tt, J 14.4, 7.1); MSm/z 371 (M + H).Compound 51: 2-(5-bromo-furan-2-yl)-6-pyrrolidin-1-ylmethyl-thieno[2,3-d]pyrimidin-4-ylamine
5-Bromofuran-2-carbonitrile was used in place of 5-chlorofuran-2-carbonitrile. 1H NMR (300 MHz, chloroform-d): δH 7.20 (1H, d, J 3.4), 7.07 (1H, s), 6.47 (1H, d, J 3.6), 5.47 (2H, br. s), 3.91 (2H, s), 2.61–2.74 (4H, m), 1.85 (4H, dt, J 6.7, 3.2); MSm/z 380 (M + H).Compound 52: 2-(5-bromo-furan-2-yl)-6-morpholin-4-ylmethyl-thieno[2,3-d]pyrimidin-4-ylamine
5-Bromofuran-2-carbonitrile was used in place of 5-chlorofuran-2-carbonitrile. 1H NMR (400 MHz, chloroform-d): δH 7.20 (1H, d, J 3.4), 6.97 (1H, s), 6.48 (1H, d, J 3.4), 5.40 (2H, br. s), 3.61–3.86 (6H, m), 2.40–2.65 (4H, m); MSm/z 396 (M + H). HRMS calcd for C15H15BrN4O2S 394.0099, found 395.0147 (M+ + H).Compound 53: 2-(5-bromo-furan-2-yl)-6-(2,6-dimethyl-piperidin-1-ylmethyl)-thieno[2,3-d]pyrimidin-4-ylamine
5-Bromofuran-2-carbonitrile was used in place of 5-chlorofuran-2-carbonitrile. 1H NMR (400 MHz, chloroform-d): δH 7.19 (1H, d, J 3.4), 6.95 (1H, s), 6.48 (1H, d, J 3.7), 5.44 (2H, br. s), 4.11 (2H, s), 2.45–2.66 (2H, m), 1.55–1.70 (2H, m), 1.26–1.39 (4H, m), 1.19 (6H, d, J 6.1); MSm/z 422 (M + H).Compound 32: 2-(5-ethyl-furan-2-yl)-6-morpholin-4-ylmethyl-thieno[2,3-d]pyrimidin-4-ylamine
A 1 M THF solution of Et2Zn (0.6 mL, 0.60 mmol) was added to a THF solution (1.5 mL) of Pd(dppf)Cl2 (10 mg, 0.01 mmol) and 2-(5-bromo-furan-2-yl)-6-morpholin-4-ylmethyl-thieno[2,3-d]pyrimidin-4-ylamine 52 (60 mg, 0.15 mmol) and the mixture was refluxed. After 4 h the mixture was cooled and carefully diluted with EtOAc and water. The aqueous phase was extracted with EtOAc and the combined organics were washed with water and brine, dried (Na2SO4), and dry packed onto silica gel. Column chromatography gave 33 mg of the title compound. 1H NMR (300 MHz, chloroform-d): δH 7.19 (1H, d, J 3.4), 6.95 (1H, s), 6.17 (1H, d, J 3.4), 5.32 (2H, s), 3.68–3.77 (6H, m), 2.81 (2H, q, J 7.5), 2.44–2.58 (4H, m), 1.25–1.34 (3H, m); MSm/z 345 (M + H).The procedure to prepare compound 32 was also used to prepare the following compounds:
Compound 33: 6-(2,6-dimethyl-piperidin-1-ylmethyl)-2-(5-ethyl-furan-2-yl)-thieno[2,3-d]pyrimidin-4-ylamine
Compound 53 was used in place of compound 52. 1H NMR (300 MHz, chloroform-d): δH 7.18 (1H, d, J 3.4), 6.95 (1H, s), 6.16 (1H, d, J 3.4), 5.34 (2H, br. s), 4.13 (2H, s), 2.81 (2H, q, J 7.4), 2.57 (2H, br. s), 1.78 (4H, br. s), 1.51–1.70 (2H, m), 1.23–1.35 (3H, m), 1.21 (6H, d, J 6.0); MSm/z 371 (M + H).Compound 34: 2-(5-isopropyl-furan-2-yl)-6-morpholin-4-ylmethyl-thieno[2,3-d]pyrimidin-4-ylamine
i-PrZnBr was used in place of Et2Zn. 1H NMR (400 MHz, chloroform-d): δH 7.18 (1H, d, J 3.4), 6.95 (1H, s), 6.15 (1H, d, J 3.4), 5.26 (2H, br. s), 3.68–3.79 (6H, m), 3.07–3.19 (1H, m), 2.45–2.59 (4H, m), 1.32 (6H, d, J 7.1); MSm/z 359 (M + H).Compound 35: 6-(2,6-dimethyl-piperidin-1-ylmethyl)-2-(5-isopropyl-furan-2-yl)-thieno[2,3-d]pyrimidin-4-ylamine
i-PrZnBr and compound 53 were used in place of Et2Zn and compound 52, respectively. 1H NMR (300 MHz, chloroform-d): δH 7.17 (1H, d, J 3.4), 6.95 (1H, s), 6.14 (1H, d, J 3.0), 5.34 (2H, br. s), 4.13 (2H, s), 3.12 (1H, quin, J 6.9), 2.57 (2H, br. s), 1.51–1.80 (6H, m), 1.32 (6H, d, J 6.8), 1.2 (6H, d, J 6.0); MSm/z 385 (M + H).Compound 37: 2-(5-cyclopropyl-furan-2-yl)-6-(2,6-dimethyl-piperidin-1-ylmethyl)-thieno[2,3-d]pyrimidin-4-ylamine
Solid cyclopropylboronic acid (31 mg, 0.36 mmol) was added to a toluene (1 mL)/water (0.05 mL) suspension of 2-(5-bromo-furan-2-yl)-6-(2,6-dimethyl-piperidin-1-ylmethyl)-thieno[2,3-d]pyrimidin-4-ylamine 53 (60 mg, 0.14 mmol), Pd(OAc)2 (2 mg, 0.01 mmol), P(Cy)3 (5 mg, 0.02 mmol) and K3PO4 (104 mg, 0.49 mmol) and the mixture was heated to 100 °C. After 4 h the mixture was cooled, diluted with EtOAc, washed with water and brine, dried (Na2SO4) and dry packed onto silica gel. Column chromatography gave 30 mg of 2-(5-cyclopropyl-furan-2-yl)-6-(2,6-dimethyl-piperidin-1-ylmethyl)-thieno[2,3-d]pyrimidin-4-ylamine 37. 1H NMR (300 MHz, chloroform-d): δH 7.15 (1H, d, J 3.4), 6.87–6.99 (1H, m), 6.03 (1H, d, J 3.0), 5.27 (2H, s), 4.12 (2H, s), 2.56 (2H, br. s), 2.00–2.11 (1H, m), 1.58–1.71 (2H, m), 1.23–1.41 (4H, m), 1.21 (3H, s), 1.19 (3H, s), 0.89–0.99 (2H, m), 0.79–0.88 (2H, m); MSm/z 383 (M + H).Compound 36: 2-(5-cyclopropyl-furan-2-yl)-6-morpholin-4-ylmethyl-thieno[2,3-d]pyrimidin-4-ylamine
Compound 36 was prepared according to the procedure described in compound 37 except compound 52 was used in place of compound 53. 1H NMR (300 MHz, chloroform-d): δH 7.16 (1H, d, J 3.4), 6.95 (1H, s), 6.03 (1H, d, J 3.4), 5.29 (2H, s), 3.62–3.83 (6H, m), 2.47–2.56 (4H, m), 1.99–2.12 (1H, m), 0.90–1.00 (2H, m), 0.79–0.89 (2H, m); MSm/z 357 (M + H).Notes and references
- A. M. Lozano, A. E. Lang, W. D. Hutchison and J. O. Dostrovsky, Curr. Opin. Neurobiol., 1998, 8, 783 Search PubMed.
- J. S. Fink, D. R. Weaver, S. A. Rivkees, R. A. Peterfreund, A. E. Pollack, E. M. Adler and S. M. Reppert, Mol. Brain Res., 1992, 14, 186 Search PubMed.
- S. N. Schiffmann, F. Lipert, G. Vassart and J. J. Vanderhaeghen, Neurosci. Lett., 1991, 130, 177 Search PubMed.
- (a) A. Antonini, E. Tolosa, Y. Mizuno, M. Yamamoto and W. H. Poewe, Lancet Neurol., 2009, 8, 929 Search PubMed; (b) M. Yamamoto and A. H. V. Schapira, Expert Rev. Neurother., 2008, 8, 671 Search PubMed.
- (a) B. R. Neustadt, H. Liu, J. Hao, W. J. Greenlee, A. W. Stamford, C. Foster, L. Arik, J. Lachowicz, H. Zhang, R. Bertorelli, S. Fredduzzi, G. Varty, M. Cohen-Williams and K. Ng, Bioorg. Med. Chem. Lett., 2009, 19, 967 Search PubMed; (b) D. H. Slee, X. Zhang, M. Moorjani, E. Lin, M. C. Lanier, Y. Chen, J. K. Rueter, S. M. Lechner, S. Markison, S. Malany, T. Joswig, M. Santos, R. S. Gross, J. P. Williams, J. C. Castro-Palomino, M. I. Crespo, M. Prat, S. Gual, J. L. Diaz, J. Wen, Z. O'Brien and J. Saunders, J. Med. Chem., 2008, 51, 400 Search PubMed; (c) Y. Shao, A. G. Cole, M. R. Brescia, L. Y. Qin, J. Duo, T. M. Stauffer, L. L. Rokosz, B. F. McGuinness and I. Henderson, Bioorg. Med. Chem. Lett., 2009, 19, 1399 Search PubMed; (d) C. B. Vu, B. Peng, G. Kumaravel, G. Smits, X. Jin, D. Phadke, T. Engber, C. Huang, J. Reilly, S. Tam, D. Grant, G. Hetu, L. Chen, J. Zhang and R. C. Petter, J. Med. Chem., 2004, 47, 4291 Search PubMed; (e) R. J. Gillespie, I. A. Cliffe, C. E. Dawson, C. T. Dourish, S. Gaur, P. R. Giles, A. M. Jordan, A. R. Knight, A. Lawrence, J. Lerpiniere, A. Misra, R. M. Pratt, R. S. Todd, R. Upton, S. M. Weiss and D. S. Williamson, Bioorg. Med. Chem. Lett., 2008, 18, 2920 Search PubMed.
- (a) S. Latini and F. Pedata, J. Neurochem., 2001, 79, 463 CAS; (b) T. V. Dunwiddie and S. A. Masino, Annu. Rev. Neurosci., 2001, 24, 31 CrossRef CAS.
- G. L. Stiles, J. Biol. Chem., 1992, 267, 6451 Search PubMed.
- (a) D. L. Rosin, A. Robeva, R. L. Woodard, P. G. Guyenet and J. Linden, J. Comp. Neurol., 1998, 401, 163 Search PubMed; (b) B. B. Fredholm and P. Svenningsson, Trends Pharmacol. Sci., 1998, 19, 46 Search PubMed.
- W. Bara-Himenez, A. Sherzai, T. Dimitrova, A. Favit, F. Bibbiani, M. Gillespie, M. J. Morris, M. M. Mouradian and T. N. Chase, Neurology, 2003, 61, 293 CAS.
- (a) K. A. Moore, R. A. Nicoll and D. Schmitz, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 14397 Search PubMed; (b) N. Rebola, P. C. Pinheiro, C. R. Oliveira, J. O. Malva and R. A. Cunha, Brain Res., 2003, 987, 49 Search PubMed.
- (a) B. C. Shook, S. Rassnick, D. Hall, K. C. Rupert, G. R. Heintzelman, K. Hansen, D. Chakravarty, J. L. Bullington, R. Scannevin, B. Magliaro, L. Westover, K. Carroll, L. Lampron, R. Russell, S. Branum, K. Wells, S. Damon, S. Youells, X. Li, M. Osbourne, K. Demarest, Y. Tang, K. Rhodes and P. F. Jackson, Bioorg. Med. Chem. Lett., 2010, 20, 2864 Search PubMed; (b) B. C. Shook, S. Rassnick, D. Chakravarty, N. Wallace, M. Ault, J. Crooke, J. K. Barbay, A. Wang, K. Leonard, M. T. Powell, V. Alford, D. Hall, K. C. Rupert, G. R. Heintzelman, K. Hansen, J. L. Bullington, R. Scannevin, K. Carroll, L. Lampron, L. Westover, R. Russell, S. Branum, K. Wells, S. Damon, S. Youells, D. Beauchamp, X. Li, K. Rhodes and P. F. Jackson, Bioorg. Med. Chem. Lett., 2010, 20, 2868 Search PubMed; (c) B. C. Shook, S. Rassnick, M. C. Osborne, S. Davis, L. Westover, J. Boulet, D. Hall, K. C. Rupert, G. R. Heintzelman, K. Hansen, D. Charavarty, J. L. Bullington, R. Russell, S. Branum, K. M. Wells, S. Damon, S. Youells, X. Li, D. A. Beauchamp, D. Palmer, M. Reyes, K. Demarest, Y. Tang, K. Rhodes and P. F. Jackson, J. Med. Chem., 2010, 53, 8104 CrossRef CAS.
- (a) J. K. Barbay, D. Chakravarty, B. C. Shook, and A. Wang, WO 2010/045006; (b) J. A. Seijas, M. P. Vazquez-Tato and M. M. Martinez, Tetrahedron Lett., 2000, 41, 2215 CrossRef CAS.
- M. R. Zarrindast, M. Modabber and M. Sabetkasai, Psychopharmacology, 1993, 113, 257 Search PubMed.
- The pKa values were calculated using Third Dimensional Explorer software.
- Third Dimensional Explorer software does not take into account the steric effects surrounding the basic atoms and may therefore not give an accurate adjustment for the pKa value. The known pKa of piperidine is 11.2 (H. K. Hall Jr, J. Am. Chem. Soc., 1957, 79, 5441) and the known pKa of cis-2,6-dimethyl-piperidine is 10.9 (H. K. Hall Jr, J. Am. Chem. Soc., 1957, 79, 5439). Also, the polarity of 25 is significantly less than the corresponding piperidine 16 based on TLC analysis.
- D. H. Slee, Y. Chen, X. Zhang, M. Moorjani, M. C. Lanier, E. Lin, J. K. Rueter, J. P. Williams, S. M. Lechner, S. Markinson, S. Malany, M. Santos, R. S. Gross, K. Jalali, Y. Sai, Z. Zuo, C. Yang, J. C. Castro-Palomino, M. I. Crespo, M. Prat, S. Gual, J. L. Diaz and J. Saunders, J. Med. Chem., 2008, 51, 1719 Search PubMed.
- M. R. Collins, and V. Natarajan, PCT Int. Appl., WO 2006043145 A1, 2006.
| This journal is © The Royal Society of Chemistry 2011 |