DOI:
10.1039/C1MD00069A
(Concise Article)
Med. Chem. Commun., 2011,
2, 509-523
New substituted aryl esters and aryl amides of 3,4-dihydro-2H-1,2,4-benzothiadiazine 1,1-dioxides as positive allosteric modulators of AMPA receptors
Received
3rd March 2011
, Accepted 3rd March 2011
First published on 24th March 2011
Abstract
AMPA receptor potentiators belonging to 3,4-dihydro-2H-1,2,4-benzothiadiazine 1,1-dioxides have been found to be of great interest as cognitive enhancers. Previous structure–activity relationships have demonstrated the importance for activity of the nature of the substituent at the 7-position of the heterocycle. This work aims to explore the impact on AMPA potentiation of the introduction of different aryl and aralkyl ester or aryl amide groups at the 7-position. The new synthesized compounds were evaluated as AMPA receptor potentiators by examining their effect on rat brain primary cell cultures on AMPA-evoked membrane depolarisation using fluorescent membrane potential dyes and on imaging-based plate reader. The most potent compound of this series was COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound2-methylphenyl 4-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide 16c which provoked a strong potentiation of AMPA current with a potency close to that reported for the best reference compounds of the benzothiadiazine class (i.ecyclothiazide). This work also revealed that only the ortho-substitution of the phenyl group of 1,2,4-benzothiadiazine-7-carboxylate esters provided potent AMPA receptor potentiators opening the way to further chemical exploration.
Abbreviations
1. Introduction
Glutamate is, with GABA and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundglycine, the main neurotransmitter belonging to the amino-acid family. Its activity, together with other transmitters, maintains an equilibrium between neuronal excitation and inhibition.
Two types of receptors are associated with glutamate and are located in the central nervous system : the ionotropic and the metabotropic receptors. The first class of receptors could be classified in three main subtypes according to their affinity for different exogenous agonists : the KA (kainate), the NMDA (N-methyl-D-aspartate) and the AMPA (α-amino-3-hydroxy-5-methylisoxazole-4-propionate) receptors. Among these, the AMPA receptors are involved in several important biological processes such as the expression and the maintenance of long-term potentiation (LTP), the development of the central nervous system1 (CNS), synaptic plasticity, learning and memory processes.2,3 It has been proven that a disorder in the stimulation of these receptors could have negative consequences, as their excessive stimulation is linked to excitotoxicity4 and leads to several disorders such as epilepsy and neurogenerative disorders (i.e. : Huntington's and Alzheimer's diseases). On the contrary, a decrease of glutamate stimulation plays an important role in the cognition deficits. AMPA agonists have been proposed as possible therapeutic agents but they induce a marked excitotoxicity and remain simply as valuable pharmacological tools. Due to lower risk of toxicity in case of overdose, positive allosteric modulators of the AMPA receptors (AMPApams) have been developed.5–7 This latter approach seems better than a direct stimulation since positive allosteric modulators need the presence of the endogenous neurotransmitter to be active and to amplify the AMPA signals. They are therefore less toxic than the agonists. Beside their great interest as cognitive enhancers, AMPA receptor potentiators could also be important candidates for the treatment of schizophrenia or depression.6 The most important family of AMPApams include the 1,2,4-benzothiadiazine 1,1-dioxides with compounds 1, 2 and 3 as reference drugs.8,9 Among these drugs, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcyclothiazide (1) and the other benzothiadiazines, such as IDRA-21 (3), have been shown to bind and to act differently on the AMPA receptors, suggesting mechanistic differences in this series.10,11 Many synthetic developments have been made around this family leading to the discovery of very potent pyrido- and benzothiadiazine dioxides such as 4,125,126,137 (S18986)14 and 8 (Fig. 1).15 These compounds are structurally characterised by a saturated thiadiazine ring substituted at the 4-position by a small alkyl group.13,15,16 In the benzenic series, the 7-position seems to be critical for activity and several small groups have been introduced at this position but little has been done to enhance the steric hindrance (in this position). The present work aims at continuing the exploration of the impact on the AMPA receptor potentiation by the introduction of different aryl and aralkyl ester or aryl amide groups at the 7-position (with 9 as a general structure) while keeping a short alkyl chain at the 4-position like a methyl or an ethyl chain.
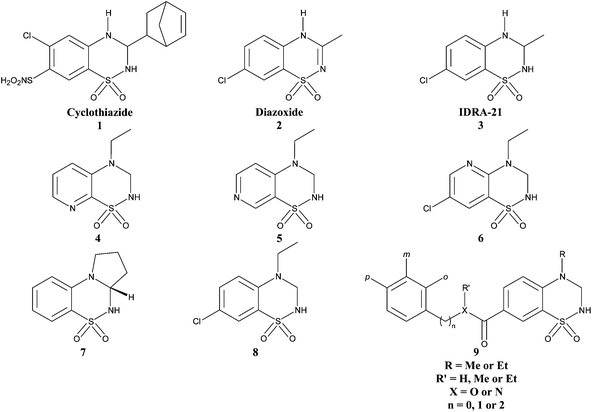 |
| | Fig. 1 Chemical structure of some positive allosteric modulators of AMPA receptors (1–8) and the general structure of the newly synthetized 1,2,4-benzothiadiazine 1,1-dioxides. | |
2. Chemistry
The synthetic pathways used to prepare the different products in the 3,4-dihydro-2H-benzothiadiazine 1,1-dioxide series are described in Schemes 1, 2 and 3.
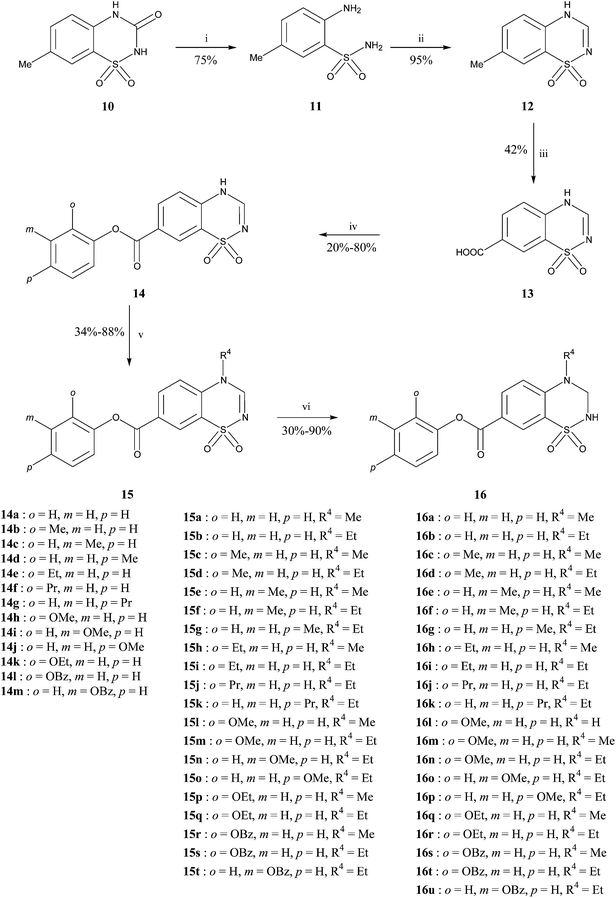 |
| | Scheme 1 Reagents and conditions : (i) H2SO4 50%, Δ; (ii) CH(OCH2CH3)3, Δ; (iii) KMnO4, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundwater, Δ; (iv) appropriate COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundphenol, CDI, DMF, Δ; (v) NaH, DMF, R4-I; (vi) NaBH4, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound2-propanol, Δ. | |
7-Methyl-3-oxo-3,4-dihydro-2H-1,2,4-benzothiadiazine 1,1-dioxide
10 was submitted to ring opening in acidic conditions to give the corresponding aminobenzenesulfonamide 11. The latter reacted with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundtriethyl orthoformate to give intermediate 12 by ring closure. Oxidation of the methyl group of 12 was then accomplished by action of KMnO4. Esterification of compound 13 was achieved on the 7-carboxylic acid function by using the appropriate phenol in the presence of CDI. The resulting benzothiadiazines 14 were then alkylated at the 4-position by the appropriate alkyl iodide. The last step was the saturation of the double bond at the 2,3-positions. This was achieved by action of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsodium borohydride leading to the final products 16 (Scheme 1).
For the synthesis of compounds bearing a benzyloxy- or phenethyloxycarbonyl group, esterification occurred on the carboxylic acid function 13 with the corresponding benzyl alcohol or COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundphenethyl alcohol in the presence of CDI. The benzothiadiazines 17 were then alkylated by COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl iodide in the presence of potassium carbonate. The reduction with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsodium borohydride in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound2-propanol gave the final products 19 (Scheme 2).
Concerning 3,4-dihydro-2H-1,2,4-benzothiadiazine 1,1-dioxides substituted at the 7-position by a phenylaminocarbonyl group, the amide link was introduced starting from compound 13. The reaction was achieved by using BOP in presence of NaHCO3. The expected products 20 were then alkylated with the appropriate alkyl iodide. Finally, compounds of general formula 21 were converted into their saturated counterparts by reduction with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsodium borohydride (Scheme 3).
3. Pharmacology
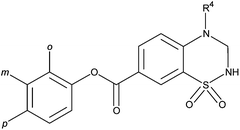
The diversely 7-substituted 3,4-dihydro-2H-1,2,4-benzothiadiazine 1,1-dioxides were tested as AMPA receptor potentiators. The functional assay consisted of examining the effect of the AMPA receptor potentiators on AMPA-evoked membrane depolarisation, measured by fluorescent membrane potential dyes and imaging based plate reader (FDSS), on rat brain primary cell cultures. Results are reported in Table 1, 2 and 3 and compared to the activity of compounds 18,9 and 412 used as reference.
Table 1 The effects of 7-phenoxycarbonyl-substituted 3,4-dihydro-2H-1,2,4-benzothiadiazine 1,1-dioxides on AMPA-evoked membrane depolarisation, measured by fluorescence membrane potential dyes and an imaging-based plate reader (FDSS) on rat brain primary cell cultures
|
|
|
Cmpd
|
o
|
m
|
p
|
R4 |
EC2Xa (μM) |
EC50b (μM) |
Max increasec (%) |
|
Concentration of drug evoking a 2-fold increase of all AMPA-mediated responses (between 3–100 μM AMPA; mean ± SEM; n ≥ 2).
Concentration of compound evoking 50% of the maximal effect of the compound.
Maximum effect of the drug on the AMPA-evoked current (expressed in % of the current evoked by AMPA, taken as 100%).
nd : not determined.
Compound described in the ref.15 and tested as AMPA receptor potentiator using voltage-clamp recordings of AMPA-induced currents on XenopusOocytes.
|
|
16a
|
H |
H |
H |
Me |
3.66 ± 0.16 |
3.65 ± 0.34 |
550 |
|
16b
e
|
H |
H |
H |
Et |
32.36 ± 1.15 |
ndd |
>670 |
|
16c
|
Me |
H |
H |
Me |
0.94 ± 0.15 |
1.72 ± 0.29 |
410 |
|
16d
|
Me |
H |
H |
Et |
3.48 ± 0.29 |
3.91 ± 0.59 |
330 |
|
16e
|
H |
Me |
H |
Me |
>100 |
ndd |
<200 |
|
16f
|
H |
Me |
H |
Et |
>100 |
ndd |
<200 |
|
16g
|
H |
H |
Me |
Et |
>100 |
ndd |
<200 |
|
16h
|
Et |
H |
H |
Me |
>100 |
ndd |
<200 |
|
16i
|
Et |
H |
H |
Et |
>100 |
ndd |
<200 |
|
16j
|
Pr
|
H |
H |
Et |
>100 |
ndd |
<200 |
|
16k
|
H |
H |
Pr
|
Et |
>100 |
ndd |
<200 |
|
16l
|
OMe
|
H |
H |
H |
>100 |
ndd |
<200 |
|
16m
|
OMe
|
H |
H |
Me |
6.40 ± 0.47 |
7.27 ± 2.30 |
410 |
|
16n
|
OMe
|
H |
H |
Et |
10.63 ± 9.10 |
ndd |
380 |
|
16o
|
H |
OMe
|
H |
Et |
>35 |
ndd |
200 |
|
16p
|
H |
H |
OMe
|
Et |
>100 |
ndd |
<200 |
|
16q
|
OEt
|
H |
H |
Me |
7.08 ± 1.99 |
ndd |
>360 |
|
16r
|
OEt
|
H |
H |
Et |
21.57 ± 8.04 |
ndd |
>270 |
|
16s
|
OBz
|
H |
H |
Me |
>100 |
ndd |
<200 |
|
16t
|
OBz
|
H |
H |
Et |
>100 |
ndd |
<200 |
|
16u
|
H |
OBz
|
H |
Et |
>100 |
ndd |
<200 |
|
1
e
|
|
|
|
H |
1.7 ± 0.4 |
7.1 ± 0.7 |
844 |
|
4
e
|
|
|
|
Et |
7.9 ± 2.2 |
22 ± 12 |
1478 |
Table 2 The effects of 7-benzyloxy or 7-phenethyloxycarbonyl-substituted 3,4-dihydro-2H-1,2,4-benzothiadiazine 1,1-dioxides on AMPA-evoked membrane depolarisation, measured by fluorescence membrane potential dyes and an imaging-based plate reader (FDSS), on rat brain primary cell cultures
|

|
|
Cmpd
|
n |
EC2Xa (μM) |
EC50b (μM) |
Max increasec (%) |
|
Concentration of drug evoking a 2-fold increase of all AMPA-mediated responses (between 3–100 μM AMPA; mean ± SEM; n ≥ 3).
Concentration of compound evoking 50% of the maximal effect of the compound.
Maximum effect of the drug on the AMPA-evoked current (expressed in % of the current evoked by AMPA, taken as 100%).
nd : not determined.
|
|
19a
|
1 |
>100 |
ndd |
<200 |
|
19b
|
2 |
>100 |
ndd |
<200 |
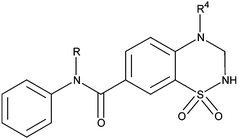
Table 3 The effects of 7-phenylaminocarbonyl-substituted 3,4-dihydro-2H-1,2,4-benzothiadiazine 1,1-dioxides on AMPA-evoked membrane depolarisation, measured by fluorescence membrane potential dyes and an imaging-based plate reader (FDSS), on rat brain primary cell cultures
|
|
|
Cmpd
|
R4 |
R
|
EC2Xa (μM) |
EC50b (μM) |
Max increasec (%) |
|
Concentration of drug evoking a 2-fold increase of all AMPA-mediated responses (between 3–100 μM AMPA; mean ± SEM; n ≥ 2).
Concentration of compound evoking 50% of the maximal effect of the compound.
Maximum effect of the drug on the AMPA-evoked current (expressed in % of the current evoked by AMPA, taken as 100%).
nd : not determined.
|
|
22a
|
Me |
H |
>100 |
ndd |
<200 |
|
22b
|
Me |
Me |
>100 |
ndd |
<200 |
|
22c
|
Me |
Et |
>100 |
ndd |
<200 |
|
22d
|
Et |
H |
>100 |
ndd |
<200 |
4. Results and discussion
Contrary to previously described results demonstrating the weak activity of compounds substituted at the 7-position by a carboxylic group or its methyl ester or amide counterparts, the introduction of a phenoxycarbonyl group at the 7-position led to potent AMPA receptor potentiators (16a, 16c, 16d, 16m and 16q). These results are quite surprising because, in all the benzothiadiazine series that we developed around IDRA-21 structure, the presence of hindered groups on the benzenic or on the thiadiazine rings provoked a strong decrease of affinity for AMPA receptors.
The nature of the substituent at the 4-position also seemed to be important for activity. Indeed, in all series, 4-methyl derivatives were more potent than the 4-ethyl counterparts (compare 16a with 16b, 16c with 16d, 16m with 16n and 16q with 16r). These results were rather different to the results previously described12,15 but underline the fact that the steric hindrance on the thiadiazine ring have a great influence on activity. However, the absence of a substituent in this position (16l) led to a drastic decrease in AMPA current potentiation.
The introduction of small electron donating groups (Me and OMe) in the ortho-, meta- and para-position of the phenyl group was realized in order to identify the best position for a putative substitution (Table 1).
Examination of the results obtained with methyl, methoxy or ethoxy groups demonstrated that only ortho substitution of 4-methyl-substituted compounds allowed the maintenance of a marked AMPA potentiation (16a, 16c, 16m and 16q).
Indeed, all meta or para substituted derivatives appeared to be less active or inactive in the chosen pharmacological model (16e, 16f, 16g, 16k, 16o, 16p and 16u).
Moreover, an increase of the size of the ortho (Et and Pr) substituent led also to a rapid loss of potency (16h, 16i, 16j, 16s and 16t).
These results highlight the negative impact of the steric hindrance on activity and confirm the very sensitive cut-off that links activity and substitution of the thiadiazine ring system in our series. The data underline also the poor steric latitude at some crucial positions of the thiadiazine ring or the phenyl ester group.
Concerning the maximum increase of the AMPA current observed with the most active compounds (see Table 1) it can be noticed that these derivatives seems to exhibit lower maximal effect than COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcyclothiazide and 4 but enhance both significantly and potently the current induced by AMPA.
Since the lead compounds are aryl esters expected to show some sensitivity to hydrolysis, it was important to appreciate their chemical stability in aqueous solution. Thus, a degradation experiment of compound (16a) was realized in aqueous medium under neutral (pH = 7.4) and acidic (pH = 1) conditions and monitored by HPLC. The results indicated no significant degradation of this product on both conditions even after 24 h at 25 °C.
Table 2, indicates that by separating the phenyl ring with one or two carbon atoms from the ester function at the 7-position, a complete loss of activity was observed (compare 19a and 19b with 16b).
Replacement of an ester function by an amide function also seemed to be unfavourable to activity, whether the nitrogen atom was or was not substituted (22a, 22b, 22c and 22d) (Table 3). These compounds were prepared with the aim of providing analogues of the active ester exhibiting a greater metabolic stability. This loss of activity could be due to the change of the conformation between the ester and the amide function including a change in the phenyl ring orientation.
By comparing our SAR data to those of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcyclothiazide and its analogues on AMPA receptors, we can expect that our compounds are not able to occupy the same binding sites. Indeed, the absence of a hindered substituent at the 3-position and the absence of a hydrogen atom at the 4-position (which seem to be required for COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcyclothiazide activity and its binding to the GluR2 ligand-binding domain10,11) prohibited the same interactions between our molecules and the iGluR2 subunits. So, in spite of an evident structural analogy, the SAR deduced from COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcyclothiazide analogues could not be applied to our derivatives. The new compounds show closer analogy to IDRA-21 suggesting a similar positioning in the binding pocket. However, because an aryl ester function appears to be required in order to maintain a high level of activity, the phenyl group probably enters into a specific hydrophobic pocket of restricted size and capacity. It is tempting to speculate that our molecules could probably have a binding mode between that of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcyclothiazide and IDRA-21.
As AMPA receptors are located in the CNS, it seems important to evaluate the ability of our lead compounds to cross the blood-brain barrier (BBB). It is well described that some physicochemical properties such as acidity (pKa) or lipophilicity (i.e. log P) constants greatly influence this penetration. As the 3,4-dihydro-2H-1,2,4-benzothiadiazine 1,1-dioxide ring are known to exhibit estimated or measured pKa values >9,16 it could be postulated that COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcyclothiazide, IDRA-21 and our compounds are poorly or not ionized at the physiological pH which is an important requirement for CNS drugs. Concerning lipophilicity, it is generally admitted that log P values between 2 and 4 are favourable to a BBB crossing. We have estimated the log P values of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcyclothiazide, IDRA-21 and 16a, 16c and 16m using ClogP programme17 (Biobyte Corp. 201 W. Fourth Street, Suite ≠ 204 Claremont, CA 91711-4707; USA). Calculated log P value of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcyclothiazide was found to be equal to 1.97 which is very close to the described measured value (lit. : 1.95).18 Despite of this value, cyclothiazide is reported to not readily cross the BBB.19IDRA-21 has the same calculated log P value than COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcyclothiazide (2.07) but is decribed to have in vivo effect in memory tests suggesting a penetration in the brain.20 Compared to the references, our lead compounds (16a, 16c and 16m) show an interesting increase of lipophilicity value with an estimated log P value ≥3 (respectively 3.27, 3.77 and 2.94). Taking into account pKa and calculated log P values we can postulate that our drugs could present physicochemical properties compatible with CNS required drug like profile.
5. Conclusions
A series of 7-substituted 3,4-dihydro-2H-1,2,4-benzothiadiazine 1,1-dioxides were synthesised and evaluated as positive allosteric modulators of the AMPA receptors. The biological results obtained with the new molecules showed that the modulation of the nature of the substituent at the 7-position had a marked effect on in vitro activity. Among the new described products, compounds bearing a phenoxycarbonyl group at the 7-position, and more specifically a 2-methylphenoxycarbonyl group (compound 16c), appeared to possess a very interesting in vitro activity on the AMPA receptors. From a general point of view, it appears that the introduction of a methyl chain on the nitrogen atom at the 4-position is better for the activity than an ethyl chain. In the same way, the presence of a short alkyl or O-alkyl group at the ortho-position of the phenyloxycarbonyl group is also favorable for activity. The rank order of potency is as follows Me > OMe > OEt. If the same alkyl or O-alkyl group was bore on the meta or para-position, a decrease or a loss of activity was observed. The same observation was made when the benzene ring was separated from the carboxylic group by one or two carbon atoms. Finally, it appeared that the replacement of the ester group by an amido group at the 7-position conducted to a loss of activity. This work indicates that the introduction at the 7-position of bulky groups such as a phenoxycarbonyl group can provide very potent AMPA receptor potentiators even if modulation of the substitution on the phenyl ring seems to have a marked affect on the activity of the AMPA receptors. In particular, ortho-substitution of the ester aromatic ring appears to be quite interesting for the AMPA current modulation and opens new perspectives that could allow for the improvement of pharmacodynamic and pharmacokinetic parameters in this promising series.
This series of compounds could be placed between COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcyclothiazide and IDRA-21 in terms of structure but demonstrated that the presence of a hydrogen atom at the 4-position and a hindered group at the 3-position are not required to maintain an interesting activity. This opens new perspectives for the development of more bulky and probably more subunit selective AMPA receptor potentiators in the benzothiadiazine family.
6. Experimental protocols
6.1. Chemistry
Melting points were determined on a Stuart SMP3 capillary apparatus and are uncorrected. IR spectra were recorded as KBr pellets on a Perkin Elmer 1000 FTIR-spectrophotometer. The 1H NMR spectra were taken on a Bruker Advance 500 (500 MHz) instrument in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6 with TMS as an internal standard; chemical shifts are reported in δ values (ppm) relative to internal TMS. The abbreviation s = singlet, d = doublet, t = triplet, q = quadruplet, dd = doublet of doublet, td = triplet of doublet, m = multiplet, b = broad and Cq = quaternary carbon are used throughout. Elemental analyses (C, H, N, S) were realized on a FlashEA 1112 series (Thermo-Interscience) and where within ±0.4% of the theoretical values. All reactions were routinely checked by TLC on COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsilica gel Merck 60F254.
2-Amino-5-methylbenzenesulfonamide (11).
A solution of 7-methyl-3-oxo-3,4-dihydro-2H-1,2,4-benzothiadiazine 1,1-dioxide (10, 10 g, 47,12 mmol) in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsulfuric acid 50% (150 mL) was refluxed until complete dissolution of the suspension. At the end of the reaction, the solution was adjusted to pH 7 with an aqueous solution of concentrated NaOH. The solvent was removed by distillation under reduced pressure. The residue was triturated with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundmethanol and the salts were eliminated by filtration. The filtrate was concentrated to dryness under reduced pressure and the residue was triturated in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundwater (a minimum). The resulting precipitate was collected by filtration, washed with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundwater and dried (6.58 g, 75%); mp 113–121 °C; (Found: C, 45.23; H, 5.40; N, 15.03; S, 17.30. Calc. for C7H10N2O2S: C, 45.14; H, 5.41; N, 15.04; S, 17.21%); ν/cm−1 3440, 3395, 3361, 3287, 1536, 1500, 1315, 1141; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 2.17 (s, 3H, CH3), 5.63 (s, 2H, NH2), 6.71 (d, 1H, J = 8.3 Hz, 3-H), 7.06 (dd, 1H, J = 1.8 Hz and 8.3 Hz, 4-H), 7.14 (s, 2H, SO2NH2), 7.35 (d, 1H, J = 1.8 Hz, 6-H).
7-Methyl-4H-1,2,4-benzothiadiazine 1,1-dioxide (12).
2-Amino-5-methylbenzenesulfonamide (11, 6 g, 32,21 mmol) was heated in triethyl orthoformiate (30 mL) to reflux for 20 min. After cooling on an ice bath, the title compound was collected by filtration, washed with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compounddiethyl ether and dried (6 g, 95%); mp > 300 °C; (Found: C, 49.08; H, 4.14; N, 14.31; S, 16.19. Calc. for C8H8N2O2S: C, 48.97; H, 4.11; N, 14.28; S, 16.34%); δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 2.38 (s, 3H, CH3), 7.23 (d, 1H, J = 8.4 Hz, 5-H), 7.50 (dd, 1H, J = 1.4 Hz and 8.4 Hz, 6-H) 7.62 (d, 1H, J = 1.4 Hz, 8-H), 7.94 (s, 1H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 12.19 (bs, 1H, 4-NH).
N), 12.19 (bs, 1H, 4-NH).
4H-1,2,4-Benzothiadiazine-7-carboxylic acid 1,1-dioxide (13).
7-Methyl-4H-1,2,4-benzothiadiazine 1,1-dioxide (12, 3,9 g, 19,9 mmol) was heated at 70 °C in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundwater (60 mL). A solution of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundNaOH 10% was added dropwise to the mixture until dissolution of the suspension. Potassium permanganate (11,7 g, 74,1 mmol) was added portionwise and the mixture was kept at 70 °C for 4 h. At the end of the reaction, the insoluble material was removed by filtration and the filtrate was treated with charcoal. After the elimination of charcoal, the solution was adjusted to pH 1 with concentrated HCl and the resulting precipitate was collected by filtration, washed with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundwater and dried (1.89 g, 42%); mp > 300 °C; (Found: C, 42.06; H, 2.74; N, 12.18; S, 13.78. Calc. for C8H6N2O4S: C, 42.48; H, 2.67; N, 12.38; S, 14.17%); δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 7.44 (d, 1H, J = 8.6 Hz, 5-H), 8.07 (s, 1H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 8.18 (dd, 1H, J = 1.8 Hz and 8.6 Hz, 6-H) 8.25 (d, 1H, J = 1.8 Hz, 8-H), 12.61 (bs, 1H, 4-NH), 13.41 (bs, 1H, COOH).
N), 8.18 (dd, 1H, J = 1.8 Hz and 8.6 Hz, 6-H) 8.25 (d, 1H, J = 1.8 Hz, 8-H), 12.61 (bs, 1H, 4-NH), 13.41 (bs, 1H, COOH).
Phenyl 4H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (14a).
(0.28 g, 41%); mp 252–255 °C; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 7.33 (m, 3H, p-H and o-H), 7.49 (m, 2H, m–H), 7.53 (d, 1H, J = 8.5 Hz, 5-H), 8.12 (s, 1H, CHN), 8.35 (d, 1H, J = 8.5 Hz, 6-H), 8.45 (s, 1H, 8-H), 12.78 (bs, 1H, 4-NH).
2-Methylphenyl 4H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (14b).
(0.22 g, 31%); mp 199–204 °C; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 2.17 (s, 3H, 2′-CH3), 7.27 (m, 4H, 3′-H, 4′-H, 5′-H and 6′-H), 7.54 (d, 1H, J = 8.9 Hz, 5-H), 8.11 (s, 1H, CHN), 8.38 (dd, 1H, J = 1.8 Hz and 8.9 Hz, 6-H), 8.46 (d, 1H, J = 1.8 Hz, 8-H), 12.65 (bs, 1H, 4-NH).
3-Methylphenyl 4H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (14c).
(0.23 g, 65%); mp 240–242 °C; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 2.35 (s, 3H, 3′-CH3), 7.34 (m, 4H, 2′-H, 4′-H, 5′-H and 6′-H), 7.51 (d, 1H, J = 8 Hz, 5-H), 8.11 (s, 1H, CHN), 8.34 (dd, 1H, J = 1.7 Hz and 8 Hz, 6-H), 8.43 (d, 1H, J = 1.7 Hz, 8-H), 12.72 (bs, 1H, 4-NH).
4-Methylphenyl 4H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (14d).
(0.45 g, 64%); δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 2.32 (s, 3H, 4′-CH3), 7.20 (d, 2H, J = 9 Hz, 2′H and 6′H or 3′H and 5′H), 7.28 (d, 2H, J = 9 Hz, 2′H and 6′H or 3′H and 5′H), 7.52 (d, 1H, J = 8.7 Hz, 5-H), 8.12 (s, 1H, CHN), 8.35 (dd, 1H, J = 0.9 Hz and 8.7 Hz, 6-H), 8.43 (d, 1H, J = 0.9 Hz, 8-H), 12.76 (bs, 1H, 4-NH).
2-Ethylphenyl 4H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (14e).
(0.22 g, 20%); mp 161–165 °C; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 1.13 (t, 3H, J = 7.5 Hz, 2′-CH2CH3), 2.54 (q, 2H, J = 7.5 Hz, 2′-CH2CH3), 7.28 (m, 3H, 3′-H or 6′-H, 4′-H and 5′-H), 7.38 (d, 1H, J = 6.9 Hz, 3′-H or 6′-H), 7.54 (d, 1H, J = 8.7 Hz, 5-H), 8.11 (s, 1H, CHN), 8.38 (dd, 1H, J = 1.9 Hz and 8.7 Hz, 6-H), 8.45 (d, 1H, J = 1.9 Hz, 8-H), 12.67 (bs, 1H, 4-NH).
2-Propylphenyl 4H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (14f).
(0.2 g, 26%); mp 203–205 °C; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 0.84 (t, 3H, J = 7.3 Hz, 2′-CH2CH2CH3), 1.55 (m, 2H, J = 7.3 Hz, 2′-CH2CH2CH3), 2.51 (t, 2H, J = 7.3 Hz, 2′-CH2CH2CH3), 7.31 (m, 4H, 3′-H, 4′-H, 5′-H and 6′-H), 7.54 (d, 1H, J = 8.5 Hz, 5-H), 8.10 (s, 1H, CHN), 8.36 (dd, 1H, J = 1.6 Hz and 8.5 Hz, 6-H), 8.44 (d, 1H, 8-H), 12.68 (bs, 1H, 4-NH).
4-Propylphenyl 4H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (14g).
(0.58 g, 71%); mp 219–221 °C; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 0.91 (t, 3H, J = 7.5 Hz, 4′-CH2CH2CH3), 1.62 (m, 2H, J = 7.5 Hz, 4′-CH2CH2CH3), 2.59 (t, 2H, J = 7.5 Hz, 4′-CH2CH2CH3), 7.22 (d, 2H, J = 8.2 Hz, 2′-H and 6′-H or 3′-H and 5′-H), 7.29 (d, 2H, J = 8.2 Hz, 2′-H and 6′-H or 3′-H and 5′-H), 7.52 (d, 1H, J = 8.8 Hz, 5-H), 8.11 (s, 1H, CHN), 8.34 (dd, 1H, J = 1.7 Hz and 8.8 Hz, 6-H), 8.43 (d, 1H, J = 1.7 Hz, 8-H), 12.68 (bs, 1H, 4-NH).
2-Methoxyphenyl 4H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (14h).
(0.51 g, 69%); δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 3.77 (s, 3H, 2′-OCH3), 7.03 (td, 1H, J = 1.1 Hz and 8.2 Hz, 4′-H or 5′-H), 7.20 (dd, 1H, J = 1.1 Hz and 8.2 Hz, 3′-H or 6′-H), 7.29 (td, 1H, J = 1.1 Hz and 8.2 Hz, 4′-H or 5′-H), 7.32 (dd, 1H, J = 1.1 Hz and 8.2 Hz, 3′-H or 6′-H), 7.53 (d, 1H, J = 8.7 Hz, 5-H), 8.12 (s, 1H, CHN), 8.34 (dd, 1H, J = 1.9 Hz and 8.7 Hz, 6-H), 8.42 (d, 1H, J = 1.9 Hz, 8-H), 12.71 (bs, 1H, 4-NH).
3-Methoxyphenyl 4H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (14i).
(1.12 g, 76%); mp 226–229 °C; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 3.79 (s, 3H, 3′-OCH3), 6.91 (d, 2H, J 8.3 Hz, 4′-H and 6′-H), 6.97 (s, 1H, 2′-H), 7.38 (t, 1H, J = 8.3 Hz, 5′-H), 7.52 (d, 1H, J = 8.7 Hz, 5-H), 8.10 (s, 1H, CHN), 8.33 (dd, 1H, J = 1.5 Hz and 8.7 Hz, 6-H), 8.44 (d, 1H, J = 1.5 Hz, 8-H), 12.68 (bs, 1H, 4-NH).
4-Methoxyphenyl 4H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (14j).
(0.27 g, 36%); δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 3.79 (s, 3H, 4′-OCH3), 7.01 (d, 2H, J = 9 Hz, 2′-H and 6′-H or 3′-H and 5′-H), 7.26 (d, 2H, J = 9 Hz, 2′-H and 6′-H or 3′-H and 5′-H), 7.52 (d, 1H, J = 8.7 Hz, 5-H), 8.11 (s, 1H, CHN), 8.34 (dd, 1H, J = 1.6 Hz and 8.7 Hz, 6-H), 8.43 (d, 1H, J = 1.6 Hz, 8-H), 12.70 (bs, 1H, 4-NH).
2-Ethoxyphenyl 4H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (14k).
(0.92 g, 80%); mp 191–193 °C; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 1.19 (t, 3H, J = 6.9 Hz, 2′-OCH2CH3), 4.05 (q, 2H, J = 6.9 Hz, 2′-OCH2CH3), 7.01 (t, 1H, J = 8 Hz, 4′-H or 5′-H), 7.18 (d, 1H, J = 8 Hz, 3′-H or 6′-H), 7.27 (d, 1H, J = 8 Hz, 3′-H or 6′-H), 7.29 (t, 1H, J = 8 Hz, 4′-H or 5′-H), 7.53 (d, 1H, J = 8.6 Hz, 5-H), 8.11 (s, 1H, CHN), 8.34 (dd, 1H, J = 1.7 Hz and 8.6 Hz, 6-H), 8.42 (d, 1H, J = 1.7 Hz, 8-H), 12.69 (bs, 1H, 4-NH).
2-Benzyloxyphenyl 4H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (14l).
(0.55 g, 60%); mp 206–210 °C; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 5.15 (s, 2H, OCH2), 7.04 (t, 1H, J = 7.5 Hz, H-aromatic), 7.28 (m, 8H, H-aromatic), 7.51 (d, 1H, J = 8.7 hz, 5-H), 8.11 (s, 1H, CHN), 8.34 (dd, 1H, J = 1.8 Hz and 8.7 Hz, 6-H), 8.45 (d, 1H, J = 1.8 Hz, 8-H), 12.63 (bs, 1H, 4-NH).
3-Benzyloxyphenyl 4H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (14m).
(0.96 g, 53%); mp 146–150 °C; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 5.12 (s, 2H, OCH2), 6.92 (d, 1H, J = 8.1 Hz, 4′-H or 6′-H), 6.98 (d, 1H, J = 8.1 Hz, 4′-H or 6′-H), 7.06 (s, 1H, 2′-H), 7.40 (m, 7H, 5-H, 5′-H and H-aromatic), 7.96 (s, 1H, CHN), 8.26 (dd, 1H, J = 1.7 Hz and 8.7 Hz, 6-H), 8.41 (d, 1H, J = 1.7 Hz, 8-H), 12.70 (bs, 1H, 4-NH).
Benzyl 4H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (17a).
(0.33 g, 53%); δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 5.38 (s, 2H, OCH2), 5.43 (m, 6H, 5-H and H-aromatic), 8.08 (s, 1H, CHN), 8.22 (dd, 1H, J = 1.9 Hz and 8.6 Hz, 6-H), 8.29 (d, 1H, J = 1.9 Hz, 8-H), 12.68 (bs, 1H, 4-NH).
Phenethyl 4H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (17b).
(0.26 g, 88%); δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 3.06 (t, 2H, J = 6.8 Hz, OCH2CH2), 4.51 (t, 2H, J = 6.8 Hz, OCH2CH2), 7.28 (m, 5H, H-aromatic), 7.44 (d, 1H, J = 8.8 Hz, 5-H), 8.05 (s, 1H, CHN), 8.14 (dd, 1H, J = 1.9 Hz and 8.8 Hz, 6-H), 8.23 (d, 1H, J = 1.9 Hz, 8-H), 12.62 (bs, 1H, 4-NH).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundPhenyl 4-methyl-4H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (15a).
(0.36 g, 86%); δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 3.69 (s, 3H, NCH3), 7.34 (d, 2H, J = 7.9 Hz, 2′-H and 6′-H), 7.36 (t, 1H, J = 7.9 Hz, 4′-H), 7.50 (t, 2H, J = 7.9 Hz, 3′-H and 5′-H), 7.71 (d, 1H, J = 7.9 Hz, 5-H), 8.21 (s, 1H, CHN), 8.43 (dd, 1H, J = 1.6 Hz and 9 Hz, 6-H), 8.49 (d, 1H, J = 1.6 Hz, 8-H).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound2-Methylphenyl 4-methyl-4H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (15c).
(0.15 g, 65%); δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 2.18 (s, 3H, 2′-CH3), 3.71 (s, 3H, NCH3), 7.29 (m, 4H, 3′-H, 4′-H, 5′-H and 6′-H), 7.72 (d, 1H, J = 8.8 Hz, 5-H), 8.20 (s, 1H, CHN), 8.48 (dd, 1H, J = 2 Hz and 8.8 Hz, 6-H), 8.51 (d, 1H, J = 2 Hz, 8-H).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound2-Methylphenyl 4-ethyl-4H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (15d).
(0.18 g, 82%); mp 208–214 °C; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 1.36 (t, 3H, J = 7 Hz, NCH2CH3), 2.18 (s, 3H, 2′-CH3), 4.23 (q, 2H, J = 7 Hz, NCH2CH3), 7.31 (m, 4H, 3′-H, 4′-H, 5′-H and 6′-H), 7.83 (d, 1H, J = 9.2 Hz, 5-H), 8.27 (s, 1H, CHN), 8.45 (dd, 1H, J = 1.9 Hz and 9.2 Hz, 6-H), 8.51 (d, 1H, J = 1.9 Hz, 8-H).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound3-Methylphenyl 4-methyl-4H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (15e).
(0.19 g, 82%); mp 161–165 °C; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 2.39 (s, 3H, 3′-CH3), 3.69 (s, 3H, NCH3), 7.16 (m, 2H, 4′-H and 6′-H), 7.18 (s, 1H, 2′-H), 7.38 (t, 1H, J = 8.2 Hz, 5′-H), 7.71 (d, 1H, J = 8.8 Hz, 5-H), 8.20 (s, 1H, CHN), 8.42 (dd, 1H, J = 1.8 Hz and 8.8 Hz, 6-H), 8.48 (d, 1H, J = 1.8 Hz, 8-H).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound3-Methylphenyl 4-ethyl-4H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (15f).
(0.3 g, 68%); mp 149–152 °C; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 1.34 (t, 3H, J = 7.2 Hz, NCH2CH3), 2.37 (s, 3H, 3′-CH3), 4.23 (q, 2H, J = 7.2 Hz, NCH2CH3), 7.39 (m, 4H, 3′-H, 4′-H, 5′-H and 6′-H), 7.81 (d, 1H, J = 8.9 Hz, 5-H), 8.28 (s, 1H, CHN), 8.41 (dd, 1H, J = 1.9 Hz and 8.9 Hz, 6-H), 8.49 (d, 1H, J = 1.9 Hz, 8-H).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound4-Methylphenyl 4-ethyl-4H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (15g).
(0.23 g, 72%); δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 1.36 (t, 3H, J = 6.9 Hz, NCH2CH3), 2.35 (s, 3H, 4′-CH3), 4.23 (q, 2H, J = 6.9 Hz, NCH2CH3), 7.25 (m, 4H, 3′-H, 4′-H, 5′-H and 6′-H), 7.81 (d, 1H, J = 8.9 Hz, 5-H), 8.26 (s, 1H, CHN), 8.40 (dd, 1H, J = 2 Hz and 8.9 Hz, 6-H), 8.48 (d, 1H, J 2 Hz, 8-H).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound2-Ethylphenyl 4-methyl-4H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (15h).
(0.2 g, 85%); mp 165–169 °C; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 1.13 (t, 3H, J = 7.8 Hz, 2′-CH2CH3), 2.55 (q, 2H, J = 7.8 Hz, 2′-CH2CH3), 3.71 (s, 3H, NCH3), 7.34 (m, 4H, 3′-H, 4′-H, 5′-H and 6′-H), 7.73 (d, 1H, J = 8.7 Hz, 5-H), 8.21 (s, 1H, CHN), 8.47 (dd, 1H, J = 2 Hz and 8.7 Hz, 6-H), 8.49 (d, 1H, J = 2 Hz, 8-H).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound2-Ethylphenyl 4-ethyl-4H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (15i).
(0.16 g, 66%); mp 89–94 °C; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 1.13 (t, 3H, J = 7.7 Hz, 2′-CH2CH3), 1.36 (t, 3H, J = 6.9 Hz, NCH2CH3), 2.55 (q, 2H, J = 7.7 Hz, 2′-CH2CH3), 4.23 (q, J = 6.9 Hz, 2H, NCH2CH3), 7.34 (m, 4H, 3′-H, 4′-H, 5′-H and 6′-H), 7.83 (d, 1H, J = 8.8 Hz, 5-H), 8.27 (s, 1H, CHN), 8.44 (dd, 1H, J = 1.9 Hz and 8.8 Hz, 6-H), 8.50 (d, 1H, J = 1.9 Hz, 8-H).
2-Propylphenyl 4-ethyl-4H-1,2,4-benzothiadiazine 1,1-dioxide-7-carboxylate (15j).
(0.17 g, 78%); δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 0.85 (t, 3H, J = 7.2 Hz, 2′-CH2CH2CH3), 1.37 (t, 3H, J = 6.9 Hz, NCH2CH3), 1.56 (m, 2H, J = 7.2 Hz, 2′-CH2CH2CH3), 2.52 (t, 2H, J = 7.2 Hz, 2′-CH2CH2CH3), 4.24 (q, 2H, J = 6.9 Hz, NCH2CH3), 7.34 (m, 4H, 3′-H, 4′-H, 5′-H and 6′-H), 7.84 (d, 1H, J = 8.9 Hz, 5-H), 8.27 (s, 1H, CHN), 8.44 (dd, 1H, J = 1.8 Hz and 8.9 Hz, 6-H), 8.50 (d, 1H, 8-H).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound4-Propylphenyl 4-ethyl-4H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (15k).
(0.16 g, 74%); mp 143–146 °C; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 0.92 (t, 3H, J = 7.3 Hz, 4′-CH2CH2CH3), 1.36 (t, 3H, J = 7 Hz, NCH2CH3), 1.62 (m, 2H, J = 7.3 Hz, 4′-CH2CH2CH3), 2.60 (t, 2H, J = 7.3 Hz, 4′-CH2CH2CH3), 4.23 (q, 2H, J = 7 Hz, NCH2CH3), 7.24 (d, 2H, J = 8.2 Hz, 2′-H and 6′-H or 3′-H and 5′-H), 7.29 (d, 2H, J = 8.2 Hz, 2′-H and 6′-H or 3′-H and 5′-H), 7.81 (d, 1H, J = 9 Hz, 5-H), 8.26 (s, 1H, CHN), 8.40 (dd, 1H, J = 2 Hz and 9 Hz, 6-H), 8.48 (d, 1H, J = 2 Hz, 8-H).
2-Methoxyphenyl 4-methyl-4H-1,2,4-benzothiadiazine 1,1-dioxide-7-carboxylate (15l).
(0.09 g, 61%); δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 3.70 (s, 3H, NCH3), 3.77 (s, 3H, 2′-OCH3), 7.03 (t, 1H, J = 7.7 Hz, 4′-H or 5′-H), 7.21 (d, J = 7.7 Hz, 1H, 3′-H or 6′-H), 7.31 (d, 1H, J = 7.7 Hz, 3′-H or 6′-H), 7.32 (t, 1H, J = 7.7 Hz, 4′-H or 5′-H), 7.71 (d, 1H, J = 8.9 Hz, 5-H), 8.21 (s, 1H, CHN), 8.42 (dd, 1H, J = 2 Hz and 8.9 Hz, 6-H), 8.46 (d, 1H, J = 2 Hz, 8-H).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound2-Methoxyphenyl 4-ethyl-4H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (15m).
(0.18 g, 68%); mp 156–158 °C; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 1.36 (t, 3H, J = 7 Hz, NCH2CH3), 3.77 (s, 3H, 2′-OCH3), 4.23 (q, 2H, J = 7 Hz, NCH2CH3), 7.03 (t, 1H, J = 7.7 Hz, 4′-H or 5′-H), 7.21 (d, 1H, J = 7.7 Hz, 3′-H or 6′-H), 7.30 (d, 1H, J = 7.7 Hz, 3′-H or 6′-H), 7.32 (t, 1H, J = 7.7 Hz, 4′-H or 5′-H), 7.82 (d, 1H, J = 8.9 Hz, 5-H), 8.27 (s, 1H, CHN), 8.40 (dd, 1H, J = 1.8 Hz and 8.9 Hz, 6-H), 8.47 (d, 1H, J = 1.8 Hz, 8-H).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound3-Methoxyphenyl 4-ethyl-4H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (15n).
(0.24 g, 44%); mp 166–168 °C; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 1.36 (t, 3H, J = 6.9 Hz, NCH2CH3), 3.79 (s, 3H, 3′-OCH3), 4.23 (q, 2H, J = 6.9 Hz, NCH2CH3), 6.91 (m, 2H, 4′-H and 6′-H), 6.98 (s, 1H, 2′-H), 7.38 (t, 1H, J = 8.5 Hz, 5′-H), 7.81 (d, 1H, J = 8.9 Hz, 5-H), 8.26 (s, 1H, CHN), 8.40 (dd, 1H, J = 2.1 Hz and 8.9 Hz, 6-H), 8.48 (d, 1H, J = 2.1 Hz, 8-H).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound2-Ethoxyphenyl 4-methyl-4H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (15p).
(0.18 g, 88%); δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 1.18 (t, 3H, J = 6.7 Hz, 2′-OCH2CH3), 3.70 (s, 3H, NCH3), 4.06 (q, 2H, J = 6.7 Hz, 2′-OCH2CH3), 7.01 (t, 1H, J = 7.9 Hz, 4′-H or 5′-H), 7.19 (d, 1H, J = 7.9 Hz, 3′-H or 6′-H), 7.29 (d, 1H, J = 7.9 Hz, 3′-H or 6′-H), 7.31 (t, 1H, J = 7.9 Hz, 4′-H or 5′-H), 7.71 (d, 1H, J = 8.9 Hz, 5-H), 8.20 (s, 1H, CHN), 8.42 (dd, 1H, J = 1.7 Hz and 8.9 Hz, 6-H), 8.47 (d, 1H, J = 1.7 Hz, 8-H).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound2-Ethoxyphenyl 4-ethyl-4H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (15q).
(0.18 g, 73%); mp 161–164 °C; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 1.18 (t, 3H, J = 7 Hz, 2′-OCH2CH3), 1.38 (t, 3H, J = 7.4 Hz, NCH2CH3), 4.06 (q, 2H, J = 7 Hz, 2′-OCH2CH3), 4.23 (q, 2H, J = 7.4 Hz, NCH2CH3), 7.01 (t, 1H, J = 7.7 Hz, 4′-H or 5′-H), 7.20 (d, 1H, J = 7.7 Hz, 3′-H or 6′-H), 7.30 (m, 2H, 3′-H or 6′-H and 4′-H or 5′-H), 7.81 (d, 1H, J = 8.9 Hz, 5-H), 8.27 (s, 1H, CHN), 8.40 (dd, 1H, J = 2 Hz and 8.9 Hz, 6-H), 8.49 (d, 1H, J = 2 Hz, 8-H).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound2-Benzyloxyphenyl 4-ethyl-4H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (15s).
(0.13 g, 48%); δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 1.36 (t, 3H, J = 7.1 Hz, NCH2CH3), 4.23 (q, 2H, J = 7.1 Hz, NCH2CH3), 5.16 (s, 2H, OCH2), 7.05 (t, 1H, J = 7.5 Hz, H-aromatic), 7.27 (m, 8H, H-aromatic), 7.80 (d, 1H, J = 8.6 Hz, 5-H), 8.27 (s, 1H, CHN), 8.40 (dd, 1H, J = 2 Hz and 8.6 Hz, 6-H), 8.49 (d, 1H, J = 2 Hz, 8-H).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound3-Benzyloxyphenyl 4-ethyl-4H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (15t).
(0.35 g, 81%); δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 1.38 (t, 3H, J = 7.1 Hz, NCH2CH3), 4.22 (q, 2H, J = 7.1 Hz, NCH2CH3), 5.14 (s, 2H, OCH2), 6.94 (d, 1H, J = 8.6 Hz, H-aromatic), 7.01 (d, 1H, J = 8.2 Hz, H-aromatic), 7.08 (s, 1H, H-aromatic), 7.41 (m, 6H, H-aromatic), 7.82 (d, 1H, J = 9 Hz, 5-H), 8.27 (s, 1H, CHN), 8.41 (dd, 1H, J = 1.9 Hz and 9 Hz, 6-H), 8.49 (d, 1H, J = 1.9 Hz, 8-H).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundPhenyl 4-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (16a).
(0.25 g, 71%); mp 171–174 °C; (Found: C, 56.56; H, 4.40; N, 8.94; S, 9.32. Calc. for C15H14N2O4S: C, 56.59; H, 4.43; N, 8.80; S, 10.07%); ν/cm−1 3443, 3208, 3361, 1731, 1609, 1524, 1394, 1244, 1148 cm−1; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 3.08 (s, 3H, NCH3), 4.79 (d, 2H, J = 8.1 Hz, 3-CH2), 7.01 (d, 1H, J = 8.9 Hz, 5-H), 7.27 (d, 2H, J = 7.5 Hz, 2′-H and 6′-H), 7.32 (t, 1H, J = 7.5 Hz, 4′-H), 7.45 (t, 2H, J = 7.5 Hz, 3′-H and 5′-H), 8.07 (dd, 1H, J = 2.1 Hz and 8.9 Hz, 6-H), 8.19 (d, 1H, J = 2.1 Hz, 8-H), 8.29 (t, 1H, J = 8.1 Hz, 2-NH); δC (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 36.22 (1C, NCH3), 62.19 (1C, 3-CH2), 113.58 (1C, 5-CH), 115.16 (1Cq), 121.63 (1Cq), 121.94 (2C, 2′-CH and 6′-CH or 3′-CH and 5′-CH), 125.81 (1C, 4′-CH), 126.17 (1C, 8-CH), 129.50 (2C, 2′-CH and 6′-CH or 3′-CH and 5′-CH), 134.29 (1C, 6-CH), 147.36 (1Cq), 150.63 (1Cq), 163.53 (1Cq).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound2-Methylphenyl 4-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (16c).
(0.11 g, 74%); mp 162–163 °C; (Found: C, 57.98; H, 5.06; N, 8.58 S, 9.91. Calc. for C16H16N2O4S: C, 57.82; H, 4.85; N, 8.43; S, 9.65%); ν/cm−1 3207, 2922, 1693, 1605, 1524, 1335, 1241, 1173 cm−1; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 2.14 (s, 3H, 2′-CH3), 3.07 (s, 3H, NCH3), 4.83 (s, 2H, 3-CH2), 7.01 (d, 1H, J = 8.9 Hz, 5-H), 7.18 (d, 1H, J = 7.6 Hz, 3′-H or 6′-H), 7.21 (t, 1H, J = 7.6 Hz, 4′-H or 5′-H), 7.28 (t, 1H, J = 7.6 Hz, 4′-H or 5′-H), 7.34 (d, 1H, J = 7.6 Hz, 3′-H or 6′-H), 8.09 (dd, 1H, J = 2.2 Hz and 8.9 Hz, 6-H), 8.20 (d, 1H, J = 2.2 Hz, 8-H), 8.28 (bs, 1H, 2-NH); δC (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 15.67 (1C, 2′-CH3), 36.23 (1C, NCH3), 62.20 (1C, 3-CH2), 113.63 (1C, 5-CH), 114.95 (1Cq), 121.69 (1Cq), 122.24 (1C, 3′-CH or 4′-CH or 5′-CH or 6′-CH), 125.98 (1C, 3′-CH or 4′-CH or 5′-CH or 6′-CH), 126.10 (1C, 8-CH), 127.00 (1C, 3′-CH or 4′-CH or 5′-CH or 6′-CH), 129.93 (1Cq), 130.93 (1C, 3′-CH or 4′-CH or 5′-CH or 6′-CH), 134.29 (1C, 6-CH), 147.37 (1Cq), 149.19 (1Cq), 163.22 (1Cq).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound2-Methylphenyl 4-ethyl-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (16d).
(0.11 g, 75%); mp 136–140 °C; (Found: C, 59.00; H, 5.32; N, 8.18; S, 9.57. Calc. for C17H18N2O4S: C, 58.94; H, 5.24; N, 8.09; S, 9.25%); ν/cm−1 3290, 2972, 1726, 1604, 1519, 1337, 1220, 1162 cm−1; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 1.16 (t, 3H, J = 7 Hz, NCH2CH3), 2.18 (s, 3H, 2′-CH3), 3.58 (q, 2H, J = 7 Hz, NCH2CH3), 4.82 (d, 2H, J = 7.7 Hz, 3-CH2), 7.08 (d, 1H, J = 9 Hz, 5-H), 7.19 (d, 1H, J = 7.6 Hz, 3′-H or 6′-H), 7.22 (t, 1H, J = 7.6 Hz, 4′-H or 5′-H), 7.28 (t, 1H, J = 7.65 Hz, 4′-H or 5′-H), 7.36 (d, 1H, J = 7.6 Hz, 3′-H or 6′-H), 8.09 (dd, 1H, J = 2.2 Hz and 9 Hz, 6-H), 8.21 (d, 1H, J = 2.2 Hz, 8-H), 8.26 (d, 1H, J = 7.7 Hz, 2-NH); δC (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 11.58 (1C, NCH2CH3), 15.72 (1C, 2′-CH3), 43.25 (1C, NCH2CH3), 60.56 (1C, 3-CH2), 113.43 (1C, 5-CH), 114.63 (1Cq), 121.52 (1Cq), 122.21 (1C, 3′-CH or 4′-CH or 5′-CH or 6′-CH), 125.97 (1C, 3′-CH or 4′-CH or 5′-CH or 6′-CH), 126.52 (1C, 8-CH), 127.00 (1C, 3′-CH or 4′-CH or 5′-CH or 6′-CH), 129.93 (1Cq), 130.94 (1C, 3′-CH or 4′-CH or 5′-CH or 6′-CH), 134.26 (1C, 6-CH), 146.24 (1Cq), 149.19 (1Cq), 163.18 (1Cq).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound3-Methylphenyl 4-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (16e).
(0.13 g, 85%); mp 152–154 °C; (Found: C, 58.03; H, 4.93; N, 8.45; S, 9.12. Calc. for C16H16N2O4S: C, 57.82; H, 4.85; N, 8.43; S, 9.65%); ν/cm−1 3238, 2923, 1731, 1609, 1525, 1297, 1234, 1142 cm−1; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 2.34 (s, 3H, 3′-CH3), 3.07 (s, 3H, NCH3), 4.83 (s, 2H, 3-CH2), 7.00 (d, 1H, J = 9 Hz, 5-H), 7.05 (d, 1H, J = 7.9 Hz, 4′-H or 6′-H), 7.09 (s, 1H, 2′-H), 7.11 (d, 1H, J = 7.9 Hz, 4′-H or 6′-H), 7.33 (t, 1H, J = 7.9 Hz, 5′-H), 8.06 (dd, 1H, J = 2.2 Hz and 9 Hz, 6-H), 8.18 (d, 1H, J = 2.2 Hz, 8-H), 8.29 (bs, 1H, 2-NH); δC (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 20.90 (1C, 3′-CH3), 36.20 (1C, NCH3), 62.10 (1C, 3-CH2), 113.57 (1C, 5-CH), 115.23 (1Cq), 118.99 (1C, 4′-CH or 6′-CH), 121.65 (1Cq), 122.42 (1C, 2′-CH), 126.13 (1C, 8-CH), 126.51 (1C, 4′-CH or 6′-CH), 129.22 (1C, 5′-CH), 134.31 (1C, 6-CH), 139.23 (1Cq), 147.30 (1Cq), 150.62 (1Cq), 163.61 (1Cq).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound3-Methylphenyl 4-ethyl-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (16f).
(0.21 g, 86%); mp 147–150 °C; (Found: C, 59.07; H, 5.32; N, 8.27; S, 9.21. Calc. for C17H18N2O4S: C, 58.94; H, 5.24; N, 8.09; S, 9.25%); ν/cm−1 3254, 2975, 1716, 1607, 1523, 1334, 1230, 1162 cm−1; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 1.12 (t, 3H, J = 7 Hz, NCH2CH3), 2.36 (s, 3H, 3′-CH3), 3.52 (q, 2H, J = 7 Hz, NCH2CH3), 4.83 (s, 2H, 3-CH2), 7.06 (d, 1H, J = 9 Hz, 5-H), 7.12 (m, 3H, 2′-H, 4′-H and 6′-H), 7.35 (t, 1H, J = 7.8 Hz, 5′-H), 8.03 (dd, 1H, J = 2.1 Hz and 9 Hz, 6-H), 8.19 (d, 1H, J = 2.1 Hz, 8-H), 8.29 (bs, 1H, 2-NH); δC (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 11.57 (1C, NCH2CH3), 20.77 (1C, 3′-CH3), 43.24 (1C, NCH2CH3), 60.57 (1C, 3-CH2), 113.37 (1C, 5-CH), 114.83 (1Cq), 118.91 (1C, 2′-CH or 4′-CH or 6′-CH), 121.54 (1Cq), 121.60 (1C, 2′-CH or 4′-CH or 6′-CH), 122.35 (1C, 5′-CH), 126.51 (1C, 8-CH), 129.17 (1C, 2′-CH or 4′-CH or 6′-CH), 134.22 (1C, 6-CH), 139.22 (1Cq), 146.19 (1Cq), 150.58 (1Cq), 163.53 (1Cq).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound4-Methylphenyl 4-ethyl-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (16g).
(0.16 g, 71%); mp 176–180 °C; (Found: C, 58.84; H, 5.25; N, 8.30; S, 9.36. Calc. for C17H18N2O4S: C, 58.94; H, 5.24; N, 8.09; S, 9.25%); ν/cm−1 3181, 2980, 1733, 1609, 1521, 1296, 1180, 1159 cm−1; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 1.14 (t, 3H, J = 7.1 Hz, NCH2CH3), 2.31 (s, 3H, 4′-CH3), 3.54 (q, 2H, J = 7.1 Hz, NCH2CH3), 4.88 (d, 2H, J = 8.2 Hz, 3-CH2), 7.07 (d, 1H, J = 9.2 Hz, 5-H), 7.13 (d, 2H, J = 8.3 Hz, 2′-H and 6′-H or 3′H and 5′-H), 7.26 (d, 2H, J = 8.3 Hz, 2′-H and 6′-H or 3′-H and 5′-H), 8.02 (dd, 1H, J = 2 Hz and 9.2 Hz, 6-H), 8.19 (d, 1H, J = 2 Hz, 8-H), 8.29 (t, 1H, J = 8.2 Hz, 2-NH); δC (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 11.60 (1C, NCH2CH3), 20.41 (1C, 4′-CH3), 43.27 (1C, NCH2CH3), 60.55 (1C, 3-CH2), 113.33 (1C, 5-CH), 114.86 (1Cq), 121.48 (1Cq), 121.60 (2C, 2′-CH and 6′-CH or 3′-CH and 5′-CH), 126.50 (1C, 8-CH), 129.81 (2C, 2′-CH and 6′-CH or 3′-CH and 5′-CH), 134.30 (1C, 6-CH), 134.95 (1Cq), 146.18 (1Cq), 148.42 (1Cq), 163.67 (1Cq).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound2-Ethylphenyl 4-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (16h).
(0.13 g, 85%); mp 208–210 °C; (Found: C, 58.83; H, 5.28; N, 8.14; S, 8.83. Calc. for C17H18N2O4S: C, 58.94; H, 5.24; N, 8.09; S, 9.25%); ν/cm−1 3289, 2945, 1715, 608, 1525, 1337, 1235, 1171 cm−1; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 1.12 (t, 3H, J = 7.6 Hz, 2′-CH2CH3), 2.51 (q, 2H, J = 7.6 Hz, 2′-CH2CH3), 3.07 (s, 3H, NCH3), 4.83 (s, 2H, 3-CH2), 7.01 (d, 1H, J = 9.2 Hz, 5-H), 7.18 (d, 1H, J = 7.2 Hz, 3′-H or 6′-H), 7.26 (m, 2H, 4′-H and 5′-H), 7.36 (d, 1H, J = 7.2 Hz, 3′-H or 6′-H), 8.09 (dd, 1H, J = 2.3 Hz and 9.2 Hz, 6-H), 8.20 (d, 1H, J = 2.3 Hz, 8-H), 8.28 (bs, 1H, 2-NH); δC (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 14.26 (1C, 2′-CH2CH3), 22.77 (1C, 2′-CH2CH3), 36.19 (1C, NCH3), 62.29 (1C, 3-CH2), 113.73 (1C, 5-CH), 114.94 (1Cq), 121.69 (1Cq), 122.60 (1C, 3′-CH or 6′-CH), 126.07 (1C, 4′-CH or 5′-CH), 126.17 (1C, 8-CH), 126.98 (1C, 4′-CH or 5′-CH), 129.42 (1C, 3′-CH or 6′-CH), 134.29 (1C, 6-CH), 135.56 (1Cq), 147.36 (1Cq), 148.67 (1Cq), 163.51 (1Cq).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound2-Ethylphenyl 4-ethyl-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (16i).
(0.07 g, 44%); mp 115–116 °C; (Found: C, 60.16; H, 5.65; N, 7.82; S, 9.08. Calc. for C18H20N2O4S: C, 59.98; H, 5.59; N, 7.77; S, 8.89%); ν/cm−1 3242, 2973, 1706, 1607, 1530, 1336, 1235, 1168 cm−1; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 1.12 (t, 3H, J = 7.4 Hz, 2′-CH2CH3), 1.16 (t, 3H, J = 7 Hz, NCH2CH3), 2.51 (q, 2H, J = 7.4 Hz, 2′-CH2CH3), 3.54 (q, 2H, J = 7 Hz, NCH2CH3), 4.84 (s, 2H, 3-CH2), 7.07 (d, 1H, J = 9.1 Hz, 5-H), 7.18 (d, 1H, J = 7.5 Hz, 3′-H or 6′-H), 7.26 (m, 2H, 4′-H and 5′-H), 7.35 (d, 1H, J = 7.5 Hz, 3′-H or 6′-H), 8.06 (dd, 1H, J = 2.1 Hz and 9.1 Hz, 6-H), 8.20 (d, 1H, J = 2.1 Hz, 8-H), 8.23 (bs, 1H, 2-NH); δC (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 11.60 (1C, NCH2CH3), 14.29 (1C, 2′-CH2CH3), 22.78 (1C, 2′-CH2CH3), 43.28 (1C, NCH2CH3), 60.56 (1C, 3-CH2), 113.52 (1C, 5-CH), 114.66 (1Cq), 121.53 (1Cq), 122.61 (1C, 3′-CH or 6′-CH), 126.20 (1C, 4′-CH or 5′-CH), 126.50 (1C, 8-CH), 127.03 (1C, 4′-CH or 5′-CH), 129.49 (1C, 3′-CH or 6′-CH), 134.27 (1C, 6-CH), 135.64 (1Cq), 146.28 (1Cq), 148.73 (1Cq), 163.50 (1Cq).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound2-Propylphenyl 4-ethyl-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (16j).
(0.09 g, 56%); mp 86–90 °C; (Found: C, 61.43; H, 6.07; N, 7.27; S, 7.90. Calc. for C19H22N2O4S: C, 61.83; H, 6.23; N, 7.21; S, 8.25%); ν/cm−1 3237, 2960, 1725, 1607, 1521, 1334, 1241, 1168 cm−1; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 0.83 (t, 3H, J = 7.4 Hz, 2′-CH2CH2CH3), 1.17 (t, 3H, J = 6.9 Hz, NCH2CH3), 1.54 (m, 2H, j = 7.4 Hz, 2′-CH2CH2CH3), 2.44 (t, 2H, J = 7.4 Hz, 2′-CH2CH2CH3), 3.56 (q, 2H, J = 6.9 Hz, NCH2CH3), 4.87 (s, 2H, 3-CH2), 7.08 (d, 1H, J = 9.2 Hz, 5-H), 7.19 (d, 1H, J = 7.4 Hz, 3′-H or 6′-H), 7.25 (t, 1H, J = 7.4 Hz, 4′-H or 5′-H), 7.29 (t, 1H, J = 7.4 Hz, 4′-H or 5′-H), 7.36 (d, 1H, J = 7.4 Hz, 3′-H or 6′-H), 8.07 (dd, 1H, J = 1.9 Hz and 9.2 Hz, 6-H), 8.21 (d, 1H, J = 1.9 Hz, 8-H), 8.23 (bs, 1H, 2-NH); δC (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 11.58 (1C, NCH2CH3), 13.68 (1C, 2′-CH2CH2CH3), 22.84 (1C, 2′-CH2CH2CH3), 31.68 (1C, 2′-CH2CH2CH3), 43.26 (1C, NCH2CH3), 60.56 (1C, 3-CH2), 113.48 (1C, 5-CH), 114.62 (1Cq), 121.53 (1Cq), 122.67 (1C, 3′-CH or 6′-CH), 125.96 (1C, 4′-CH or 5′-CH), 126.42 (1C, 8-CH), 127.01 (1C, 4′-CH or 5′-CH), 130.24 (1C, 3′-CH or 6′-CH), 133.99 (1Cq), 134.18 (1C, 6-CH), 146.23 (1Cq), 148.801Cq), 163.44 (1Cq).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound4-Propylphenyl 4-ethyl-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (16k).
(0.1 g, 62%); mp 146–148 °C; (Found: C, 60.66; H, 5.95; N, 7.71; S, 8.50. Calc. for C19H22N2O4S: C, 60.94; H, 5.92; N, 7.48; S, 8.56%); ν/cm−1 3235, 2954, 1705, 1607, 1515, 1337, 1243, 1167 cm−1; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 0.91 (t, 3H, J = 7.5 Hz, 4′-CH2CH2CH3), 1.16 (t, 3H, J = 7.1 Hz, NCH2CH3), 1.61 (m, 2H, J = 7.5 Hz, 4′-CH2CH2CH3), 2.58 (t, 2H, J = 7.5 Hz, 4′-CH2CH2CH3), 3.54 (q, 2H, J = 7.1 Hz, NCH2CH3), 4.84 (d, 2H, J = 8.1 Hz, 3-CH2), 7.06 (d, 1H, J = 9.1 Hz, 5-H), 7.15 (d, 2H, J = 8.4 Hz, 2′-H and 6′-H or 3′-H and 5′-H), 7.26 (d, 2H, J = 8.4 Hz, 2′-H and 6′-H or 3′-H and 5′-H), 8.03 (dd, J = 1.8 Hz and 9.1 Hz, 1H, 6-H), 8.18 (d, J = 1.8 Hz, 1H, 8-H), 8.24 (t, 1H, J = 8.1 Hz, 2-NH); δC (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 11.55 (1C, NCH2CH3), 13.58 (1C, 4′-CH2CH2CH3), 24.12 (1C, 4′-CH2CH2CH3), 36.55 (1C, 4′-CH2CH2CH3), 43.28 (1C, NCH2CH3), 60.54 (1C, 3-CH2), 113.41 (1C, 5-CH), 114.87 (1Cq), 121.47 (1Cq), 121.59 (2C, 2′-CH and 6′-CH or 3′-CH and 5′-CH), 126.54 (1C, 8-CH), 129.21 (2C, 2′-CH and 6′-CH or 3′-CH and 5′-CH), 134.29 (1C, 6-CH), 139.68 (1Cq), 146.15 (1Cq), 148.56 (1Cq), 163.67 (1Cq).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound2-Methoxyphenyl 3,4-dihydro-2H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (16l).
(0.06 g, 54%); mp 122–123 °C; ν/cm−1 3338, 2926, 1714, 1606, 1524, 1326, 1294, 1164 cm−1; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 3.75 (s, 3H, 2′-OCH3), 4.74 (s, 2H, 3-CH2), 6.91 (d, 2H, J = 8.9 Hz, 5-H), 6.99 (t, 1H, J = 7.6 Hz, 4′-H or 5′-H), 7.16 (d, 1H, J = 8.2 Hz, 3′-H or 6′-H), 7.20 (d, 1H, J = 7.6 Hz, 3′-H or 6′-H), 7.27 (t, 1H, J = 8.2 Hz, 4′-H or 5′-H), 7.89 (bs, 1H, 2-NH or 4-NH), 7.93 (dd, 1H, J = 1.8 Hz, and 8.9 Hz, 6-H), 8.11 (bs, 1H, 2-NH or 4-NH), 8.13 (d, 1H, J = 1.8 Hz, 8-H); δC (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 54.28 (1C, 3-CH2), 55.72 (1C, 2′-OCH3), 112.79 (1C, 3′-CH or 6′-CH), 115.04 (1Cq), 115.86 (1C, 5-CH), 120.60 (2C, 4′-CH or 5′-CH), 123.08 (1C, 3′-CH or 6′-CH), 126.46 (1C, 8-CH), 126.97 (1C, 4′-CH or 5′-CH), 133.78 (1C, 6-CH), 139.37 (1Cq), 147.69 (1Cq), 151.03 (1Cq), 157.71 (1Cq), 163.09 (1Cq).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound2-Methoxyphenyl 4-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (16m).
(0.06 g, 67%); mp 147–149 °C; (Found: C, 55.34; H, 4.80; N, 7.82; S, 8.74. Calc. for C16H16N2O5S: C, 55.16; H, 4.63; N, 8.04; S, 9.20%); ν/cm−1 3298, 2923, 1710, 1607, 1526, 1335, 1236, 1154 cm−1; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 3.07 (s, 3H, NCH3), 3.75 (s, 3H, 2′-OCH3), 4.83 (d, 2H, J = 8.2 Hz, 3-CH2), 6.99 (d, 2H, J = 8.9 Hz, 5-H), 7.01 (t, 1H, J = 7.7 Hz, 4′-H or 5′-H), 7.17 (d, 1H, J = 7.7 Hz, 3′-H or 6′-H), 7.21 (d, 1H, J = 7.7 Hz, 3′-H or 6′-H), 7.28 (t, 1H, J = 7.7 Hz, 4′-H or 5′-H), 8.05 (dd, 1H, J = 1.9 Hz and 8.9 Hz, 6-H), 8.16 (d, 1H, J = 1.9 Hz, 8-H), 8.29 (t, 1H, J = 8.2 Hz, 2-NH); δC (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 36.1 (1C, NCH3), 55.70 (1C, 2′-OCH3), 62.20 (1C, 3-CH2), 112.80 (1C, 3′-CH or 6′-CH), 113.70 (1C, 5-CH), 115 (1Cq), 120.60 (2C, 4′-CH or 5′-CH), 121.60 (1Cq), 123 (1C, 3′-CH or 6′-CH), 126.20 (1C, 8-CH), 127 (1C, 4′-CH or 5′-CH), 134.40 (1C, 6-CH), 139.30 (1Cq), 147.30 (1Cq), 151 (1Cq), 163 (1Cq).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound2-Methoxyphenyl 4-ethyl-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (16n).
(0.12 g, 82%); mp 184–186 °C; (Found: C, 56.28; H, 5.03; N, 7.97; S, 8.60. Calc. for C17H18N2O5S: C, 56.34; H, 5.01; N, 7.73; S, 8.85%); ν/cm−1 3225, 2979, 1708, 1608, 1501, 1333, 1241, 1170 cm−1; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 1.16 (t, 3H, J = 6.7 Hz, NCH2CH3), 3.54 (q, 2H, J = 6.7 Hz, NCH2CH3), 3.75 (s, 3H, 2′-OCH3), 4.84 (d, 2H, J = 8 Hz, 3-CH2), 7.00 (t, 1H, J = 7.8 Hz, 4′-H or 5′-H), 7.06 (d, 2H, J = 9.2 Hz, 5-H), 7.16 (d, 1H, J = 7.8 Hz, 3′-H or 6′-H), 7.20 (d, 1H, J = 7.8 Hz, 3′-H or 6′-H), 7.27 (t, 1H, J = 7.8 Hz, 4′-H or 5′-H), 8.02 (dd, 1H, J = 1.8 Hz and 9.2 Hz, 6-H), 8.17 (d, 1H, J = 1.8 Hz, 8-H), 8.23 (t, 1H, J = 8 Hz, 2-NH); δC (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 11.60 (1C, NCH2CH3), 43.20 (1C, NCH2CH3), 55.80 (1C, 2′-OCH3), 60.60 (1C, 3-CH2), 112.79 (1C, 3′-CH or 6′-CH), 113.41 (1C, 5-CH), 114.53 (1Cq), 120.59 (1C, 4′-CH or 5′-CH), 121.49 (1Cq), 123.06 (1C, 3′-CH or 6′-CH), 126.54 (1C, 8-CH), 126.99 (1C, 4′-CH or 5′-CH), 134.34 (1C, 6-CH), 139.33 (1Cq), 146.18 (1Cq), 150.95 (1Cq), 162.96 (1Cq).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound3-Methoxyphenyl 4-ethyl-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (16o).
(0.07 g, 34%); mp 73–98 °C; (Found: C, 56.35; H, 5.01; N, 8.07; S, 8.98. Calc. for C17H18N2O5S: C, 56.34; H, 5.01; N, 7.73; S, 8.85%); ν/cm−1 3229, 2982, 1727, 1606, 1518, 1335, 1229, 1144 cm−1; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 1.16 (t, 3H, J = 7 Hz, NCH2CH3), 3.54 (q, 2H, J = 7 Hz, NCH2CH3), 3.78 (s, 3H, 3′-OCH3), 4.84 (d, 2H, J = 8.2 Hz, 3-CH2), 6.83 (d, 2H, J = 8.5 Hz, 4′-H and 6′-H), 6.88 (s, 1H, 2′-H), 7.07 (d, 2H, J = 9.1 Hz, 5-H), 7.35 (t, 1H, J = 8.5 Hz, 5′-H), 8.03 (dd, 1H, J = 2 Hz and 9.1 Hz, 6-H), 8.19 (d, 1H, J = 2 Hz, 8-H), 8.24 (t, 1H, J = 8.2 Hz, 2-NH); δC (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 11.60 (1C, NCH2CH3), 43.40 (1C, NCH2CH3), 55.50 (1C, 3′-OCH3), 60.60 (1C, 3-CH2), 108 (1C, 2′-CH), 111.80 (1C, 4′-CH or 6′-CH), 113.40 (1C, 5-CH), 114.20 (1C, 4′-CH or 6′-CH), 114.80 (1Cq), 121.50 (1Cq), 126.51 (1C, 8-CH), 129.86 (1C, 5′-CH), 134.29 (1C, 6-CH), 146.18 (1Cq), 151.62 (1Cq), 160.16 (1Cq), 163.39 (1Cq).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound4-Methoxyphenyl 4-ethyl-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (16p).
(0.18 g, 90%); mp 187–189 °C; (Found: C, 56.55; H, 5.04; N, 8.08; S, 9.02. Calc. for C17H18N2O5S: C, 56.34; H, 5.01; N, 7.73; S, 8.85%); ν/cm−1 3225, 2976, 1718, 1606, 1519, 1339, 1236, 1193 cm−1; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 1.17 (t, 3H, J = 7 Hz, NCH2CH3), 3.54 (q, 2H, J = 7 Hz, NCH2CH3), 3.78 (s, 3H, 4′-OCH3), 4.84 (d, 2H, J = 8.3 Hz, 3-CH2), 6.99 (d, 2H, J = 8.5 Hz, 2′-H and 6′-H or 3′-H and 5′-H), 7.06 (d, 2H, J = 9.2 Hz, 5-H), 7.18 (d, 2H, J = 8.5 Hz, 2′-H and 6′-H or 3′-H and 5′-H), 8.03 (dd, 1H, J = 1.9 Hz and 9.2 Hz, 6-H), 8.18 (d, 1H, J = 1.9 Hz, 8-H), 8.23 (t, 1H, J = 8.3 Hz, 2-NH); δC (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 11.63 (1C, NCH2CH3), 43.29 (1C, NCH2CH3), 55.48 (1C, 4′-OCH3), 60.57 (1C, 3-CH2), 113.36 (1C, 5-CH), 114.42 (2C, 2′-H and 6′-H or 3′-H and 5′-H), 114.92 (1Cq), 121.47 (1Cq), 122.75 (2C, 2′-H and 6′-H or 3′-H and 5′-H), 126.50 (1C, 8-CH), 134.27 (1C, 6-CH), 143.95 (1Cq), 146.19 (1Cq), 156.92 (1Cq), 163.86 (1Cq).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound2-Ethoxyphenyl 4-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (16q).
(0.12 g, 80%); mp 165–167 °C; (Found: C, 56.35; H, 5.03; N, 7.78; S, 8.50. Calc. for C17H18N2O5S: C, 56.34; H, 5.01; N, 7.73; S, 8.85%); ν/cm−1 3296, 2930, 1713, 1607, 1525, 1336, 1236, 1189 cm−1; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 1.18 (t, 3H, J = 6.9 Hz, 2′-OCH2CH3), 3.07 (s, 3H, NCH3), 4.03 (q, 2H, J = 6.9 Hz, 2′-OCH2CH3), 4.83 (d, 2H, J = 8.2 Hz, 3-CH2), 6.98 (t, 1H, J = 8.2 Hz, 4′-H or 5′-H), 6.99 (d, 1H, J = 8.8 Hz, 5-H), 7.15 (d, 1H, J = 8.2 Hz, 3′-H or 6′-H), 7.21 (d, 1H, J = 8.2 Hz, 3′-H or 6′-H), 7.25 (t, 1H, J = 8.2 Hz, 4′-H or 5′-H), 8.05 (dd, 1H, J = 2 Hz and 8.8 Hz, 6-H), 8.18 (d, 1H, J = 2 Hz, 8-H), 8.28 (t, 1H, J = 8.2 Hz, 2-NH); δC (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 14.53 (1C, NCH3), 36.25 (1C, 2′-OCH2CH3), 62.25 (1C, 3-CH2), 63.90 (1C, 2′-OCH2CH3), 113.64 (1C, 5-CH), 114.01 (1C, 3′-CH or 6′-CH), 115.04 (1Cq), 120.66 (1C, 4′-CH or 5′-CH), 121.51 (1Cq), 122.98 (1C, 3′-CH or 6′-CH), 126.09 (1C, 8-CH), 126.88 (1C, 4′-CH or 5′-CH), 134.27 (1C, 6-CH), 139.82 (1Cq), 147.21 (1Cq), 150.26 (1Cq), 163.07 (1Cq).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound2-Ethoxyphenyl 4-ethyl-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (16r).
(0.12 g, 80%); mp 211–212 °C; (Found: C, 57.27; H, 5.39; N, 7.41; S, 8.33. Calc. for C18H20N2O5S: C, 57.43; H, 5.36; N, 7.44; S, 8.52%); ν/cm−1 3223, 2979, 1711, 1607, 1544, 1333, 1245, 1167 cm−1; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 1.15 (t, 3H, J = 7 Hz, NCH2CH3), 1.19 (t, 3H, J = 6.8 Hz, 2′-OCH2CH3), 3.54 (q, 2H,J = 7 Hz, NCH2CH3), 4.03 (q, 2H, J = 6.8 Hz, 2′-OCH2CH3), 4.84 (d, 2H, J = 8.3 Hz, 3-CH2), 6.99 (t, 1H, J = 7.8 Hz, 4′-H or 5′-H), 7.06 (d, 1H, J = 9 Hz, 5-H), 7.15 (d, 1H, J = 7.8 Hz, 3′-H or 6′-H), 7.21 (d, 1H, J = 7.8 Hz, 3′-H or 6′-H), 7.25 (t, 1H, J = 7.8 Hz, 4′-H or 5′-H), 8.02 (dd, 1H, J = 1.8 Hz and 9 Hz, 6-H), 8.18 (d, 1H, J = 1.8 Hz, 8-H), 8.22 (t, 1H, J = 8.3 Hz, 2-NH); δC (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 11.63 (1C, 2′-OCH2CH3), 14.58 (1C, NCH2CH3), 43.27 (1C, NCH2CH3), 60.57 (1C, 3-CH2), 64.04 (1C, 2′-OCH2CH3), 113.38 (1C, 5-CH), 114.02 (1C, 3′-CH or 6′-CH), 114.71 (1Cq), 120.61 (1C, 4′-CH or 5′-CH), 121.42 (1Cq), 122.98 (1C, 3′-CH or 6′-CH), 126.45 (1C, 8-CH), 126.86 (1C, 4′-CH or 5′-CH), 134.20 (1C, 6-CH), 139.82 (1Cq), 146.12 (1Cq), 150.23 (1Cq), 163.01 (1Cq).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound2-Benzyloxyphenyl 4-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (16s).
(0.02 g, 30%); mp 162–163 °C; (Found: C, 61.95; H, 5.00; N, 6.28; S, 7.11. Calc. for C22H20N2O5S: C, 62.25; H, 4.75; N, 6.60; S, 7.55%); ν/cm−1 3260, 2916, 1720, 1608, 1525, 1332, 1298, 1166 cm−1; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 3.07 (s, 3H, NCH3), 4.83 (d, 2H, J = 8.2 Hz, 3-CH2), 5.14 (s, 2H, OCH2), 6.98 (d, 2H, J = 9.7 Hz, 5-H), 7.02 (t, 1H, J = 7.2 Hz, 4′-H or 5′-H), 7.28 (m, 8H, H-aromatic), 8.06 (dd, 1H, J = 1.8 Hz and 9.7 Hz, 6-H), 8.20 (d, 1H, J = 1.8 Hz, 8-H), 8.28 (t, 1H, J = 8.2 Hz, 2-NH); δC (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 36.21 (1C, NCH3), 62.20 (1C, 3-CH2), 69.70 (1C, OCH2), 113.63 (1C, 5-CH), 114.49 (1C aromatic), 115.02 (1Cq), 121.04 (1C, 4′-CH or 5′-CH), 121.62 (1Cq), 123.11 (1C aromatic), 126.20 (1C, 8-CH), 126.89 (2C aromatic), 127.64 (1C aromatic), 128.28 (2C aromatic), 134.30 (1C, 6-CH), 136.80 (1Cq), 139.89 (1Cq), 147.24 (1Cq), 149.90 (1Cq), 163.10 (1Cq).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound2-Benzyloxyphenyl 4-ethyl-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (16t).
(0.09 g, 74%); mp 76–77 °C; (Found: C, 62.60; H, 5.20; N, 6.28; S, 7.15. Calc. for C23H22N2O5S: C, 63.00; H, 5.06; N, 6.39; S, 7.31%); ν/cm−1 3228, 2933, 1727, 1607, 1521, 1333, 1298, 1166 cm−1; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 1.15 (t, 3H, J = 6.9 Hz, NCH2CH3), 3.53 (q, 2H, J = 6.9 Hz, NCH2CH3), 4.84 (s, 2H, 3-CH2), 5.14 (s, 2H, OCH2), 7.01 (t, 1H, J = 7.5 Hz, 4′-H or 5′-H), 7.05 (d, 2H, J = 9.2 Hz, 5-H), 7.28 (m, 8H, H-aromatic), 8.03 (dd, 1H, J = 1.8 Hz and 9.2 Hz, 6-H), 8.20 (d, 1H, J = 1.8 Hz, 8-H), 8.25 (bs, 1H, 2-NH); δC (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 11.60 (1C, NCH2CH3), 43.26 (1C, NCH2CH3), 60.62 (1C, 3-CH2), 69.66 (1C, OCH2), 113.37 (1C, 5-CH), 114.46 (1C aromatic), 114.62 (1Cq), 121.11 (1C, 4′-CH or 5′-CH), 121.56 (1Cq), 123.10 (1C aromatic), 126.54 (1C, 8-CH), 126.91 (2C aromatic), 127.68 (1C aromatic), 128.29 (2C aromatic), 134.25 (1C, 6-CH), 136.80 (1Cq), 139.92 (1Cq), 146.17 (1Cq), 149.90 (1Cq), 163.11 (1Cq).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound3-Benzyloxyphenyl 4-ethyl-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (16u).
(0.17 g, 48%); mp 159–163 °C; ν/cm−1 3225, 2929, 1718, 1610, 1526, 1316, 1238, 1138 cm−1; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 1.15 (t, 3H, J = 7.1 Hz, NCH2CH3), 3.52 (q, 2H, J = 7.1 Hz, NCH2CH3), 4.84 (s, 2H, 3-CH2), 5.12 (s, 2H, OCH2), 6.84 (d, 1H, J = 8.3 Hz, 4′-H or 6′-H), 6.95 (d, 1H, J = 8.3 Hz, 4′-H or 6′-H), 6.98 (s, 1H, 2′-H), 7.04 (d, 2H, j = 9.2 Hz, 5-H), 7.39 (m, 6H, 5′-H and H-aromatic), 8.02 (dd, 1H, J = 1.7 Hz and 9.2 Hz, 6-H), 8.17 (d, 1H, J = 1.7 Hz, 8-H), 8.28 (bs, 1H, 2-NH); δC (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 11.54 (1C, NCH2CH3), 43.21 (1C, NCH2CH3), 60.73 (1C, 3-CH2), 69.48 (1C, OCH2), 108.77 (1C, 2′-CH), 112.46 (1C, 4′-CH or 6′-CH), 113.31 (1C, 5-CH), 114.39 (1C, 4′-CH or 6′-CH), 114.66 (1Cq), 121.63 (1Cq), 126.49 (1C, 8-CH), 127.76 (2C aromatic), 127.90 (1C aromatic), 128.44 (2C aromatic), 129.90 (1C aromatic), 134.22 (1C, 6-CH), 136.79 (1Cq), 146.24 (1Cq), 151.61 (1Cq), 159.23 (1Cq), 163.43 (1Cq).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundBenzyl 4-ethyl-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (19a).
(0.13 g, 92%); mp 182–184 °C; (Found: C, 58.73; H, 5.35; N, 8.09; S, 9.32. calc. for C17H18N2O4S: C, 58.94; H, 5.24; N, 8.09; S, 9.25%); ν/cm−1 3228, 2978, 1699, 1609, 1514, 1333, 1241, 1168 cm−1; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 1.13 (t, 3H, 7.1 Hz, NCH2CH3), 3.50 (q, 2H, J = 7.1 Hz, NCH2CH3), 4.80 (d, 2H, J = 8.4 Hz, 3-CH2), 5.31 (s, 2H, OCH2), 7.00 (d, 1H, J = 9 Hz, 5-H), 7.35 (t, 1H, J = 7.2 Hz, 4′-H), 7.41 (t, 2H, J = 7.2 Hz, 3′-H and 5′-H), 7.45 (d, 2H, J = 7.2 Hz, 2′-H and 6′-H), 7.93 (dd, 1H, J = 2 Hz and 9 Hz, 6-H), 8.01 (d, 1H, J = 2 Hz, 8-H), 8.17 (t, 1H, J = 8.4 Hz, 2-NH); δC (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 11.56 (1C, NCH2CH3), 43.19 (1C, NCH2CH3), 60.51 (1C, 3-CH2), 65.88 (1C, OCH2), 113.24 (1C, 5-CH), 115.59 (1Cq), 121.35 (1Cq), 125.86 (1C, 8-CH), 127.94 (2C aromatic), 128.08 (1C aromatic), 128.50 (2C aromatic), 133.82 (1C, 6-CH), 136.28 (1Cq), 145.81 (1Cq), 164.53 (1Cq).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundPhenethyl 4-ethyl-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (19b).
(0.11 g, 56%); mp 95–97 °C; (Found: C, 59.98; H, 5.63; N, 7.85; S, 9.38. Calc. for C18H20N2O4S: C, 59.98; H, 5.59; N, 7.77; S, 8.89%); ν/cm−1 3296, 2975, 1708, 1604, 1515, 1340, 1237, 1167 cm−1; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 1.12 (t, 3H, J = 7 Hz, NCH2CH3), 3.02 (t, 2H, J = 6.7 Hz, OCH2CH2), 3.49 (q, 2H, J = 7 Hz, NCH2CH3), 4.43 (t, 2H, J = 6.7 Hz, OCH2CH2), 4.79 (s, 2H, 3-CH2), 6.98 (d, 1H, J = 9.2 Hz, 5-H), 7.26 (m, 5H, H-aromatic), 7.84 (dd, 1H, J = 2 Hz and 9.2 Hz, 6-H), 8.02 (d, 1H, J = 2 Hz, 8-H), 8.15 (bs, 1H, 2-NH); δC (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 11.59 (1C, NCH2CH3), 34.49 (1C, OCH2CH2), 43.20 (1C, NCH2CH3), 60.55 (1C, 3-CH2), 64.93 (1C, OCH2CH2), 113.21 (1C, 5-CH), 115.81 (1Cq), 121.37 (1Cq), 125.82 (1C, 8-CH), 126.37 (1C aromatic), 128.36 (2C aromatic), 128.91 (2C aromatic), 133.64 (1C, 6-CH), 138.10 (1Cq), 145.71 (1Cq), 164.56 (1Cq).
N-Phenyl-4-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-carboxamide 1,1-dioxide (22a).
(0.08 g, 39%); mp 246–249 °C; (Found: C, 56.88; H, 4.74; N, 13.26; S, 10.29. Calc. for C15H15N3O3S: C, 56.77; H, 4.76; N, 13.24; S, 10.10%); ν/cm−1 3339, 3070, 1647, 1610, 1520, 1438, 1308, 1154 cm−1; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 3.03 (s, 3H, NCH3), 4.86 (d, 2H, J = 8.3 Hz, 3-CH2), 6.95 (d, 1H, J = 9.1 Hz, 5-H), 7.08 (t, 1H, J = 7.5 Hz, 4′-H), 7.33 (t, 2H, J = 7.5 Hz, 3′-H and 5′-H), 7.78 (d, 2H, J = 7.5 Hz, 2′-H and 6′-H), 8.08 (dd, 1H, J = 1.8 Hz and 9.1 Hz, 6-H), 8.19 (t, 1H, J = 8.3 Hz 2-NH), 8.29 (d, 1H, J = 1.8 Hz, 8-H); δC (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 36.18 (1C, NCH3), 62.17 (1C, 3-CH2), 113.19 (1C, 5-CH), 120.42 (2C, 2′-CH and 6′-CH), 121.27 (1Cq), 121.51 (1Cq), 125.86 (1C, 4′-CH), 124.04 (1C, 8-CH), 128.56 (2C, 3′-CH and 5′-CH), 132.78 (1C, 6-CH), 139.24 (1Cq), 145.93 (1Cq), 163.66 (1Cq).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundN-Methyl-N-phenyl-4-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-carboxamide 1,1-dioxide (22b).
(0.04 g, 56%); mp 143–144 °C; (Found: C, 57.72; H, 5.15; N, 12.80; S, 9.43. Calc. for C16H17N3O3S: C, 57.99; H, 5.17; N, 12.68; S, 9.67%); ν/cm−1 3104, 2910, 1611, 1523, 1377, 1324, 1158 cm−1; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 2.88 (s, 3H, NCH3), 3.31 (s, 3H, 7-CONCH3), 4.64 (d, 2H, J = 8.1 Hz, 3-CH2), 6.60 (d, 1H, J = 9.2 Hz, 5-H), 7.19 (m, 4H, 2′-H, 4′-H, 6′-H and 6-H), 7.32 (t, 2H, J = 7.5 Hz, 3′-H and 5′-H), 7.48 (d, 1H, J = 2 Hz, 8-H), 8.07 (t, 1H, J = 8.1 Hz, 2-NH); δC (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 35.98 (1C, NCH3), 38.36 (1C, 7-CONCH3), 62.04 (1C, 3-CH2), 112.30 (1C, 5-CH), 121.23 (1Cq), 122.55 (1Cq), 125.46 (1C, 8-CH), 126.52 (1C, 4′-CH), 126.85 (2C, 2′-CH and 6′-CH), 129.36 (2C, 3′-CH and 5′-CH), 133.66 (1C, 6-CH), 144.44 (1Cq), 144.97 (1Cq), 167.72 (1Cq).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundN-Ethyl-N-phenyl-4-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-carboxamide 1,1-dioxide (22c).
(0.03 g, 69%); mp 227–229 °C; (Found: C, 58.21; H, 5.49; N, 11.98; S, 9.35. Calc. for C17H19N3O3S: C, 59.11; H, 5.54; N, 12.16; S, 9.28%); ν/cm−1 3107, 2988, 1610, 1586, 1402, 1325, 1166 cm−1; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 1.08 (t, 3H, J = 7.1 Hz, 7-CONCH2CH3), 2.88 (s, 3H, NCH3), 3.84 (q, 2H, J = 7.1 Hz, 7-CONCH2CH3), 4.64 (s, 2H, 3-CH2), 6.57 (d, 1H, J = 9.2 Hz, 5-H), 7.16 (d, 2H, J = 8 Hz, 2′-H and 6′-H), 7.19 (dd, 1H, J = 2 Hz and 9.2 Hz, 6-H), 7.21 (t, 1H, J = 8 Hz, 4′-H), 7.33 (t, 2H, J = 8 Hz, 3′-H and 5′-H), 7.48 (d, 1H, J = 2 Hz, 8-H), 8.04 (bs, 1H, 2-NH); δC (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 12.52 (1C, 7-CONCH2CH3), 35.95 (1C, NCH3), 45.01 (1C, 7-CONCH2CH3), 62.03 (1C, 3-CH2), 112.28 (1C, 5-CH), 121.19 (1Cq), 122.82 (1Cq), 125.47 (1C, 8-CH), 126.65 (1C, 4′-CH), 127.75 (2C, 2′-CH and 6′-CH), 129.37 (2C, 3′-CH and 5′-CH), 133.57 (1C, 6-CH), 143.15 (1Cq), 144.48 (1Cq), 167.31 (1Cq).
N-Phenyl-4-ethyl-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-carboxamide 1,1-dioxide (22d).
(0.1 g, 69%); mp 231–233 °C; (Found: C, 58.05; H, 5.09; N, 12.72; S, 9.64. Calc. for C16H17N3O3S: C, 57.99; H, 5.17; N, 12.68; S, 9.67%); ν/cm−1 3388, 3173, 1649, 1608, 1509, 1331, 1258, 1166 cm−1; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 1.12 (t, 3H, J = 6.9 Hz, 7-CONCH2CH3), 3.56 (q, 2H, J = 6.9 Hz, 7-CONCH2CH3), 4.88 (s, 2H, 3-CH2), 7.01 (d, 1H, J = 8.8 Hz, 5-H), 7.07 (t, 1H, J = 7.7 Hz, 4′-H), 7.33 (t, 2H, J = 7.7 Hz, 3′-H and 5′-H), 7.75 (d, 2H, J = 7.7 Hz, 2′-H and 6′-H), 8.03 (dd, 1H, J = 2.1 Hz and 8.8 Hz, 6-H), 8.1 (bs, 1H, 2-NH), 8.29 (d, 1H, J = 2.1 Hz, 8-H); δC (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 11.66 (1C, NCH2CH3), 43.15 (1C, NCH2CH3), 60.48 (1C, 3-CH2), 112.91 (1C, 5-CH), 120.35 (2C, 2′-CH and 6′-CH), 120.84 (1Cq), 121.33 (1Cq), 123.40 (1C, 4′-CH), 124.38 (1C, 8-CH), 128.53 (2C, 3′-CH and 5′-CH), 132.74 (1C, 6-CH), 139.27 (1Cq), 144.76 (1Cq), 163.68 (1Cq).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundBenzyl 4-ethyl-4H-1,2,4-benzothiadiazine-7-carboxylate 1,1-dioxide (18a).
(0.14 g, 64%); mp 109–111 °C; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 1.36 (t, 3H, J = 7.1 Hz, NCH2CH3), 4.24 (q, 2H, J = 7.1 Hz, NCH2CH3), 5.45 (s, 2H, OCH2), 7.49 (m, 5H, H-aromatic), 7.72 (d, 1H, J = 8.9 Hz, 5-H), 8.04 (s, 1H, CHN), 8.26 (dd, 1H, 2.1 Hz, and 8.9 Hz, 6-H), 8.33 (d, 1H, J = 2.1 Hz, 8-H).
Phenethyl 4-ethyl-4H-1,2,4-benzothiadiazine 1,1-dioxide-7-carboxylate (18b).
(0.19 g, 70%); δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 1.36 (t, 3H, J = 6.8 Hz, NCH2CH3), 3.07 (t, 2H, J = 6.2 Hz, OCH2CH2), 4.17 (q, 2H, J = 6.8 Hz, NCH2CH3), 4.51 (t, 2H, J = 6.2 Hz, OCH2CH2), 7.29 (m, 5H, H-aromatic), 7.73 (d, 1H, J = 9 Hz, 5-H), 8.20 (dd, 1H, J = 1.8 Hz and 9 Hz, 6-H), 8.21 (s, 1H, CHN), 8.29 (d, 1H, J = 1.8 Hz, 8-H).
N-Phenyl-4-methyl-4H-1,2,4-benzothiadiazine-7-carboxamide 1,1-dioxide (21a).
(0.15 g, 47%); δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 3.72 (s, 3H, NCH3), 7.13 (t, 1H, J = 7.5 Hz, 4′-H), 7.36 (t, 2H, J = 7.5 Hz, 3′-H and 5′-H), 7.65 (d, 1H, J = 8.7 Hz, 5-H), 7.78 (d, 2H, J = 7.5 Hz, 2′-H and 6′-H), 8.15 (s, 1H, CHN), 8.36 (d, 1H, J = 8.7 Hz, 6-H), 8.58 (s, 1H, 8-H), 10.66 (s, 1H, 7-CONH).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundN-Methyl-N-phenyl-4-methyl-4H-1,2,4-benzothiadiazine-7-carboxamide 1,1-dioxide (21b).
(0.08 g, 50%); mp 262–264 °C; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 3.39 (s, 3H, NCH3), 3.54 (s, 3H, 7-CONCH3), 7.20 (t, 1H, J = 7.2 Hz, 4′-H), 7.25 (d, 2H, J = 7.2 Hz, 2′-H and 6′-H), 7.30 (t, 2H, J = 7.2 Hz, 3′-H and 5′-H), 7.33 (d, 1H, 9 Hz, 5-H), 7.60 (dd, 1H, J = 1.2 Hz and 9 Hz, 6-H), 7.73 (d, 1H, J = 1.2 Hz, 8-H), 8.02 (s, 1H, CHN).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundN-Ethyl-N-phenyl-4-methyl-4H-1,2,4-benzothiadiazine-7-carboxamide 1,1-dioxide (21c).
(0.06 g, 20%); mp 263–266 °C; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 1.12 (t, 3H, J = 7.1 Hz, 7-CONCH2CH3), 3.54 (s, 3H, NCH3), 3.88 (q, 2H, J = 7.1 Hz, 7-CONCH2CH3), 7.22 (m, 3H, H-aromatic), 7.31 (m, 3H, 5-H and H-aromatic), 7.59 (d, 1H, J = 8.7 Hz, 6-H), 8.02 (s, 1H, 8-H).
N-Phenyl-4-ethyl-4H-1,2,4-benzothiadiazine-7-carboxamide 1,1-dioxide (21d).
(0.15 g, 45%); δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 1.35 (t, 3H, J = 6.7 Hz, NCH2CH3), 4.21 (q, 2H, j = 6.7 Hz, CONCH2CH3), 7.12 (t, 1H, 7.4 Hz, 4′-H), 7.37 (t, 2H, J = 7.4 Hz, 3′-H and 5′-H), 7.75 (d, 1H, J = 9 Hz, 5-H), 7.78 (d, 2H, J = 7.4 Hz, 2′-H and 6′-H), 8.21 (s, 1H, CHN), 8.35 (d, 1H, J = 9 Hz, 6-H), 8.59 (s, 1H, 8-H), 10.56 (s, 1H, 7-CONH).
General procedure for the synthesis of compounds 20.
A mixture of 4H-1,2,4-benzothiadiazine-7-carboxylic acid 1,1-dioxide (13, 4,4 mmol), NaHCO3 (22 mmol), BOP (4,4 mmol), the appropriate benzylamine (4,4 mmol) and DMF (10 mL) was stirred at room temperature over night. The suspension was supplemented with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundwater (20 mL) and extracted 3-fold (3 × 20 mL) with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate. The combined layers were dried over MgSO4 and filtered. The filtrate was concentred to dryness under reduced pressure and the residue of the title compound was recrystallized in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundmethanol–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundwater 1/3.
N-Phenyl-4H-1,2,4-benzothiadiazine-7-carboxamide 1,1-dioxide (20a).
(0.94 g, 35%); δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 7.13 (t, 1H, J = 7.6 Hz, 4′-H), 7.37 (t, 2H, J = 7.6 Hz, 3′-H and 5′-H), 7.46 (d, 1H, J = 8.7 Hz, 5-H), 7.78 (d, 2H, J = 7.6 Hz, 2′-H and 6′-H), 8.07 (s, 1H, CHN), 8.26 (dd, 1H, J = 1.9 Hz and 8.7 Hz, 6-H), 8.54 (d, 1H, J = 1.9 Hz, 8-H), 10.49 (s, 1H, 7-CONH), 12.54 (bs, 1H, 4-NH).
N-Methyl-N-phenyl-4H-1,2,4-benzothiadiazine-7-carboxamide 1,1-dioxide (20b).
(0.19 g, 13%); mp 230–236 °C; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 3.39 (s, 3H, 7-CONCH3), 7.14 (d, 1H, J = 8.5 Hz, 5-H), 7.19 (t, 1H, J = 7.6 Hz, 4′-H), 7.23 (d, 2H, J = 7.6 Hz, 2′-H and 6′-H), 7.3 (t, 2H, J = 7.6 Hz, 3′-H and 5′-H), 7.51 (d, 1H, J = 8.5 Hz, 6-H), 7.65 (s, 1H, CHN), 7.94 (s, 1H, 8-H), 12.36 (bs, 1H, 4-NH).
N-Ethyl-N-phenyl-4H-1,2,4-benzothiadiazine-7-carboxamide 1,1-dioxide (20c).
(0.5 g, 34%); mp 227–229 °C; δH (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO-d6, Me4Si) 1.11 (t, 3H, J = 7 Hz, 7-CONCH2CH3), 3.87 (q, 2H, J = 7 Hz, 7-CONCH2CH3), 7.11 (d, 1H, J = 8.6 Hz, 5-H), 7.21 (m, 3H, 2′-H, 4′-H and 6′-H), 7.30 (t, 2H, J = 7.7 Hz, 3′-H and 5′-H), 7.49 (d, 1H, J = 8.6 Hz, 6-H), 7.65 (s, 1H, 8-H), 7.93 (s, 1H, CHN), 12.31 (bs, 1H, 4-NH).
6.2. Biological assay
Effects of the compounds on the AMPA-evoked membrane depolarisation of brain cells.
Rat brain cell cultures were prepared from embryonic rat (E17) and were added to poly-D-lysine coated 384-well culture plates for 18 days at 37 °C/5% CO2 (density 20 000 cells/well). On the day of the experiment, ground media was removed from the cells and was replaced by 20 μL/well of membrane potential dye loading solution (Molecular devices), reconstituted according to the manufacturer's instructions. Plates were incubated for 1 h at room temperature and then directly transferred to the FDSS (fluorescence imaging based plate reader). Baseline fluorescence was monitored for 10 s followed by the addition of AMPA (3–100 μM) for 3 min and then the compound in presence of AMPA for 3 min. Subsequent monitoring of fluorescence changes was performed during these two periods of 3 min. Responses were averaged over the last 15 s of each 3 min-recording period. AMPA concentration-response curves were calculated in absence or presence of different concentrations of the compound. Results were expressed as the area under the curve of AMPA-mediated concentration-response effect in absence or presence of compound. The activities of our compounds were expressed as the EC2X value which is the concentration of drug evoking a 2-fold increase of all AMPA-mediated responses (between 3–100 μM AMPA). EC50 corresponds to the concentration of compound that evoked 50% of the maximal effect of the compound. Max increase is an arithmetic average which expresses the maximum effect to the drug on the AMPA-evoked current (expressed in the % of the current evoked by AMPA, taken 100%). This value is obtained at the last dose tested.
6.3. Stability assay
The LC separations were carried out on an Agilent 1100 series LC system from Agilent (Waldbronn, Germany) equipped with a quaternary pump, a column thermostat, on autosampler, and a diode array detector. The analyte separations were performed on an Alltech Hypersil BDS C18 column (150 × 4.6 mm, i.d.; particule size = 3 μm) from Alltech (Breda, The Netherlands) using mobile phase A : 90% COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundwater and mobile phase B : 10% ACN with a flow rate of 0.8 ml min−1 and the following linear gradient : 0 min, 10% ACN; 24 min, 60% ACN; 27 min, 80% ACN and 32 min, 10% ACN. The column temperature was set to 30 °C.
Stability solution : 99 ml of PBS were added with 1 ml of a solution 1 mM of tested compound in ACN. This solution is incubated at 25 °C and analysed by the method described above at different times from 0 h to 24 h.
Acknowledgements
This study was supported in part by a grant from Servier and the National Fund for Scientific Research (F.N.R.S., Belgium) from which Pascal de Tullio is a Senior Research associate. The technical assistance of Yvette Abrassart and Stéphane Counerotte is gratefully acknowledged.
References
- M. Crair, Curr. Opin. Neurobiol., 1999, 9, 88–93 CrossRef CAS.
- S. Maren and M. Baudry, Neurobiology of Learning and Memory, 1995, 63, 1–18 Search PubMed.
- E. Graindorge, P. Francotte, S. Boverie, P. de Tullio and B. Pirotte, Curr. Med. Chem., 2004, 4, 95–103 CAS.
- M. Beal, Curr. Opin. Neurobiol., 1992, 2, 657–662 CrossRef CAS.
- G. Lynch, Curr. Opin. Pharmacol., 2004, 4, 4–11 CrossRef CAS.
- M. D. Black, A Review of Preclinical Data, Psychopharmacology., 2005, 179, 154–163 Search PubMed.
- P. Francotte, P. de Tullio, P. Fraikin, S. Counerotte, E. Goffin and B. Pirotte, Recent Pat. CNS Drug Discovery, 2006, 1, 239–246 Search PubMed.
- K. Partin, D. Patneau, C. Winters, M. Mayer and A. Buonanno, Neuron, 1993, 11, 1069–1082 CrossRef CAS.
- M. Bertolino, M. Baraldi, C. Parenti, D. Braghiroli, M. Di Bella, S. Vicini and E. Costa, Receptor Channel., 1993, 1, 267–278 Search PubMed.
- C. P. Ptak, A. H. Ahmed and R. E. Oswald, Biochemistry, 2009, 48, 8594–8602 CrossRef CAS.
- H. Hald, P. K. Ahring, D. B. Timmermann, T. Liljefors, M. Gajhede and J. S. Kastrup, J. Mol. Biol., 2009, 391, 906–917 CrossRef CAS.
- B. Pirotte, T. Podona, O. Diouf, P. de Tullio, P. Lebrun, L. Dupont, F. Somers, J. Delarge, P. Morain, P. Lestage, J. Lepagnol and M. Spedding, J. Med. Chem., 1998, 41, 2946–2959 CrossRef CAS.
- P. Francotte, P. de Tullio, T. Podona, O. Diouf, P. Fraikin, P. Lestage, L. Danober, J. Y. Thomas, D. H. Caignard and B. Pirotte, Bioorg. Med. Chem., 2008, 16, 9948–9956 CrossRef CAS.
- P. Lestage, L. Danober, B. Lockhart, A. Roger, C. Lebrun, J. L. Robin, P. Desos and A. Cordi, Res. Pract. Alzheimer's Dis., 2002, 6, 253–259 Search PubMed.
- P. Francotte, P. de Tullio, E. Goffin, G. Dintilhac, E. Graindorge, P. Fraikin, P. Lestage, L. Danober, J. Y. Thomas, D. H. Caignard and B. Pirotte, J. Med. Chem., 2007, 50, 3153–3157 CrossRef CAS.
- P. Francotte, E. Goffin, P. Frainkin, P. Lestage, J. C. Van Heugen, L. Danober, J. Y. Thomas, P. Chiap, D. H. Caignard, B. Pirotte and P. de Tullio, J. Med. Chem., 2010, 53, 1700–1711 CrossRef CAS.
- J. T. Chou and P. C. Jurs, Journal of Chemical Information and Modeling, 1979, 19, 172–178 Search PubMed.
- Y. Zhao, J. Jona, D. T. Chow, H. Rong, D. Semin, X. Xia, R. Zanon, C. Spancake and Ed. Maliski, Rapid Commun. Mass Spectrom., 2002, 16, 1548–1555 CrossRef CAS.
- C. F. Bigge and S. S. Nikam, Expert Opin. Ther. Pat., 1997, 7, 1099–1114 Search PubMed.
- J. J. Buccafusco, T. Weiser, K. Winter, K. Klinder and A. V. Terry Jr, Neuropharmacology, 2004, 46, 10–22 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2011 |
Click here to see how this site uses Cookies. View our privacy policy here. 

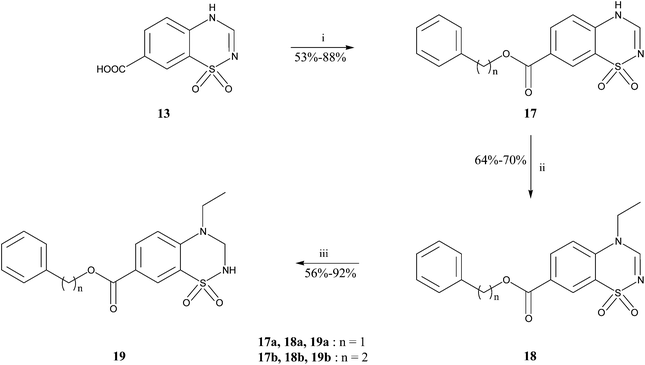
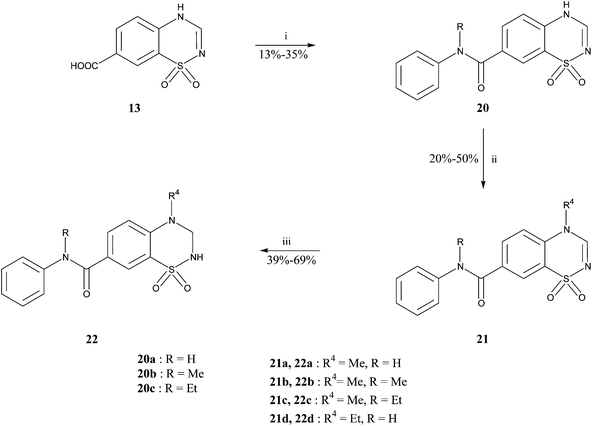
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 12.19 (bs, 1H, 4-NH).
N), 12.19 (bs, 1H, 4-NH).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 8.18 (dd, 1H, J = 1.8 Hz and 8.6 Hz, 6-H) 8.25 (d, 1H, J = 1.8 Hz, 8-H), 12.61 (bs, 1H, 4-NH), 13.41 (bs, 1H, COOH).
N), 8.18 (dd, 1H, J = 1.8 Hz and 8.6 Hz, 6-H) 8.25 (d, 1H, J = 1.8 Hz, 8-H), 12.61 (bs, 1H, 4-NH), 13.41 (bs, 1H, COOH).



