Antimycobacterial activity of spirooxindolo-pyrrolidine, pyrrolizine and pyrrolothiazole hybrids obtained by a three-component regio- and stereoselective 1,3-dipolar cycloaddition†
Stephen Michael
Rajesh
a,
Subbu
Perumal
*a,
J. Carlos
Menéndez
*b,
Perumal
Yogeeswari
c and
Dharmarajan
Sriram
c
aDepartment of Organic Chemistry, School of Chemistry, Madurai Kamaraj University, Madurai, 625 021, India. E-mail: subbu.perum@gmail.com
bDepartamento de Química Orgánica y Farmacéutica, Facultad de Farmacia, Universidad Complutense, 28040, Madrid, Spain. E-mail: josecm@farm.ucm.es
cMedicinal Chemistry & Antimycobacterial Research Laboratory, Pharmacy Group, Birla Institute of Technology & Science—Pilani, Hyderabad Campus, Jawahar Nagar, Hyderabad, 500 078, Andhra Pradesh, India
First published on 12th May 2011
Abstract
Spirooxindolo-pyrrolidine, pyrrolizine and pyrrolothiazole hybrid compounds were obtained in excellent yields from the regio- and stereoselective reaction between β-nitrostyrenes and non-stabilized azomethine ylides, generated in situ from isatin and phenylglycine/proline/thiaproline. These compounds were evaluated for their in vitro activity against Mycobacterium tuberculosisH37Rv (MTB). Eleven compounds were more active than pyrazinamide and one of them, namely 6′-(3-nitrophenyl)-7′-nitro-3′,6′,7′,7a′-tetrahydro-1′H-spiro-[indoline-3,5′-pyrrolo-[1,2-c]thiazol]-2-one, displayed a 7.6 μM MIC value, which represents a potency similar to that of the first-line anti-TB drug ethambutol and 6.7 times higher than that of pyrazinamide.
Tuberculosis (TB), caused by Mycobacterium tuberculosis (MTB), has become an important world-wide public health problem with one-third of the world's population infected by the TB bacillus. About 9 million people, mostly from developing countries, develop active TB yearly, and the death toll is about 2 million in each year.1 The pathogenic synergy of tuberculosis with HIV2 enhances the overall incidence of TB in HIV-positive patients by 50 times relative to the rate for HIV-negative individuals.3 Single agent TB therapy rapidly leads to drug-resistant organisms,4 while multi-drug treatment needs to be prolonged as MTB divides slowly and it is metabolically capable of becoming drug insensitive and/or bacilli may become sequestered.5 The emergence of multidrug and extensively drug resistant tuberculosis (MDR-TB and XDR-TB) further aggravates the problems associated with TB treatment, as patients could become virtually untreatable using currently available anti-TB drugs. Furthermore, no new drugs have been introduced in the last four decades, except the recently introduced fluoroquinolones,6 which testifies to the lack of significant research in this area in the pharmaceutical industry. Hence the development of new drugs, capable of overcoming MDR- and XDR-TB, to efficiently treat this disease is imperative.
Due to the extension of the TB problem in less developed countries, it is of paramount importance that these drug candidates are affordable and this requirement must be taken into account when designing synthetic routes, which must be able to generate molecular complexity and diversity from simple and inexpensive materials. Prompted by these considerations, we have recently embarked on a research programme aimed at studying the application of multi-component, domino and cycloaddition reactions to the construction of novel heterocycles,7 including spiro compounds,8 and their screening for antimycobacterial activities, which has unearthed several compounds with activities comparable to or superior to those of some of the currently employed first-line drugs for TB.8,9 In this context, we report here our findings on the antitubercular properties of three families of spirooxindole–pyrrolidine compounds. This heterocyclic ring system can be found in several bioactive alkaloids. For instance, coerulescine,10 the simplest spirooxindole–pyrrolidine hybrid found in nature, displays local anesthetic effect, pteropodine modulates the function of muscarinic serotonin receptors.11 The spirotryprostatins have antimitotic properties and are of interest as anticancer lead compounds,12 and the recently discovered small-molecule MDM2 inhibitor MI-219 and its analogues are in advanced preclinical development for cancer therapeutics (Fig. 1).13
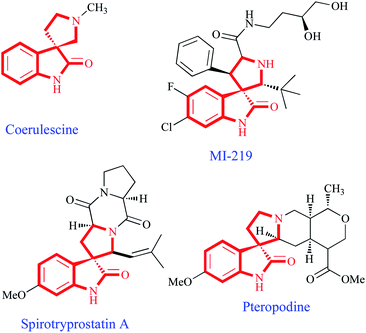 | ||
| Fig. 1 Representative bioactive natural and unnatural products having a spirooxindolo-pyrrolidine core motif. | ||
Our synthetic approach was based on a three-component strategy involving the 1,3-dipolar cycloaddition between β-nitrostyrenes 1a–j and azomethine ylides generated in situ from phenylglycine, proline15a or thiaproline (Scheme 1). This reaction proceeded in a highly regio- and stereocontrolled fashion and afforded compounds 3a–i, 4a–j and 5a–e in excellent yields (80–94%), in a process that generated three C–C bonds and four contiguous stereocenters, one of which is quaternary, in a single operation. All these reactions were effected by refluxing equimolar amounts of the reactants in methanol, and crude products were obtained in pure form without the need for further purification.14 The absence of purification steps, combined with the high yields obtained and the fact that the only side products are water and carbon dioxide render this protocol attractive from an environmental point of view. Interestingly, its regiochemistry was the opposite to the one observed in the previously reported analogous reaction of isatin, sarcosine and β-nitrostyrene.15b
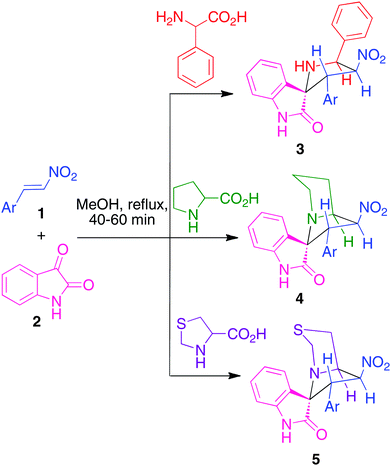 | ||
| Scheme 1 One pot, three-component synthesis of hybrid spiroheterocycles 3–5. | ||
Because of the potential for a large number of regio- and diastereoisomers, in order to obtain meaningful structure–activity relationships a careful structural study was needed. The structures proposed for all compounds 3–5 were in agreement with their NMR spectroscopic data as discussed for a representative example, 3a (Fig. 2). The 1H NMR spectrum of 3a has a doublet at 2.75 ppm (J = 5.7 Hz) and a singlet at 9.90 ppm ascribable to the NH proton of the pyrrolidine and oxindole rings respectively, which vanished upon D2O treatment. The doublet of doublets appearing at 5.86 ppm (J = 10.0, 5.7 Hz) is readily assigned to H-5′ on the basis of the J value of 5.7 Hz suggesting it to be a coupling partner of NH at the 1′ position. This assignment is also supported by the fact that the doublet of doublets due to H-5′ is simplified into a doublet with a J value of 10.0 Hz upon addition of D2O. The triplet at 6.36 ppm (J = 10.1 Hz) is readily assigned to H-4′, as it shows a H,H-COSY correlation with H-5′. The doublet at 4.52 ppm (J = 10.1 Hz) is assigned to H-3′ on the basis of its H,H-correlation with H-4′ and HMBC with the carbonyl of the oxindole ring. The regiochemistry of the cycloaddition is also evident from HMBCs of H-3′ with the oxindole carbonyl carbon and the carbon meta to the methoxy group of the p-anisyl ring. The structure deduced from NMR data was further confirmed by the X-ray study of a single crystal of 3a (Fig. 3), which reveals that the pyrrolidine ring adopts an envelope form with the spiro carbon being out of plane. For the structural study of compounds 4 and 5, see the ESI†.
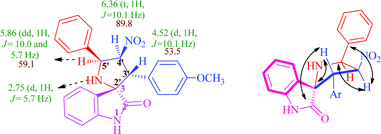 | ||
| Fig. 2 (a) Selected 1H and 13C NMR chemical shifts of 3a. (b) Selected HMBCs of 3a. | ||
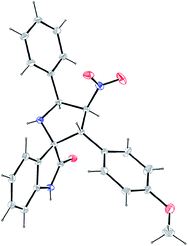 | ||
| Fig. 3 Single crystal X-ray diffraction study of compound 3a. | ||
A plausible mechanism for the regio- and stereoselective formation of the hybrid heterocycles 3–5 is proposed below. The azomethine ylides generated from the reaction of isatin with the amino acids have two nucleophilic carbons, each of which can add to the electron deficient β-carbon of the dipolarophile during the cycloaddition, potentially resulting in two regioisomers. Both of these possibilities have been observed in practice since, as previously mentioned, the regiochemistry of compounds 3–5 described in the present study is the opposite to the one reported previously for the reaction of isatin, sarcosine and β-nitrostyrene.15b The regioselectivity observed in the formation of compounds 3–5 is explicable on the basis that the transition state (TS1) leading to the observed products is likely to be more stable than the other possible one (TS2), which would be destabilized by interactions between the aryl ring from the styrene derivative and the amino acid chain (Scheme 2). In contrast, the transition state of the reaction involving sarcosine does not suffer from unfavourable interactions due to the absence of the amino acid side chain, explaining its reversed regiochemistry.
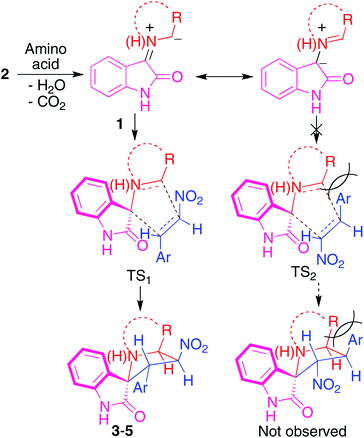 | ||
| Scheme 2 Explanation of the observed regiochemistry. | ||
The spiro heterocycles 3–5 are also formed with complete stereoselectivity, despite the presence of four stereocentres, with the aryl ring attached to the pyrrolidine ring adjacent to the spiro carbon of 3–5 being cis to the carbonyl of the oxindole moiety and trans to the nitro group. This stereoselectivity suggests that the transition state TS3 leading to the unobserved stereoisomers suffers from unfavourable interactions between the phenyl ring and the benzo group of the oxindole moiety (Scheme 3).
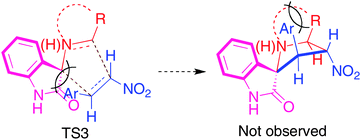 | ||
| Scheme 3 Explanation of the observed stereochemistry. | ||
The twenty four-compound library was screened for in vitro antimycobacterial activity against Mycobacterium tuberculosisH37Rv using the agar dilution method for the determination of MIC (minimum concentration of the compound required to inhibit 99% of bacterial growth), in duplicate. The MIC values (μM) of the synthesized compounds and three standard antitubercular drugs are presented in Table 1. Eleven compounds, namely 3b–e, 3h, 4b, 4d and 5b–e have MIC values in the micromolar range, varying from 7.6 to 32.6 μM. Among them, five compounds in series 3, viz3d, 3h, 3c, 3b and 3e are respectively 3.4, 1.9, 1.75, 1.7 and 1.6 times more active than the first-line antitubercular drug pyrazinamide (MIC = 50.8 μM). In series 4, compounds 4b and 4d are equipotent, showing MIC values of 32.6 μM and being 1.6 times more active than pyrazinamide. In series 5, four compounds (5b, 5c, 5d, 5e) are 3.3, 6.7, 6.5, 6.2 and times more potent than pyrazinamide, respectively. Compound 5c possesses the maximum activity of the library, being equipotent to the drug ethambutol (MIC value 7.6 μM). However, all the compounds were less active than isoniazid, the most active first-line anti-TB drug. Fig. 4 allows a visual comparison of the activities of the most relevant compounds of the three series and that of the three standard drugs.
| Entry | Compound | Ar | Yield (%) | MIC/μg mL−1 | MIC/μM |
|---|---|---|---|---|---|
| 1 | 3a | 4-H3COC6H4 | 86 | >25 | >60.2 |
| 2 | 3b | 4-ClC6H4 | 85 | 12.5 | 29.8 |
| 3 | 3c | 3-O2NC6H4 | 81 | 12.5 | 29.0 |
| 4 | 3d | 3-ClC6H4 | 85 | 6.25 | 14.9 |
| 5 | 3e | 4-FC6H4 | 88 | 12.5 | 31.0 |
| 6 | 3f | C6H5 | 80 | >25 | >64.9 |
| 7 | 3g | 4-H3CC6H4 | 83 | >25 | >62.6 |
| 8 | 3h | 4-BrC6H4 | 84 | 12.5 | 26.9 |
| 9 | 3i | 2,5-(CH3O)2C6H3 | 81 | >25 | >56.1 |
| 10 | 4a | 4-CH3OC6H4 | 87 | 25 | 65.9 |
| 11 | 4b | 4-ClC6H4 | 83 | 12.5 | 32.6 |
| 12 | 4c | 3-O2NC6H4 | 81 | 25 | 63.4 |
| 13 | 4d | 3-ClC6H4 | 85 | 12.5 | 32.6 |
| 14 | 4e | 4-FC6H4 | 83 | 25 | 68.1 |
| 15 | 4f | C6H5 | 90 | 25 | 71.6 |
| 16 | 4g | 4-H3CC6H4 | 87 | 25 | 68.8 |
| 17 | 4h | 4-BrC6H4 | 88 | 25 | 58.4 |
| 18 | 4i | 2,5-(CH3O)2C6H3 | 94 | >25 | >61.1 |
| 19 | 4j | 3,4,5-(CH3O)3C6H2 | 86 | >25 | >56.9 |
| 20 | 5a | 4-CH3OC6H4 | 91 | 25 | 62.9 |
| 21 | 5b | 4-ClC6H4 | 90 | 6.25 | 15.5 |
| 22 | 5c | 3-O2NC6H4 | 93 | 3.13 | 7.6 |
| 23 | 5d | 3-ClC6H4 | 81 | 3.13 | 7.8 |
| 24 | 5e | 4-FC6H4 | 82 | 3.13 | 8.2 |
| 25 | — | Isoniazid | — | 0.05 | 0.4 |
| 26 | — | Ethambutol | — | 1.56 | 7.6 |
| 27 | — | Pyrazinamide | — | 6.25 | 50.08 |
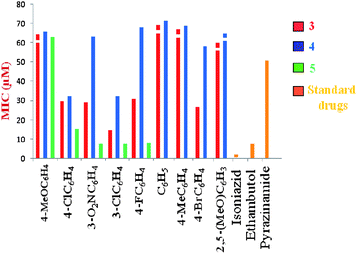 | ||
| Fig. 4 Comparison of MIC (μM) values of series 3, 4, 5 and standard antitubercular drugs. MIC values greater than the numbers given in the y-axis are represented by bars with broken tops. | ||
From the point of view of the establishment of structure–MTB activity relationships, the data in Table 1 and Fig. 4 disclose that, (i) in general, compounds of series 5 have higher activity than those in series 3, which, in turn, are more potent than the corresponding compounds in series 4; (ii) in series 3, compounds with electron-withdrawing groups in the phenyl ring, i.e.halogens and nitro substituents, show higher activity than those bearing electron-releasing groups like methyl and methoxy, the order of activity being: H < 4-Me < 4-OMe < 2,5-(OMe)2 < 4-F < 4-Cl < 3-NO2 < 4-Br < 3-Cl. In series 5, a similar trend is discerned, with the electron-withdrawing groups in the phenyl ring rendering the activity higher than that with electron-releasing methoxy group, the order being: 4-OMe < 4-Cl < 4-F < 3-Cl < 3-NO2. In contrast, no clear trend emerges in series 4, where 3- and 4-chloro substitution in the phenyl ring led to maximum activity, while all other groups in the phenyl ring, both electron-releasing and electron-withdrawing, diminish it. This suggests a blend of electronic and other factors underlying the activity.
In conclusion, the 1,3-dipolar cycloaddition of azomethine ylides to β-nitrostyrenes proceeds regio- and stereoselectively affording novel heterocyclic hybrids, with spirooxindolo-pyrrolidine/pyrrolizine/pyrrolothiazole structures in excellent yields. A library of hybrid heterocycles containing the spirooxindole moiety can be rapidly accessed using this methodology. These spiroheterocycles displayed good in vitro antimycobacterial activity against MTB, with eleven compounds showing activity in the micromolar range and being more potent than pyrazinamide and one having a potency similar to that of the first-line anti-TB drug ethambutol and 6.7 times higher than that of pyrazinamide. Of the three series of compounds, those derived from the 3′,6′,7′,7a′-tetrahydro-1′H-spiro-[indoline-3,5′-pyrrolo-[1,2-c]thiazol]-2-one system were particularly promising. The presence of several functional groups in these hybrid molecules, especially amide and nitro functionalities, enhances the potential for further transformation of these compounds into therapeutically useful candidates.
Acknowledgements
SP and JCM thank MICINN, Spain and the Department of Science and Technology, New Delhi, for funding for Indo-Spanish collaborative major research projects (grants DST/INT/SPAIN/09 and ACI2009-0956). SP and JCM also gratefully acknowledge DST for funds under IRHPA program for the purchase of a high resolution NMR spectrometer and MICINN for grant CTQ2009-12320-BQU, respectively. SMR thanks the University Grants Commission, New Delhi for the award of a Junior Research Fellowship.Notes and references
- (a) D. E. Snider, M. Raviglione and A. Kochi, in Global Burden of Tuberculosis, ed. B. Bloom, Tuberculosis: Pathogenesis, Protection and Control, ASM, Washington, DC, 1994, p. 3 Search PubMed; (b) C. Dye, S. Scheele, P. Dolin, V. Pathania and M. C. Raviglione, JAMA, J. Am. Med. Assoc., 1999, 282, 677–686 Search PubMed.
- K. M. De Cock and R. E. Chaisson, Int. J. Tubercul. Lung Dis., 1999, 3, 457–465 Search PubMed.
- E. L. Corbett, C. J. Watt, N. Walker, D. Maher, B. G. Williams, M. C. Raviglione and C. Dye, Arch. Intern. Med., 2003, 163, 1009–1021 CrossRef.
- K. Honer zu Bentrup and D. G. Russell, Trends Microbiol., 2001, 9, 597–605 Search PubMed.
- (a) C. Dye, B. G. Williams, M. A. Espinal and M. C. Raviglione, Science, 2002, 295, 2042–2046 Search PubMed; (b) T. B. Agerton, S. E. Valway, R. J. Blinkhorn, K. L. Shilkret, R. Reves, W. W. Schluter, B. Gore, C. J. Pozsik, B. B. Plikaytis, C. Woodley and I. M. Onorato, Clin. Infect. Dis., 1999, 29, 85–92 Search PubMed.
- (a) P. R. Mohapatra, Am. J. Respir. Crit. Care Med., 2004, 170, 920–921 Search PubMed; (b) H. L. Rieder, Lancet, 2009, 373, 1148–1149 Search PubMed.
- (a) S. Muthusaravanan, S. Perumal, P. Yogeeswari and D. Sriram, Tetrahedron Lett., 2010, 51, 6439–6443 Search PubMed; (b) R. S. Kumar, S. M. Rajesh, S. Perumal, P. Yogeeswari and D. Sriram, Tetrahedron: Asymmetry, 2010, 21, 1315–1327 Search PubMed; (c) K. Balamurugan, S. Perumal, A. S. Kumar Reddy, P. Yogeeswari and D. Sriram, Tetrahedron Lett., 2009, 50, 6191–6195 Search PubMed; (d) S. Indumathi, R. R. Kumar and S. Perumal, Tetrahedron, 2007, 63, 1411–1416 Search PubMed; (e) M. Srinivasan and S. Perumal, Tetrahedron, 2007, 63, 2865–2874 Search PubMed; (f) M. Kamal Nasar, R. R. Kumar and S. Perumal, Tetrahedron Lett., 2007, 48, 2155–2158 Search PubMed; (g) S. V. Karthikeyan and S. Perumal, Tetrahedron Lett., 2007, 48, 2261–2265 Search PubMed; (h) N. Savitha Devi and S. Perumal, Tetrahedron Lett., 2007, 48, 5627–5629 Search PubMed.
- (a) R. R. Kumar, S. Perumal, P. Senthilkumar, P. Yogeeswari and D. Sriram, Bioorg. Med. Chem. Lett., 2007, 17, 6459–6462 Search PubMed; (b) R. R. Kumar, S. Perumal, P. Senthilkumar, P. Yogeeswari and D. Sriram, J. Med. Chem., 2008, 51, 5731–5735 Search PubMed; (c) R. R. Kumar, S. Perumal, P. Senthilkumar, P. Yogeeswari and D. Sriram, Tetrahedron, 2008, 64, 2962–2971 CrossRef CAS; (d) S. V. Karthikeyan, S. Perumal, A. S. Krithika, P. Yogeeswari and D. Sriram, Bioorg. Med. Chem. Lett., 2009, 19, 3006–3009 Search PubMed; (e) R. R. Kumar, S. Perumal, S. C. Manju, P. Bhatt, P. Yogeeswari and D. Sriram, Bioorg. Med. Chem. Lett., 2009, 19, 3461–3465 Search PubMed; (f) R. R. Kumar, S. Perumal, P. Senthilkumar, P. Yogeeswari and D. Sriram, Eur. J. Med. Chem., 2009, 44, 3821–3829 Search PubMed; (g) K. Balamurugan, V. Jeyachandran, S. Perumal, T. H. Manjashetty, P. Yogeeswari and D. Sriram, Eur. J. Med. Chem., 2010, 45, 682–688 CrossRef CAS.
- (a) R. S. Kumar, S. Perumal, K. A. Shetty, P. Yogeeswari and D. Sriram, Eur. J. Med. Chem., 2010, 45, 124–133 Search PubMed; (b) S. V. Karthikeyan, B. Devi Bala, V. P. Alex Raja, S. Perumal, P. Yogeeswari and D. Sriram, Bioorg. Med. Chem. Lett., 2010, 20, 350–353 Search PubMed; (c) S. Indumathi, S. Perumal, D. Banerjee, P. Yogeeswari and D. Sriram, Eur. J. Med. Chem., 2009, 44, 4978–4984 CrossRef CAS; (d) K. Balamurugan, S. Perumal, A. S. K. Reddy, P. Yogeeswari and D. Sriram, Tetrahedron Lett., 2009, 50, 6191–6195 Search PubMed; (e) R. S. Kumar, S. M. Rajesh, S. Perumal, P. Yogeeswari and D. Sriram, Eur. J. Med. Chem., 2010, 45, 411–422 Search PubMed; (f) R. S. Kumar, S. M. Rajesh, S. Perumal, P. Yogeeswari and D. Sriram, Chem. Pharm. Bull., 2010, 58, 602–610 Search PubMed; (g) P. Prasanna, K. Balamurugan, S. Perumal, P. Yogeeswari and D. Sriram, Eur. J. Med. Chem., 2010, 45, 5653–5661 Search PubMed; (h) S. U. Maheswari, S. Perumal, P. Yogeeswari and D. Sriram, Bioorg. Med. Chem. Lett., 2010, 20, 7278–7282 Search PubMed.
- C. V. Galliford and K. A. Scheidt, Angew. Chem., Int. Ed., 2007, 46, 8748–8758 CrossRef CAS.
- T.-H. Kang, Eur. J. Pharmacol., 2002, 444, 39–45 CrossRef CAS.
- A. Bertamino, C. Aquino, M. Sala, N. de Simone, C. A. Mattia, L. Erra, S. Musella, P. Iannelli, A. Carotenuto, P. Grieco, E. Novellino, P. Campiglia and I. Gómez-Monterrey, Bioorg. Med. Chem., 2010, 18, 4328–4337 Search PubMed.
- K. Ding, Y. Lu, Z. Nikolovska-Coleska, G. Wang, S. Qiu, S. Shangary, W. Gao, D. Qin, J. Stuckey, K. Krajewski, P. P. Roller and S. Wang, J. Med. Chem., 2006, 49, 3432–3435 CrossRef CAS.
- General procedure. A solution in methanol (10 mL) of the suitable β-nitrostyrene 1 (1 mmol), isatin 2 (1 mmol) and phenylglycine, proline or thiaproline (1 mmol) was refluxed for 40 min (compounds 3) or 1 h (compounds 4 and 5). After completion of the reaction (TLC), the mixture was poured into crushed ice and the precipitated solid was filtered and washed with water (100 mL) to obtain pure compounds 3–5 as solids. Data for representative compounds follow. For full characterization data, see the ESI†. 3′-(4-Methoxyphenyl)-4′-nitro-5′-phenylspiro[indoline-3,2′-pyrrolidin]-2-one (3a3a). Obtained as a yellow solid. Yield: 86%; mp 231 °C. IR (KBr): 1359, 1548, 1704, 3185, 3205 cm−1; (300 MHz, DMSO-d6) δH 2.75 (d, 1H, J = 5.7 Hz, NH), 3.69 (s, 3H), 4.52 (d, 1H, J = 10.1 Hz), 5.86 (dd, 1H, J = 10.0, 5.7 Hz), 6.36 (t, 1H, J = 10.1 Hz), 6.65–6.69 (m, 2H), 6.97 (d, 2H, J = 8.7 Hz), 7.16–7.22 (m, 2H), 7.31–7.39 (m, 4H), 7.59 (d, 2H, J = 6.6 Hz), 7.72–7.75 (d, 1H, J = 7.2 Hz), 9.94 (br s, 1H, NH). 13C NMR (75 MHz, DMSO-d6) δC 53.5, 53.6, 59.1, 70.2, 89.8, 108.5, 112.3, 120.8, 122.5, 122.9, 126.5, 126.6, 126.7, 127.0, 127.6, 127.9, 137.7, 141.0, 157.6, 178.6. Anal. Calcd for C24H21N3O4: C, 69.39; H, 5.10; N, 10.11%. Found: C, 69.30; H, 5.18; N, 10.17%. 2′-(2,5-Dimethoxyphenyl)-1′-nitro-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one (4i4i). Obtained as a green solid. Yield: 94%; mp = 217 °C. IR (KBr): 1342, 1543, 1705, 3191 cm−1; (300 MHz, DMSO-d6) δH 1.40–1.48 (m, 1H), 1.72–1.75 (m, 1H), 1.96–2.02 (m, 1H), 2.05–2.12 (m, 1H), 2.72–2.77 (m, 1H), 3.26–3.27 (m, 1H), 3.51 (s, 3H), 3.66 (s, 3H), 4.69–4.77 (m, 1H), 5.22 (d, 1H, J = 10.5 Hz), 6.18 (t, 1H, J = 10.5 Hz), 6.62–6.67 (m, 3H), 6.95–7.00 (m, 2H), 7.15 (t, 1H, J = 7.5 Hz), 7.57 (d, 1H, J = 7.5 Hz), 10.26 (br s, 1H, NH). 13C NMR (75 MHz, DMSO-d6) δC 23.7, 26.1, 41.5, 49.3, 53.6, 54.2, 61.9, 73.1, 90.9, 108.1, 110.4, 111.2, 112.5, 119.3, 121.0, 123.0, 125.6, 127.9, 141.4, 150.1, 151.4, 176.1. Anal. Calcd for C22H23N3O5: C, 64.54; H, 5.66; N, 10.26%. Found C, 64.43; H, 5.75; N, 10.19%. 6′-(4-Methoxyphenyl)-7′-nitro-3′,6′,7′,7a′-tetrahydro-1′ H-spiro[indoline-3,5′-pyrrolo[1,2- c]thiazol]-2-one (5a5a).Obtained as a yellow solid. Yield: 91%; mp = 243 °C. IR (KBr): 1369, 1547, 1707, 3239 cm−1; (300 MHz, DMSO-d6) δH 2.90–2.97 (m, 1H), 3.08–3.15 (m, 1H), 3.70 (s, 3H), 3.99 (d, 1H, J = 10.2 Hz), 4.17 (d, 1H, J = 10.2 Hz), 4.25 (d, 1H, J = 11.7 Hz), 4.55–4.62 (m, 1H), 6.48 (dd, 1H, J = 11.7, 7.2 Hz), 6.64–6.71 (m, 3H), 6.81–6.84 (m, 2H), 7.07 (br s, 1H, NH), 7.18–7.26 (m, 1H), 7.29–7.34 (m, 1H), 7.82 (d, 1H, J = 7.2 Hz). 13C NMR (75 MHz, DMSO-d6) δC 32.3, 52.2, 53.7, 54.1, 66.3, 73.6, 83.5, 109.0, 112.3, 120.9, 122.5, 123.1, 124.4, 127.6, 129.2, 141.7, 157.8, 176.1. Anal. Calcd for C20H19N3O4S: C, 60.44; H, 4.82; N, 10.57%. Found C, 60.37; H, 4.89; N, 10.49%.
- (a) M. Poornachandran and R. Raghunathan, Synth. Commun., 2007, 37, 2507–2517 Search PubMed; (b) M. Poornachandran, R. Muruganantham and R. Raghunathan, Synth. Commun., 2006, 36, 141–150 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Full experimental data, details of the structural study of compounds 4 and 5, ortep diagrams for 3f and 4i and copies of spectra of representative compounds. CCDC reference numbers for 3a: 802308, 3f: 802309 and 4i: 800956. See DOI: 10.1039/c0md00239a |
| This journal is © The Royal Society of Chemistry 2011 |
