Temocene: the porphycene analogue of temoporfin (Foscan®)†‡
María
García-Díaz
a,
David
Sánchez-García
a,
Jorge
Soriano
b,
M. Lluïsa
Sagristà
c,
Margarita
Mora
c,
Ángeles
Villanueva
b,
Juan C.
Stockert
b,
Magdalena
Cañete
b and
Santi
Nonell
*a
aGrup d'Enginyeria Molecular, Institut Químic de Sarrià, Universitat Ramon Llull, Via Augusta 390, 08017, Barcelona, Spain. E-mail: santi.nonell@iqs.url.edu; Fax: +34 932 05 62 66; Tel: +34 932 67 20 00
bDepartamento de Biología, Facultad de Ciencias, Universidad Autónoma de Madrid, Darwin 2, 28049, Cantoblanco-Madrid, Spain
cDepartament de Bioquímica i Biologia Molecular, Facultat de Biologia, Universitat de Barcelona, Avinguda Diagonal 645, 08028, Barcelona, Spain
First published on 6th May 2011
Abstract
Temocene, a porphycene analogue of temoporfin, has been synthesized. Compared to temoporfin, temocene is endowed with 2.5-fold larger absorption coefficients in the red part of the spectrum while keeping its excellent photophysical and singlet oxygen photosensitization ability. While its photodynamic activity towards HeLa cells is lower than that of temoporfin, its higher photostability, lower dark toxicity and mitochondrial localisation make temocene a promising candidate for photodynamic therapy applications.
During the last two decades a substantial effort has been put into the development and scrutiny of the second-generation photosensitizers (PSs)1–4 since no single PS has yet been found to meet all the demands for successful application in oncology. Amongst these second generation PSs, a series of derivatives of m-tetrahydroxyphenyl porphyrin (m-THPP) have been particularly promising.5,6 The hydroxyl functions modulate the hydrophobic character of the macrocyclic core and therefore its solubility, and provide hydrogen bonding capability for specific interactions with receptor sites. One of the most active photosensitizers is m-tetrahydroxyphenyl chlorin (m-THPC, temoporfin).7–9 Although temoporfin is currently approved for photodynamic therapy (PDT) treatment of head and neck cancer under the trade name Foscan®,10,11 this PS is not without its own shortcomings due to its high potency and prolonged skin sensitivity.12,13 Amongst the porphyrin-based photodynamic therapy agents, porphycenes show better absorption properties than their structural isomers14,15 owing to the lower molecular symmetry. The absorption on the red part of the spectrum, where the tissues are more transparent to light,16 the demonstrated cell photoinactivation17–20 and the little photosensitivity associated21 placed the porphycenes in an excellent position as promising candidates for PDT treatments. Since the synthesis of the first porphycene in 1986,22 a variety of substituted derivatives have been prepared23 but the long and complex syntheses involved were a limiting factor until very recently. In the light of the challenge set by Bonnett,24 who suggested the investigation of the corresponding m-tetra(hydroxyphenyl) porphycene derivative, we report herewith on the synthesis, photophysics, subcellular localization, and photodynamic activity of the porphycene analogue of temoporfin, which we term temocene (m-THPPo, Fig. 1).
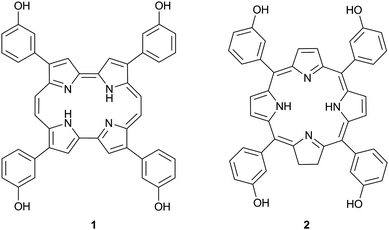 | ||
| Fig. 1 Chemical structure of temocene 1 and temoporfin 2. | ||
Temocene was synthesized using a procedure based on the four-step synthesis of porphycenes recently developed by our group25 (Scheme 1). Thus the isopropoxy ethers of porphycene 9 were deprotected to the corresponding hydroxy derivative 1 by addition of anhydrous aluminium trichloride to a dichloromethane solution of compound 9 (complete synthesis and characterization described in the ESI†). In order to compare temocene and its chlorin analogue, temoporfin was synthesized using a published procedure.26
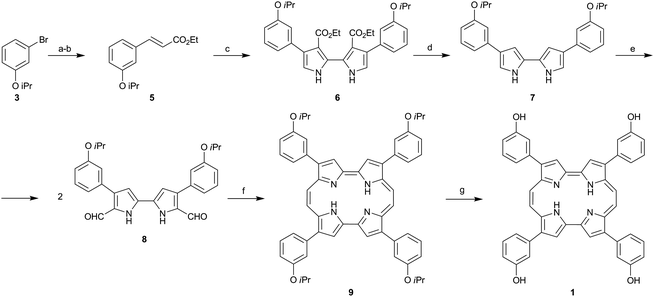 | ||
| Scheme 1 Reagents and conditions: (a) nBuLi, DMF, THF −78 °C (98%), (b) 4, nBuLi, triethyl phosphonoacetate, THF −78 °C (77%), (c) TosMiC, nBuLi, then Me3SnCl and 5, THF −78 °C then Cu(NO3)2·3H2O, THF (44%), (d) NaOH, ethylene glycol 180 °C (87%), (e) POCl3, DMF 0 °C to 60 °C then NaOAc 85 °C (89%), (f) TiCl4, Zn Cu2Cl2, THF reflux (20%) and (g) AlCl3, DCM (79%) | ||
The photophysical properties of m-THPPo and m-THPC are summarized in Table 1. As observed in Fig. 2, m-THPPo shows the typical absorption spectrum of free-base porphycenes, with three intense bands in the red part of the spectrum showing a maximum absorption coefficient of ca. 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1 at 656 nm, 2.5-fold higher than that of temoporfin.6 The fluorescence emission spectrum also matches the typical fluorescence spectrum of porphycenes, with a main band at 666 nm and a weaker shoulder at lower energies that mirror the S1 ← S0 absorption transition.15 The fluorescence quantum yield is ΦF = 0.084 ± 0.005, suggesting that temocene could be used also for fluorescence diagnostic purposes. The excited singlet state decays with a lifetime of 2.3 ± 0.1 ns and the newborn triplet state lives 260 μs in argon-saturated solutions, long enough to provide for rich photochemistry.
000 M−1 cm−1 at 656 nm, 2.5-fold higher than that of temoporfin.6 The fluorescence emission spectrum also matches the typical fluorescence spectrum of porphycenes, with a main band at 666 nm and a weaker shoulder at lower energies that mirror the S1 ← S0 absorption transition.15 The fluorescence quantum yield is ΦF = 0.084 ± 0.005, suggesting that temocene could be used also for fluorescence diagnostic purposes. The excited singlet state decays with a lifetime of 2.3 ± 0.1 ns and the newborn triplet state lives 260 μs in argon-saturated solutions, long enough to provide for rich photochemistry.
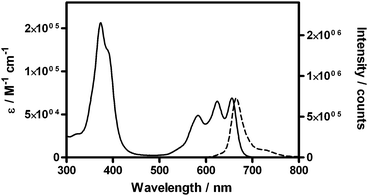 | ||
| Fig. 2 Absorption (solid line) and emission (dotted line) spectra of m-THPPo in THF. | ||
Indeed, temocene is able to photosensitize the production of singlet oxygen (1O2) in aerated solutions. The quantum yield, ΦΔ = 0.10 ± 0.01, is high enough to expect substantial phototoxicity to cells.
The kinetics of temocene and temoporfin photobleaching under the same irradiation conditions are comparatively shown in Fig. 3 (see ESI† for details). Temocene is substantially more photostable than temoporfin.
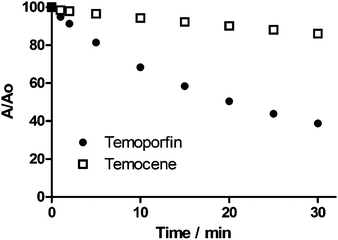 | ||
| Fig. 3 Photobleaching of temoporfin and temocene solutions in aerated acetone upon irradiation with 532 nm laser pulses. Absorbance values were recorded at 650 and 656 nm, respectively (see ESI† for details). | ||
Studies on the dark- and phototoxicity of temocene and temoporfin are summarized in Fig. 4. HeLa cells were incubated in the dark with different concentrations of m-THPPo or m-THPC in DMSO for 18 h prior to photosensitization.
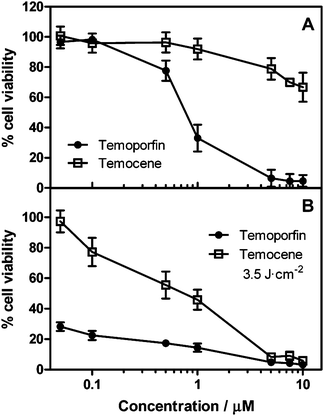 | ||
| Fig. 4 Viability of HeLa cells measured by the MTT assay after 18 h incubation with different concentrations of m-THPPo or m-THPC in DMSO. (A) Dark toxicity. (B) Photodynamic induced cytotoxicity after 3.5 J cm−2. Mean ± SD from at least four independent experiments are shown. | ||
A 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay28 was performed 24 h after treatment to establish the m-THPPo and m-THPC dark toxicity. Our studies show that temocene is substantially less toxic in the dark than temoporfin, which is advantageous for its therapeutic applications. The photodynamic damage on HeLa cells was assessed after delivery of different light doses from a LED source at 625 nm. Complete cell inactivation could be achieved at light doses of just 3.5 J cm−2 using m-THPPo concentrations higher than 5 μM. At higher light doses the same result could be obtained at concomitantly lower temocene concentrations.
Fluorescence micrographs of HeLa cells after 18 h incubation with temocene are shown in Fig. 5. Cells incubated with 0.5 μM m-THPPo showed a fluorescence pattern similar to that of control cells. With 1 μM and especially 10 μM m-THPPo, a red fluorescence could be distinguished, which colocalized with the blue mitochondrial autofluorescence (Fig. S9 in the ESI†) and with the green emission from MitoTracker®Green (Fig. 5A and merged image 5C), indicating that mitochondria are the main sites of temocene accumulation. This is fortunate as this organelle is one of the most attractive PDT targets for triggering apoptosis.29–31 In addition, a diffuse red fluorescence could be detected in the cytoplasm. No relocalization of the PS was observed when cells were exposed to prolonged irradiation.
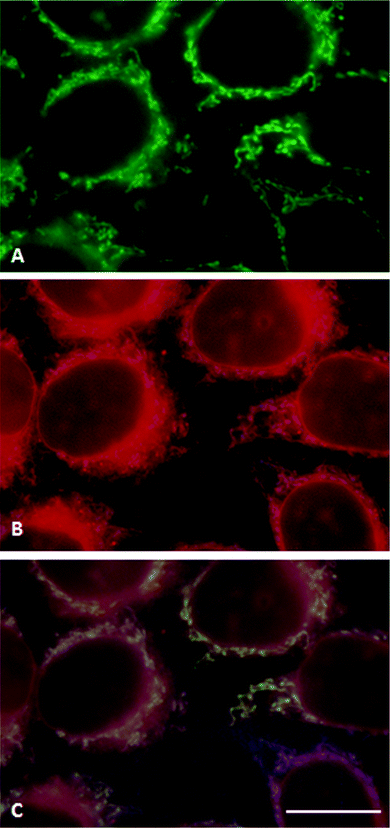 | ||
| Fig. 5 Fluorescence microscopy images of living HeLa cells incubated with MitoTracker®Green for 30 min, followed by 18 h 10 μM DMSO-loaded m-THPPo. (A) Cells observed under blue excitation. (B) Cells observed under UV excitation. (C) Merged image. Scale bar: 20 μm. | ||
Conclusions
In summary, a novel porphycene-based PS has been synthesized, which is an analogue of temoporfin. Its excellent photophysical properties, mitochondrial localization, and, above all, its photodynamic efficiency, make temocene a promising candidate for antitumoral photodynamic therapy. Compared to temoporfin, temocene shows lower activity but also lower dark toxicity and superior photostability. Taken together, temocene is endowed with potential value for photodynamic treatments and is worthy of further studies. Development of a liposome-based formulation of temocene for its improved cell delivery is currently underway.Acknowledgements
This work was supported by grants of the Spanish Ministerio de Ciencia e Innovación (CTQ2007-67763-C03/BQU and CTQ2010-20870-C03). M. G.-D. thanks the Comissionat per a Universitats i Recerca del Departament d'Innovació, Universitats i Empresa de la Generalitat de Catalunya i del Fons Social Europeu for a predoctoral fellowship. We thank Sorisa (Dr. Albert Amat) for providing us with the Sorisa Photocare® LED source.Notes and references
- M. J. Garland, C. M. Cassidy, D. Woolfson and R. F. Donnelly, Future Med. Chem., 2009, 1, 667–691 Search PubMed.
- A. E. O'Connor, W. M. Gallagher and A. T. Byrne, Photochem. Photobiol., 2009, 85, 1053–1074 CrossRef CAS.
- L. B. Josefsen and R. W. Boyle, Met.-Based Drugs, 2008, 1–24 Search PubMed.
- S. B. Brown, E. A. Brown and I. Walker, Lancet Oncol., 2004, 5, 497–508 CrossRef CAS.
- R. Bonnett, R. D. White, U. J. Winfield and M. C. Berenbaum, Biochem. J., 1989, 261, 277–280 CAS.
- R. Bonnett, P. Charlesworth, B. D. Djelal, S. Foley, D. J. McGarvey and T. G. Truscott, J. Chem. Soc., Perkin Trans. 2, 1999, 325–328 RSC.
- Q. Peng, J. H. Moan, L. W. Ma and J. M. Nesland, Cancer Res., 1995, 55, 2620–2626 CAS.
- L. Morlet, V. Vonarx-Coinsmann, P. Lenz, M. Foultier, L. X. de Brito, C. Stewart and T. Patrice, J. Photochem. Photobiol., B, 1995, 28, 25–32 CrossRef CAS.
- M. Triesscheijn, M. Ruevekamp, M. Aalders, P. Baas and F. A. Stewart, Photochem. Photobiol., 2005, 81, 1161–1167 CrossRef CAS.
- M. G. Dilkes, M. L. DeJode, A. RowntreeTaylor, J. A. McGilligan, G. S. Kenyon and P. McKelvie, Laser Med. Sci., 1996, 11, 23–29 Search PubMed.
- S. Banfi, E. Caruso, S. Caprioli, L. Mazzagatti, G. Canti, R. Ravizza, M. Gariboldi and E. Monti, Bioorg. Med. Chem., 2004, 12, 4853–4860 CrossRef CAS.
- A. M. Ronn, M. Nouri, L. A. Lofgren, B. M. Steinberg, A. Westerborn, T. Windahl, M. J. Shikowitz and A. L. Abramson, Laser Med. Sci., 1996, 11, 267–272 Search PubMed.
- R. K. Pandey, J. Porphyrins Phthalocyanines, 2000, 4, 368–373 CrossRef CAS.
- P. F. Aramendia, R. W. Redmond, S. Nonell, W. Schuster, S. E. Braslavsky, K. Schaffner and E. Vogel, Photochem. Photobiol., 1986, 44, 555–559 CrossRef CAS.
- J. C. Stockert, M. Cañete, A. Juarranz, A. Villanueva, R. W. Horobin, J. I. Borrell, J. Teixido and S. Nonell, Curr. Med. Chem., 2007, 14, 997–1026 CrossRef CAS.
- G. Jori, J. Photochem. Photobiol., A, 1992, 62, 371–378 CrossRef CAS.
- M. Guardiano, R. Biolo, G. Jori and K. Schaffner, Cancer Lett., 1989, 44, 1–6 CrossRef CAS.
- M. Cañete, M. Lapena, A. Juarranz, V. Vendrell, J. I. Borrell, J. Teixido, S. Nonell and A. Villanueva, Anti-Cancer Drug Des., 1997, 12, 543–554 CAS.
- A. Villanueva, M. Cañete, S. Nonell, J. I. Borrell, J. Teixido and A. Juarranz, Anti-Cancer Drug Des., 1996, 11, 89–99 CAS.
- M. Cañete, C. Ortega, A. Gavalda, J. Cristobal, A. Juarranz, S. Nonell, J. Teixido, J. I. Borrell, A. Villanueva, S. Rello and J. C. Stockert, Int. J. Oncol., 2004, 24, 1221–1228 CAS.
- C. Abels, R. M. Szeimies, P. Steinbach, C. Richert and A. E. Goetz, J. Photochem. Photobiol., B, 1997, 40, 305–312 CrossRef CAS.
- E. Vogel, M. Kocher, H. Schmickler and J. Lex, Angew. Chem., Int. Ed. Engl., 1986, 25, 257–259 CrossRef.
- C. Richert, J. M. Wessels, M. Muller, M. Kisters, T. Benninghaus and A. E. Goetz, J. Med. Chem., 1994, 37, 2797–2807 CrossRef CAS.
- R. Bonnett, Chemical Aspects of Photodynamic Therapy, Gordon and Breach Science Publisher, Amsterdam, 2000 Search PubMed.
- D. Sanchez-Garcia, J. I. Borrell and S. Nonell, Org. Lett., 2009, 11, 77–79 CrossRef CAS.
- R. Bonnett and M. C. Berenbaum, Eur. Pat., 337 601, 1989.
- K. Chen, M. Wacker, S. Hackbarth, C. Ludwig, K. Langer and B. Röder, J. Photochem. Photobiol., B, 2010, 101, 340–347 Search PubMed.
- T. Mosmann, J. Immunol. Methods, 1983, 65, 55–63 CrossRef CAS.
- N. L. Oleinick, R. L. Morris and I. Belichenko, Photochem. Photobiol. Sci., 2002, 1, 1–21 RSC.
- K. Plaetzer, T. Kiesslich, C. B. Oberdanner and B. Krammer, Curr. Pharm. Des., 2005, 11, 1151–1165 CrossRef CAS.
- R. Hilf, J. Bioenerg. Biomembr., 2007, 39, 85–89 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures for chemical synthesis, HPLC, NMR, photophysical characterization, photobleaching studies and microscopy studies. See DOI: 10.1039/c1md00065a |
| ‡ This paper is dedicated to Professor Raymond Bonnett in recognition of his seminal contributions in the field of photosensitisers' research. |
| This journal is © The Royal Society of Chemistry 2011 |
