RCAI-39, 41, 53, 100, 127 and 128, the analogues of KRN7000, activate mouse natural killer T cells to produce Th2-biased cytokines by their administration as liposomal particles†‡
Takuya
Tashiro
a,
Yasuyuki
Ishii
b,
Tomokuni
Shigeura
c,
Ryusuke
Nakagawa
cd,
Hiroshi
Watarai
c,
Masaru
Taniguchi
c and
Kenji
Mori
*a
aGlycosphingolipid Synthesis Group, Laboratory for Immune Regulation, Research Center for Allergy and Immunology, RIKEN, 2-1 Hirosawa, Wako-shi, Saitama 351-0198, Japan. E-mail: kjk-mori@arion.ocn.ne.jp; Fax: +81 48 467 9381; Tel: +81 48 462 1339
bLaboratory for Vaccine Design, Research Center for Allergy and Immunology, RIKEN Yokohama Institute, 1-7-22 Suehiro-cho, Tsurumi-ku, Yokohama-shi, Kanagawa 230-0045, Japan
cLaboratory for Immune Regulation, Research Center for Allergy and Immunology, RIKEN Yokohama Institute, 1-7-22 Suehiro-cho, Tsurumi-ku, Yokohama-shi, Kanagawa 230-0045, Japan
dDepartment of Immunology, Faculty of Medicine, University of Yamanashi, Yamanashi, 409-3898, Japan
First published on 12th May 2011
Abstract
α-Galactosphingolipid analogues of KRN7000 with a sulfonamide (RCAI-39), a carbamate (RCAI-41), an α,α-difluorocarboxamide (RCAI-100) or an N-methylcarboxamide linkage (RCAI-127) instead of a carboxamide bond of KRN7000 were synthesized. Their bioactivities for mouse natural killer T cells were examined. Bioactivities of truncated analogues, OCH and RCAI-53, and β-galactosphingolipid (RCAI-128) were also examined. All of these glycosphingolipids induced Th2-biased cytokine production by their administration as liposomal particles. Among them, liposomes containing RCAI-127 induced the most potent Th2-biased response.
Introduction
KRN7000 (α-GalCer, A, Fig. 1) is an anticancer drug candidate developed in 1995 by researchers at Kirin Brewery Co.2 It was obtained through the structure–activity relationship studies on agelasphins, anticancer glycosphingolipids isolated from an Okinawan marine sponge, Agelas mauritianus.3,4 These are structurally characteristic α-galactosylceramides and act as immunostimulant agents to induce anticancer activity in vivo in mice and humans. In contrast, the β-anomer of A shows almost no immunostimulatory activities.5 In 1997, it was revealed that these glycosphingolipids could be a ligand to make a complex with CD1d protein, a glycolipid presentation protein of the antigen presenting cells (APCs).5,6 In 2005, X-ray crystallographic analyses revealed the structures of the complexes of mouse and human CD1d–A.7,8 This CD1d–glycosphingolipid complex is recognized by the invariant (mouse: Vα14, human: Vα24) T cell receptor (TCR) of natural killer T (iNKT) cells and activates them. The X-ray information of human and mouse TCR–A–CD1d complexes was reported in 2007 and 2009, respectively.9,10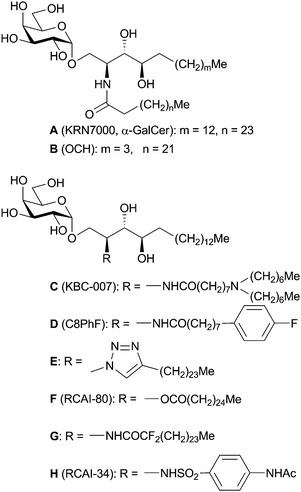 | ||
| Fig. 1 Structures of KRN7000 (A) and its typical analogues developed by modification of the lipid chains of A. | ||
iNKT cells activated with A release both helper T1-type (Th1) and Th2 cytokines at the same time in large quantities by a single injection. Th1 cytokines such as interferon (IFN)-γ mediate protective immune functions like tumor rejection, whereas Th2 cytokines such as interleukin (IL)-4 mediate regulatory immune functions to ameliorate autoimmune diseases. Additionally, Th1 and Th2 cytokines can antagonize each other's biological actions.11 Because of this antagonism, use of A for clinical therapy has been limited. To circumvent this problem, many research groups are trying to develop new glycolipids, which induce iNKT cells to produce preferentially either Th1 or Th2 cytokines. A number of analogues of A have been synthesized and assayed around the world.12 However, neither the definitive partial structures of A nor the critical factors which induce biased cytokine production by iNKT cells have been clarified yet.
The structure A contains three modifiable parts as follows: (i) the galactose–ceramide linkage, (ii) the galactose part, and (iii) the lipid chains. The representative analogues belonging to category (iii) are shown in Fig. 1. In 2001, Miyamoto et al. found that OCH (B), an analogue of A with truncated alkyl chains, caused iNKT cells to produce IL-4 predominantly in mice in vivo.13,14 Kim et al. reported their KBC-007 (C), which has a di(n-heptyl)amino group at the end of the octanamide chain, induced enhanced and biased IL-4 production in vitro (using splenocytes from mice).15,16 On the other hand, an aromatic octanamide analogue C8PhF (D) exhibited a potent Th1-biased cytokine response in vitro (using cell population from humans).17,18 Isosteric replacement of 2-amido moiety of A with a 1,2,3-triazole (E)19 or an ester group (RCAI-80, F)20 was reported, and led the analogues to Th2-type. Besides, α,α-difluoroacylated analogue (G) developed by Calenbergh and his co-workers also showed Th2-biased activity in mice in vivo.21 The analogue G has two fluorine atoms at the position α to the carbonyl group to increase acidity of amide H, which may make hydrogen bonding interaction with Thr156 of mouse CD1d (human: Thr154).7,8
In 2008, we reported that sulfonamide analogues of A (one of the potent Th2 cytokine inducer is RCAI-34, H) induced Th-2 biased cytokine production in vitro (mice), although they showed only very weak bioactivity in mice in vivo under conventional conditions.22 However, we thought them to show Th2-type bioactivity even in vivo, if we modify their drug delivery system, because one of the authors (Y.I.) has discovered that liposome particles can deliver glycolipids to the antigen presenting cells efficiently.23 We therefore synthesized sulfonamide analogue (RCAI-39, 2, Fig. 2) and others (RCAI-41, 53, 100, 127 and 128), and assayed their bioactivities by administration as liposome particles in mice. As a result, it was found that all of the liposomal glycosphingolipids induced iNKT cells to produce Th2-biased cytokines. Among them, liposomal N-methylated carboxamide analogue of A (RCAI-127, 5) induced a large and Th2-biased cytokine production.
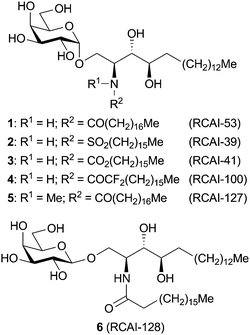 | ||
| Fig. 2 Structures of galactosphingolipids administered as liposome particles. | ||
Results and discussion
Synthesis of the analogues of KRN7000
After examining five different liposome compositions which enhance Th2-type bioactivities of RCAI-39 (2) in mice in vivo, we selected the best composition as glycolipid/DOPC/DC-Chol.24 Because the length of the acyl chains of the main component DOPC is C18, we synthesized six analogues of A as follows: octadecanamide (1, RCAI-53) as a positive control of C18-length acyl analogues, sulfonamide (2, RCAI-39),22 carbamate (3, RCAI-41) as an isoster of 1, α,α-difluorocarboxamide (4, RCAI-100) as one of the analogues with the acidic amide H, N-methylated carboxamide (5, RCAI-127) as the analogue with a methyl group instead of the amide H, and β-anomer of 1 (6, RCAI-128) as the negative control (Fig. 2).Synthesis of α,α-difluorooctadecanoyl chloride (14) is shown in Scheme 1. Epoxide 8 was coupled with a Grignard reagent prepared from 1-bromopentadecane 7 under the Schlosser conditions to give 9.25 Alcohol 9 was oxidized to 10 (89% in two steps) followed by fluorination with (diethylamino)sulfur trifluoride (DAST) to afford 11 (90%).26 After debenzylation,27 the resulting alcohol 12 was oxidized with Jones reagent to furnish acid 13 (77%), which was then converted to acyl chloride 14 in 99% yield.
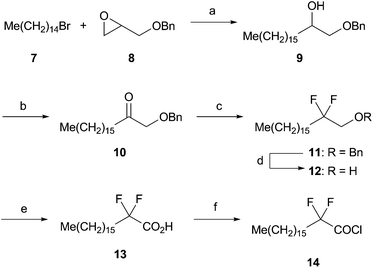 | ||
| Scheme 1 Reagents and conditions: (a) 7, Mg, I2, THF, 25 °C; then 8, Li2CuCl4 (10 mol%) −40 to 25 °C, 12 h. (b) Jones CrO3, acetone, 25 °C, 1 h, 89%, two steps. (c) DAST, CH2Cl2, reflux, 24 h, 90%. (d) H2, Pd(OH)2-C, EtOH, reflux, 24 h, 96%. (e) Jones CrO3, acetone, 70 °C, 10 h, 77%. (f) (COCl)2, benzene, 60 °C, 1.5 h, 99%. | ||
In Scheme 2, synthesis of analogues 1, 3 and 4 (RCAI-53, 41 and 100) was summarized. Synthesis of 2 (RCAI-39) has already been reported.22 Amine 15, which could be prepared from commercially available phytosphingosine,22 was converted to amide 18a–c in 79–82% yield. Protective groups of 18a–c, tert-butyldimethylsilyl (TBS) and benzyl groups, were successively removed by treatment with tetra-n-butylammonium fluoride (TBAF) and by hydrogenolysis, respectively, to furnish analogues 1, 3, and 4.28
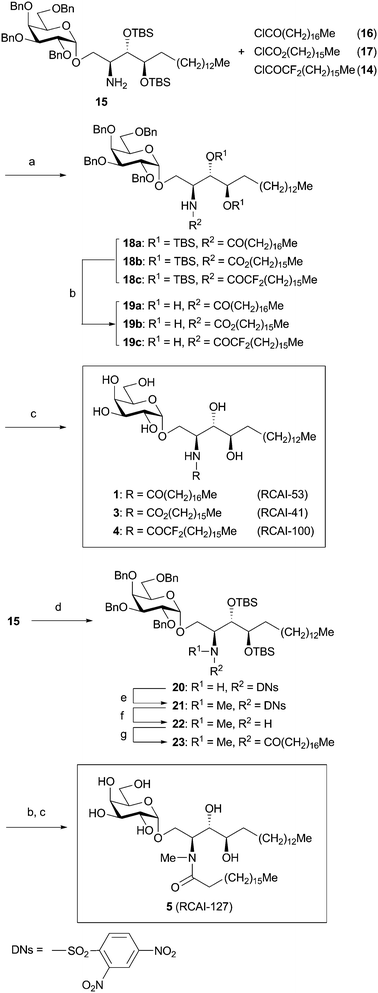 | ||
Scheme 2
Reagents and conditions: (a) Et3N, CH2Cl2, 25 °C, 1 h, 79–82%. (b) TBAF, THF, 25 °C, 24 h, 84–99%. (c) H2, Pd(OH)2–C, EtOH–CHCl3 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 25 °C, 8 h, 77–84%. (d) DNsCl, pyridine, 25 °C, 13 h, 71%. (e) MeI, K2CO3, DMF, 25 °C, 12 h, 89%. (f) Thioglycolic acid, Et3N, CH2Cl2, 25 °C, 24 h, 88%. (g) Stearic acid, EDC, DMAP, CH2Cl2, 25 °C, 17 h, 87%. 1), 25 °C, 8 h, 77–84%. (d) DNsCl, pyridine, 25 °C, 13 h, 71%. (e) MeI, K2CO3, DMF, 25 °C, 12 h, 89%. (f) Thioglycolic acid, Et3N, CH2Cl2, 25 °C, 24 h, 88%. (g) Stearic acid, EDC, DMAP, CH2Cl2, 25 °C, 17 h, 87%. | ||
Analogue 5 (RCAI-127) was synthesized as shown in the lower part of Scheme 2. Amine 15 was treated with 2,4-dinitrobenzenesulfonyl chloride (DNsCl) to give sulfonamide 20 in 71% yield. After N-methylation (89%), the resulting 21 was treated with thioglycolic acid to afford amine 22 (88%).29 Finally, amine 22 was converted to 5 by acylation with stearic acid and deprotection (two steps) similar to those employed in the synthesis of 1–4 in 72% yield (three steps).28
Results of bioassay
Fig. 3 shows the results of bioassay in mice in vivo.30 The concentrations of cytokines in sera of mice were monitored after intravenous administration of KRN7000 (A),31 OCH (B),32 or synthesized analogues (1–6) as phosphate buffered saline (PBS) solutions or liposome particles into C57BL/6 mice. In Fig. 3, the concentrations of each cytokine in sera at the peak time are shown as the relative intensity to those of mice treated with KRN7000. The left part indicates the data of IFN-γ, and the right part shows that of IL-4.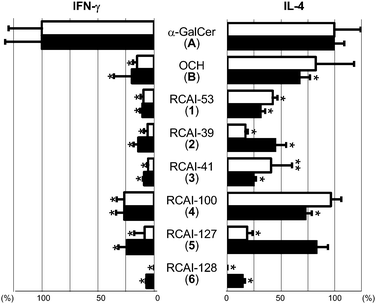 | ||
| Fig. 3 Results of bioassay in mice in vivo. The concentrations at the peak time of cytokines in sera of mice by administration of glycolipids as solutions (□) or as liposome particles (■) were measured by ELISA or CBA. The data are shown as the relative intensity to those obtained by KRN7000. Data are means ± standard deviations from three mice. Results are representative of those from three independent experiments (**P < 0.05 and *P < 0.01 vs. KRN7000). | ||
The blank bars show the results obtained by treatment with glycolipids as PBS solutions. OCH induced potent Th2-biased cytokine production similar to the reported data.13,14,33 As expected, RCAI-53 with an acyl chain shorter than that of KRN7000 together with its isoster RCAI-41 and the mimic RCAI-100 also induced predominant IL-4 production.34 On the other hand, RCAI-39 and RCAI-127 showed very weak bioactivity. In addition, as it is well known, RCAI-128 with β-galactosyl structure induced no cytokine production when it was injected as a PBS solution.
The filled bars indicate the data obtained by treatment with liposomal glycolipids. OCH, RCAI-53, 41 and 100, which were bioactive as PBS solutions, induced Th2-type response.35 By this administration method, RCAI-39 and RCAI-127 were also bioactive, and stimulated NKT cells to produce a large amount of Th2-biased cytokines, although IFN-γ production was also slightly increased. Indeed, their liposomes induced a 2–4 times larger amount of IL-4 production than their solutions. Liposomal RCAI-39 induced IL-4 production almost a half as much as KRN7000 did, and the value of produced IL-4/IFN-γ at the peak time was 3.24 (relative to the value by liposomal KRN7000). On the other hand, the value by liposomal RCAI-127 was 3.47, and the quantity of produced IL-4 was approximately at the same level as that of liposomal KRN7000. The time course of the release of cytokines with the RCAI-compounds described above was similar to that observed for KRN7000 and its known analogues (see the ESI† for KRN7000 and RCAI-127).
Discussions
Although sulfonamide analogues have amide H, which could interact with Thr156 of mouse CD1d (human CD1d: Thr154),7,8 they showed very weak bioactivity in mice in vivo. Possibly due to the nonspecific adsorption in vivo, they might not be delivered to the antigen presenting cells (APCs), although the sulfonamide analogues including RCAI-39 showed potent Th2-type bioactivity in vitro (mice).22 These results showed that RCAI-39 could stimulate iNKT cells when it makes a complex with CD1d. By using the liposome methodology, RCAI-39 would be delivered to APC efficiently, and make a complex with CD1d so as to induce iNKT cells to cytokine production. The amount of produced cytokines caused by RCAI-39, however, was not so large. According to the X-ray information of CD1d–KRN7000 complex,7,8 the amide group of KRN7000 is placed at the narrow hydrophilic site at the entrance of the binding pocket of CD1d. Because the size of the sulfonyl group is larger than that of carbonyl one, CD1d–sulfonamide analogue complex is less stable than CD1d–KRN7000. Consequently, RCAI-39 could stimulate iNKT cells only for a shorter period than KRN7000, and induced the secretion of moderate amounts of cytokines.The N-methylated analogue RCAI-127 also showed very weak bioactivity in mice in vivo when it was administered as a PBS solution, while its liposome induced iNKT cells to secrete a large amount of Th2-biased cytokines, especially IL-4. RCAI-127 has a bulky N-methyl group instead of the amide proton. In the complex of CD1d–KRN7000, the amide proton of KRN7000 makes a hydrogen bonding network with CD1d. However, this NH is not essential for stimulating iNKT cells, since the 1,2,3-triazole analogue (E, Fig. 1) or the ester one (F) is bioactive.19,20 In the case of RCAI-127, making a complex with CD1d would be inhibited by the steric hindrance caused by the additional N-methyl group. We believe liposome particles enabled RCAI-127 to bind CD1d. The complex of CD1d–RCAI-127 is not so stable, but sufficiently stable to lead iNKT cells to produce IL-4. The potent Th2-biased cytokine production was therefore induced by the administration of RCAI-127 as liposome particles.
We initially thought that Th2 bias shown by RCAI-39 and 100 might be linked to hydrogen bonding involving the amide hydrogen. However, the result with RCAI-127 appears to contradict this hypothesis, because it has no amide hydrogen. Since the van der Waals atomic radii of F (1.35 Å) and S (1.85 Å) are larger than those of H (1.2 Å) and O (1.40 Å), and of course CH3 is larger than H, Th2 bias observed with RCAI-39, 100 and 127 may be due to steric effects to decrease the binding of the amide carbonyl or sulfonamide sulfonyl with CD1d protein. Difference in the electronegativity of these atoms may also influence the solvation and binding ability of the amide region. Further studies are necessary to clarify this point.
From these results, it was found that the liposome technique for administration of glycosphingolipids is one of the useful drug delivery methods in the field of iNKT cell study. It was also clarified that liposome particles enforce low affinity glycosphingolipids to make a complex with CD1d efficiently. This technology would allow the glycolipids to induce iNKT cells to preferential production of either Th1 or Th2 cytokines by modification of the components of the liposomes.
Finally, it should be worthy of note that liposomes of RCAI-128 prompted iNKT cells to secrete a trace amount of cytokines even though it is a β-glycosphingolipid, which is known as inactive against iNKT cells.5 The detailed mechanism how β-galactosphingolipid stimulates iNKT cells is under investigation.
Conclusions
We synthesized six analogues of KRN7000 with a C18-length of acyl chain. They are a carboxamide (RCAI-53), a sulfonamide (RCAI-39), a carbamate (RCAI-41), an α,α-difluorocarboxamide (RCAI-100), and an N-methylcarboxamide (RCAI-127) α-galactosphingolipid analogues. In addition, the β-isomer of RCAI-53 (RCAI-128) was also synthesized. Their bioactivities for mouse natural killer iNKT cells were examined together with OCH, and it was found that RCAI-53, 41 and 100 showed Th2-biased cytokine production similar to OCH by administration as phosphate buffered saline (PBS) solutions. By forming liposome particles, all of the synthesized analogues including RCAI-39, 127 and 128 prompted iNKT cells to produce IL-4 predominantly. Among the liposomal analogues, RCAI-127 induced the strongest Th2-type responses.There is still room for optimization of the components of liposomes, which induce iNKT cells to produce more selective IL-4 production.
Acknowledgements
We thank Prof. K. Seino (Hokkaido University) and Mrs S. Suzuki (née Inoue, RIKEN RCAI) for their preliminary contribution. Our thanks are due to Drs T. Nakamura and Y. Hongo (RIKEN) for HRMS analyses. We are grateful to Drs K. Fuhshuku (Toyama Prefectural University), M. Shiozaki and R. Nozawa (RIKEN RCAI) for their helpful comments. This work was partly supported by Mizutani Foundation for Glycoscience.Notes and references
- T. Tashiro, R. Nakagawa, T. Hirokawa, S. Inoue, H. Watarai, M. Taniguchi and K. Mori, Bioorg. Med. Chem., 2009, 17, 6360 CrossRef CAS.
- M. Morita, K. Motoki, K. Akimoto, T. Natori, T. Sakai, E. Sawa, K. Yamaji, Y. Koezuka, E. Kobayashi and H. Fukushima, J. Med. Chem., 1995, 38, 2176 CrossRef CAS.
- T. Natori, Y. Koezuka and T. Higa, Tetrahedron Lett., 1993, 34, 5591 CrossRef CAS.
- T. Natori, M. Morita, K. Akimoto and Y. Koezuka, Tetrahedron, 1994, 50, 2771 CrossRef CAS.
- T. Kawano, J. Cui, Y. Koezuka, I. Toura, Y. Kaneko, K. Motoki, H. Ueno, R. Nakagawa, H. Sato, E. Kondo, H. Koseki and M. Taniguchi, Science, 1997, 278, 1626 CrossRef CAS.
- N. Kamada, H. Iijima, K. Kimura, M. Harada, E. Shimizu, S. Motohashi, T. Kawano, H. Shinkai, T. Nakayama, T. Sakai, L. Brossay, M. Kronenberg and M. Taniguchi, Int. Immunol., 2001, 13, 853 Search PubMed.
- D. M. Zajonc, C. CantuIII, J. Mattner, D. Zhou, P. B. Savage, A. Bendelac, I. A. Wilson and L. Teyton, Nat. Immunol., 2005, 6, 810 CrossRef CAS.
- M. Koch, V. S. Stronge, D. Shepherd, S. D. Gadola, B. Mathew, G. Ritter, A. R. Fersht, G. S. Besra, R. R. Schmidt, E. Y. Jones and V. Cerundolo, Nat. Immunol., 2005, 6, 819 CrossRef CAS.
- N. A. Borg, K. S. Wun, L. Kjer-Nielsen, M. C. J. Wilce, D. G. Pellicci, R. Koh, G. S. Besra, M. Bharadwaj, D. I. Godfrey, J. McCluskey and J. Rossjohn, Nature, 2007, 448, 44 CrossRef CAS.
- D. G. Pellicci, O. Patel, L. Kjer-Nielsen, S. S. Pang, L. C. Sullivan, K. Kyparissoudis, A. G. Brooks, H. H. Reid, S. Gras, I. S. Lucet, R. Koh, M. J. Smyth, T. Mallevaey, J. L. Matsuda, L. Gapin, J. McCluskey, D. I. Godfrey and J. Rossjohn, Immunity, 2009, 31, 47 Search PubMed.
- M.-C. Rissoan, V. Soumelis, N. Kadowaki, G. Grouard, F. Briere, R. de Waal Malefyt and Y.-J. Liu, Science, 1999, 283, 1183 CrossRef CAS.
- There are some reviews available to understand the chemistry and biology of KRN7000 and related compounds: T. Tashiro and K. Mori, Trends Glycosci. Glycotechnol., 2010, 22, 280 Search PubMed; D. Wu, M. Fujio and C.-H. Wong, Bioorg. Med. Chem., 2008, 16, 1073 Search PubMed; P. B. Savage, L. Teyton and A. Bendelac, Chem. Soc. Rev., 2006, 35, 771 CrossRef CAS; R. W. Franck and M. Tsuji, Acc. Chem. Res., 2006, 39, 692 RSC; C. R. Berkers and H. Ovaa, Trends Pharmacol. Sci., 2005, 26, 252 CrossRef CAS.
- K. Miyamoto, S. Miyake and T. Yamamura, Nature, 2001, 413, 531 CrossRef CAS.
- S. Oki, A. Chiba, T. Yamamura and S. Miyake, J. Clin. Invest., 2004, 113, 1631 CrossRef CAS.
- D. J. Baek, Y.-S. Lee, C. Lim, D. Lee, T. Lee, J.-Y. Lee, K.-A Lee, W.-J. Cho, C.-Y. Kang and S. Kim, Chem.–Asian J., 2010, 5, 1560 Search PubMed.
- Y.-S. Lee, K.-A. Lee, J.-Y. Lee, M.-H. Kang, Y. C. Song, D. J. Baek, S. Kim and C.-Y. Kang, Vaccine, 2011, 29, 417 Search PubMed.
- P.-H. Liang, M. Imamura, X. Li, D. Wu, M. Fujio, R. T. Guy, B.-C. Wu, M. Tsuji and C.-H. Wong, J. Am. Chem. Soc., 2008, 130, 12348 CrossRef CAS.
- A. Schiefner, M. Fujio, D. Wu, C.-H. Wong and I. A. Wilson, J. Mol. Biol., 2009, 394, 71 Search PubMed.
- T. Lee, M. Cho, S.-Y. Ko, H.-J. Youn, D. J. Baek, W.-J. Cho, C.-Y. Kang and S. Kim, J. Med. Chem., 2007, 50, 585 CrossRef CAS.
- M. Shiozaki, T. Tashiro, H. Koshino, R. Nakagawa, S. Inoue, T. Shigeura, H. Watarai, M. Taniguchi and K. Mori, Carbohydr. Res., 2010, 345, 1663 CrossRef CAS.
- L. Leung, C. Tomassi, K. Van Beneden, T. Decruy, M. Trappeniers, D. Elewaut, Y. Gao, T. Elliott, A. Al-Shamkhani, C. Ottensmeier, J. M. Werner, A. Williams, S. Van Calenbergh and B. Linclau, ChemMedChem, 2009, 4, 329 CrossRef CAS.
- T. Tashiro, N. Hongo, R. Nakagawa, K. Seino, H. Watarai, Y. Ishii, M. Taniguchi and K. Mori, Bioorg. Med. Chem., 2008, 16, 8896 Search PubMed.
- Y. Ishii, R. Nozawa, Y. Takamoto-Matsui, A. Teng, H. Katagiri-Matsumura, H. Nishikawa, H. Fujita, Y. Tamura and M. Taniguchi, Front. Biosci., 2008, 13, 6214 Search PubMed; Y. Tamura, A. Teng, R. Nozawa, Y. Takamoto-Matsui and Y. Ishii, Biochem. Biophys. Res. Commun., 2008, 369, 485 Search PubMed.
- DOPC: 1,2-dioleyl-sn-glycero-3-phosphocholine; DC-Chol: 3β-[N-(N′,N′-dimethylaminoethane)-carbamoyl]cholesterol.
- C. Fouquet and M. Schlosser, Angew. Chem., Int. Ed. Engl., 1974, 13, 82 CrossRef.
- Inseparable impurities were removed with activated charcoal in hot EtOH after purification by column chromatography on silica gel. Otherwise they worked as catalyst poison in the next hydrogenolysis step.
- When benzyl ether 11 was entirely pure, hydrogenolysis of 11 proceeded even at room temperature.
- RCAI-53 (1): Mp 189.0–190.0 °C; [α]D26 + 50.4 (c 0.30, pyridine); νmax (KBr): 3340 (br s, OH, NH), 1630 (s, CO), 1540 (br m), 1075 (br s, C–O), 1035 (br s, C–O) cm−1; δH (500 MHz, pyridine-d5): 8.44 (1H, d, J = 8.5 Hz), 6.70–5.84 (6H, m), 5.54 (1H, d, J = 3.5 Hz), 5.26–5.21 (1H, m), 4.64 (1H, dd, J = 10, 5.0 Hz), 4.62 (1H, dd, J = 10, 4.0 Hz), 4.52 (1H, d, J = 3.0 Hz), 4.48 (1H, t, J = 5.5 Hz), 4.42–4.37 (3H, m), 4.36 (1H, dd, J = 11, 5.5 Hz), 4.32–4.26 (2H, m), 2.42 (2H, t, J = 7.0 Hz), 2.29–2.21 (1H, m), 1.94–1.82 (2H, m), 1.82–1.74 (2H, m), 1.69–1.60 (1H, m), 1.46–1.16 (50H, m), 0.84 (6H, t, J = 7.0 Hz) ppm; HR-ESIMS: Calcd for C42H84O9N [M + H]+ 746.6141; found 746.6139. RCAI-41 (3): Mp 188.5–201.0 °C; [α]D27 + 53.7 (c 0.31, pyridine); νmax (KBr): 3340 (br s, OH, NH), 1690 (br s, CO), 1540 (br m), 1060 (br s, C–O) cm−1; δH (500 MHz, pyridine-d5): 7.86 (1H, d, J = 9.0 Hz), 6.72–5.18 (6H, m), 5.54 (1H, d, J = 3.5 Hz), 5.01–4.96 (1H, m), 4.69 (1H, dd, J = 11, 6.0 Hz),
4.62 (1H, dd, J = 10, 4.0 Hz), 4.53 (1H, d, J = 2.5), 4.46 (1H, t, J = 6.0 Hz), 4.43 (1H, dd, J = 10, 3.5 Hz), 4.40–4.34 (2H, m), 4.34–4.27 (3H, m), 4.23 (1H, dt, J = 10, 7.0 Hz), 4.17 (1H, dt, J = 10, 7.0 Hz), 2.29–2.21 (1H, m), 1.92–1.81 (2H, m), 1.68–1.58 (1H, m), 1.59 (2H, quint., J = 7.0 Hz), 1.44–1.18 (48H, m), 0.84 (6H, t, J = 7.0 Hz) ppm; HR-ESIMS: Calcd for C41H82NO10 [M + H]+ 748.5933; found 748.5939. RCAI-100 (4): Mp 174.0–175.0 °C; [α]D25 + 46.6 (c 0.30, pyridine); νmax (KBr): 3320 (br s, OH), 1675 (br s, CO), 1560 (br m), 1085 (br s, C–O), 1045 (br s, C–O) cm−1; δH (500 MHz, pyridine-d5): 9.03 (1H, d, J = 9.0 Hz), 6.93 (1H, br s), 6.78 (1H, d, J = 5.0 Hz), 6.48 (1H, br s), 6.36 (1H, br s), 6.23 (2H, br s), 5.54 (1H, d, J = 4.0 Hz), 5.31–5.26 (1H, m), 4.72 (1H, dd, J = 11, 5.5 Hz), 4.64 (1H, dd, J = 9.5, 4.0 Hz), 4.55 (1H, br d, J = 2.5 Hz), 4.47 (1H, br t, J = 6.0 Hz), 4.44 (1H, dd, J = 9.5, 3.0 Hz), 4.42–4.27 (4H, m), 4.36 (1H, dd, J = 11, 5.5 Hz), 2.32–2.21 (3H, m), 1.93–1.83 (2H, m), 1.68–1.60 (1H, m), 1.60–1.53 (2H, m), 1.46–1.16 (48H, m), 0.84 (6H, t, J = 7.0 Hz) ppm; HR-ESIMS: Calcd for C45H81O9NF2Na [M + Na]+ 804.5772; found 804.5766. RCAI-127 (5): Mp 171.0–172.0 °C; [α]D24 + 47.7 (c 0.31, pyridine); νmax (KBr): 3340 (w, OH), 1610 (br s, CO), 1070 (br s, C–O), 1050 (br s, C–O) cm−1; δH (500 MHz, pyridine-d5): rotamer ratio = ca. 2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 6.88 (0.3H, d, J = 6.5 Hz), 6.80–6.28 (3.7H, m), 6.55 (0.7H, br s), 6.49 (0.3H, br s), 6.14 (0.3H, br s), 5.45 (0.3H, d, J = 3.5 Hz), 5.42 (0.7H, d, J = 3.5 Hz), 5.40 (0.7H, br s), 5.09 (1H, quint.-like, J = 3.5 Hz), 4.78 (0.3H, dd, J = 11, 4.0 Hz), 4.72 (0.7H, dd, J = 11, 4.0 Hz), 4.70 (0.3H, dd, J = 10, 4.0 Hz), 4.66 (0.7H, dd, J = 10, 4.0 Hz), 4.64 (0.3H, br d, J = 3.0 Hz), 4.60 (0.7H, br d, J = 3.0 Hz), 4.54–4.32 (5.7H, m), 4.25–4.21 (0.3H, m), 4.16–4.11 (0.3H, m), 4.11–4.06 (0.7H, m), 3.39 (1H, s), 3.25 (1H, s), 2.93–2.82 (0.7H, m), 2.39–2.29 (1.3H, m), 2.23–2.10 (1H, m), 1.98–1.82 (2.7H, m), 1.80–1.69 (1.3H, m), 1.69–1.55 (1H, m), 1.46–1.14 (50H, m), 0.84 (6H, t, J = 7.0 Hz) ppm; HR-ESIMS: Calcd for C43H85NO9Na [M + Na]+ 782.6117; found 782.6119. RCAI-128 (6): Mp 192.0–193.0 °C; [α]D25 − 5.6 (c 0.32, pyridine); νmax (KBr): 3360 (br s, OH), 3300 (br m, NH), 1635 (br s, CO), 1540 (br m), 1080 (br m, C–O), 1045 (br m, C–O) 710 (m) cm−1; δH (500 MHz, pyridine-d5): 8.48 (1H, d, J = 8.5 Hz), 7.22 (1H, d, J = 4.0 Hz), 6.87 (1H, d, J = 6.0 Hz),6.5815 (1H, d, J = 6.5 Hz), 6.5810 (1H, t, J = 6.0 Hz), 6.44 (1H, d, J = 4.5 Hz), 5.18–5.12 (1H, m), 4.90 (1H, d, J = 7.5 Hz), 4.81 (1H, dd, J = 11, 6.0 Hz), 4.54 (1H, br t, J = 3.5 Hz), 4.51–4.46 (1H, m), 4.46 (1H, dd, J = 11, 4.0 Hz), 4.42 (2H, t, J = 6.0 Hz), 4.36 (1H, ddd, J = 6.0, 6.0, 6.0 Hz), 4.24–4.18 (1H, m), 4.14 (1H, ddd, J = 9.5, 6.0, 3.5 Hz), 4.04 (1H, t-like, J = 6.0 Hz), 2.40 (2H, t, J = 7.5 Hz), 2.23–2.17 (1H, m), 1.96–1.86 (2H, m), 1.84–1.73 (2H, m), 1.72–1.61 (1H, m), 1.48–1.16 (50H, m), 0.84 (6H, t, J = 7.0 Hz) ppm; HR-ESIMS: Calcd for C42H83NO9Na [M + Na]+ 768.5960; found 768.5955. The 1H NMR spectra of the newly synthesized analogues are shown in the ESI†.
1, 6.88 (0.3H, d, J = 6.5 Hz), 6.80–6.28 (3.7H, m), 6.55 (0.7H, br s), 6.49 (0.3H, br s), 6.14 (0.3H, br s), 5.45 (0.3H, d, J = 3.5 Hz), 5.42 (0.7H, d, J = 3.5 Hz), 5.40 (0.7H, br s), 5.09 (1H, quint.-like, J = 3.5 Hz), 4.78 (0.3H, dd, J = 11, 4.0 Hz), 4.72 (0.7H, dd, J = 11, 4.0 Hz), 4.70 (0.3H, dd, J = 10, 4.0 Hz), 4.66 (0.7H, dd, J = 10, 4.0 Hz), 4.64 (0.3H, br d, J = 3.0 Hz), 4.60 (0.7H, br d, J = 3.0 Hz), 4.54–4.32 (5.7H, m), 4.25–4.21 (0.3H, m), 4.16–4.11 (0.3H, m), 4.11–4.06 (0.7H, m), 3.39 (1H, s), 3.25 (1H, s), 2.93–2.82 (0.7H, m), 2.39–2.29 (1.3H, m), 2.23–2.10 (1H, m), 1.98–1.82 (2.7H, m), 1.80–1.69 (1.3H, m), 1.69–1.55 (1H, m), 1.46–1.14 (50H, m), 0.84 (6H, t, J = 7.0 Hz) ppm; HR-ESIMS: Calcd for C43H85NO9Na [M + Na]+ 782.6117; found 782.6119. RCAI-128 (6): Mp 192.0–193.0 °C; [α]D25 − 5.6 (c 0.32, pyridine); νmax (KBr): 3360 (br s, OH), 3300 (br m, NH), 1635 (br s, CO), 1540 (br m), 1080 (br m, C–O), 1045 (br m, C–O) 710 (m) cm−1; δH (500 MHz, pyridine-d5): 8.48 (1H, d, J = 8.5 Hz), 7.22 (1H, d, J = 4.0 Hz), 6.87 (1H, d, J = 6.0 Hz),6.5815 (1H, d, J = 6.5 Hz), 6.5810 (1H, t, J = 6.0 Hz), 6.44 (1H, d, J = 4.5 Hz), 5.18–5.12 (1H, m), 4.90 (1H, d, J = 7.5 Hz), 4.81 (1H, dd, J = 11, 6.0 Hz), 4.54 (1H, br t, J = 3.5 Hz), 4.51–4.46 (1H, m), 4.46 (1H, dd, J = 11, 4.0 Hz), 4.42 (2H, t, J = 6.0 Hz), 4.36 (1H, ddd, J = 6.0, 6.0, 6.0 Hz), 4.24–4.18 (1H, m), 4.14 (1H, ddd, J = 9.5, 6.0, 3.5 Hz), 4.04 (1H, t-like, J = 6.0 Hz), 2.40 (2H, t, J = 7.5 Hz), 2.23–2.17 (1H, m), 1.96–1.86 (2H, m), 1.84–1.73 (2H, m), 1.72–1.61 (1H, m), 1.48–1.16 (50H, m), 0.84 (6H, t, J = 7.0 Hz) ppm; HR-ESIMS: Calcd for C42H83NO9Na [M + Na]+ 768.5960; found 768.5955. The 1H NMR spectra of the newly synthesized analogues are shown in the ESI†. - T. Fukuyama, M. Cheung, C.-K. Jow, Y. Hidai and T. Kan, Tetrahedron Lett., 1997, 38, 5831 CrossRef CAS.
- The sera samples were collected at 1, 3, 6, 12, 24, 36, 48, 60, and 72 h after intravenous injection of glycolipids (2 μg per mouse). The measurement of cytokine concentration was performed by ELISA system (Thermo Fisher Scientific K.K.) for IFN-γ, and the cytometric bead array (CBA) system (BD Bioscience) for IL-2, 4, 5, 6, 10, 13, and 12p70. Only the results of IFN-γ and IL-4 are shown in Fig. 3 for the simplification of the discussion. All experiments were in accordance with protocols approved by RIKEN Animal Care and Use Committee.
- KRN7000 (A) was purchased from Funakoshi Co. (Japan).
- OCH (B) was synthesized by ourselves.22.
- R. M. Ndonye, D. P. Izmirian, M. F. Dunn, K. O. A. Yu, S. A. Porcelli, A. Khurana, M. Kronenberg, S. K. Richardson and A. R. Howell, J. Org. Chem., 2005, 70, 10260 CrossRef CAS.
- The correlation between the chain length of lipid and cytokine response was examined. According to the reports, the analogues with the shorter alkyl chains induced the more Th2-biased cytokine production T. Toba, K. Murata, K. Nakanishi, B. Takahashi, N. Takemoto, M. Akabane, T. Nakatsuka, S. Imajo, T. Yamamura, S. Miyake and H. Annoura, Bioorg. Med. Chem. Lett., 2006, 17, 2781 Search PubMed; R. D. Goff, Y. Gao, J. Mattner, D. Zhou, N. Yin, C. Cantu, L. Teyton, A. Bendelac and P. B. Savage, J. Am. Chem. Soc., 2004, 126, 13602 Search PubMed.
- Liposomes were prepared according to the procedure described in the patent: Y. Ishii, R. Nozawa, M. Taniguchi, WO 2005/120574 A1, 2005.
Footnotes |
| † Electronic Supplementary Information (ESI) available: Detailed procedure for synthesis and bioassay of all of the analogues with their 1H NMR spectra. See DOI: 10.1039/c1md00067e |
| ‡ Synthesis of sphingosine relatives, Part 33. For Part 32, see ref. 1. |
| This journal is © The Royal Society of Chemistry 2011 |
