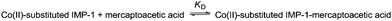Metal preference of Zn(II) and Co(II) for the dinuclear metal binding site of IMP-1 metallo-β-lactamase and spectroscopic properties of Co(II)-substituted IMP-1 with mercaptoacetic acid†
Yoshihiro
Yamaguchi‡
*a,
Kayo
Imamura
b,
Ako
Sasao
c,
Emi
Murakami
b,
Yoshichika
Arakawa§
d and
Hiromasa
Kurosaki‡
*b
aEnvironmental Safety Center, Kumamoto University, 39-1 Kurokami 2-Chome, Kumamoto, 860-8555, Japan. E-mail: yyamagu@gpo.kumamoto-u.ac.jp; Fax: +81 96 342 3237; Tel: +81 96 342 3238
bDepartment of Structure–Function Physical Chemistry, Graduate School of Pharmaceutical Sciences, Kumamoto University, 5-1 Oe-honmachi, Kumamoto, 862-0973, Japan. E-mail: ayasaya@gpo.kumamoto-u.ac.jp; Fax: +81 96 371 4314; Tel: +81 96 371 4314
cDepartment of Forensic Medicine, Graduate School of Medical Sciences, Kumamoto University, 1-1-1 Honjo, Kumamoto, 860-8556, Japan
dDepartment of Bacterial Pathogenesis and Infection Control, National Institute of Infectious Diseases, 4-7-1 Gakuen, Musashi-Murayama, Tokyo, 208-0011, Japan
First published on 2nd June 2011
Abstract
IMP-1 metallo-β-lactamase is a dinuclear Zn(II) enzyme that catalyzes the hydrolysis and inactivation of most β-lactams including carbapenems, and is involved in one of the mechanisms for generating clinical resistance to antibiotics in pathogenic bacteria. We investigated the metal preferences of Zn(II) and Co(II) for the apo-enzyme of IMP-1 metallo-β-lactamase, apo-IMP-1, which contains a dinuclear metal binding site (the Zn1 and Zn2 sites), by UV-visible spectroscopy. The UV-visible spectrum of apo-IMP-1 containing 1 equiv. of Co(II) and 1 equiv. of Zn(II) showed a high preference of Zn(II) for the Zn1 site compared to Co(II). Moreover, Zn(II) bound more strongly to the Zn2 site than Co(II). The interaction of IMP-1 metallo-β-lactamase with mercaptoacetic acid was also investigated using Co(II)-substituted IMP-1 and UV-visible spectroscopy. Possible metal binding modes of Co(II) or Zn(II) to the dinuclear metal binding site in apo-IMP-1 and of mercaptoacetic acid to Co(II)-substituted IMP-1 are proposed.
Introduction
Metallo-β-lactamases are Zn(II)-dependent enzymes that catalyze the hydrolysis of most β-lactams including carbapenems with the exception of monobactams such as aztreonam, and are now recognized as a new potential threat to human society. Some metallo-β-lactamases including IMP-1 discovered in Japan are coded by a gene cassette in an integron and are transferable to other bacteria.1,2 Since a new metallo-β-lactamase, NDM-1, has just emerged in India and Pakistan, and also been expanding in the United Kingdom3 and in other countries, further worldwide proliferation of the kind of metallo-β-lactamases is becoming a grave general concern.4 One countermeasure to these lactamases involves the development of inhibitors that block the action of the enzyme. At present, no relevant inhibitors are available for clinical use. In our continuing efforts to develop metallo-β-lactamase inhibitors, we found that mercaptocarboxylic acids are potent inhibitors for IMP-1.5 Among them, mercaptoacetic acid and 2-mercaptopropionic acid strongly and competitively inhibited IMP-1 with Ki values of 0.23 and 0.19 μM, respectively.5Based on the inhibition effect of mercaptoacetic acid against IMP-1, Arakawa et al. developed a double-disk method for detection of metallo-β-lactamase-producing Gram-negative bacteria.6 This disk (named SMA disk) is composed of two Kirby–Bauer disks containing ceftazidime and a filter disk containing sodium mercaptoacetate. By changing the shape of the growth inhibitory zone around the disk containing ceftazidime or imipenem, the presence or absence of metallo-β-lactamase-producing bacteria could be judged.
In 2000, the X-ray crystal structure of IMP-1 was determined (Fig. 1).7IMP-1 is composed of an αβ/βα fold and two distinct Zn(II) binding sites, which are located at the bottom of a wide shallow groove between the β-sheets. One of the Zn(II) ions, Zn1, is tetrahedrally coordinated by three histidine residues (His116, His118, and His196, according to BBL numbering scheme8). Another Zn(II) ion, Zn2, is trigonal-bipyramidally coordinated by Asp120, Cys221, His263, and a water molecule. In addition, the water molecule is bridged to the two Zn(II) ions (presumably, as a hydroxide ion), although the electron density of this water molecule was not observed in the X-ray crystal structure.
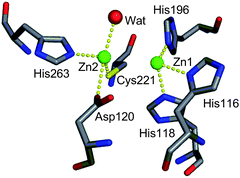 | ||
| Fig. 1 Schematic representation of the active site of IMP-1 from Pseudomonas aeruginosa (PDB code 1DDK). The Zn(II) atoms and a water molecule are shown as spheres. | ||
The relationship between the role of each metal ion in the active site and its catalytic activity in metallo-β-lactamases remains controversial. Unfortunately, spectroscopic studies to characterize the metal binding sites have been limited, due to the physicochemical properties of Zn(II). It would be beneficial to exchange Zn(II) with Co(II), which yields a structurally similar and catalytically active analog. One method to prepare Co(II)-substituted enzymes of metallo-β-lactamases is the direct addition of Co(II) to an apo-enzyme that is prepared by the use of chelators and dialysis. The preparation of apo- and Co(II)-substituted enzymes of BcII and CcrA metallo-β-lactamases has been successful.9–13 Although the preparation of the apo-enzyme of IMP-1 metallo-β-lactamase, apo-IMP-1, has been attempted, complete demetallation with chelators such as EDTA resulted in failure. Very recently, we established a preparation technique for apo-IMP-1 metallo-β-lactamase, which has restored enzyme activity upon addition of Zn(II) ions.14 Using this technique, we were able to investigate spectroscopic studies on Co(II)-substituted IMP-1.
In this paper, we investigated the metal preferences of Co(II) or Zn(II) for apo-IMP-1 and the spectral properties of mercaptoacetic acid with Co(II)-substituted IMP-1, using UV-visible spectroscopy.
Results and discussion
Metallo-β-lactamases are Zn(II)-dependent enzymes that hydrolyze most β-lactams, even in environments in which the Zn(II) concentration is extremely low. Analysis of the coordination environment around the Zn(II) active site is of great importance for elucidating the relationship between the role of individual Zn(II) ions and the catalytic mechanism of metallo-β-lactamases. Zn(II) ions are, however, “spectroscopically silent” d10 metal ions. Therefore, substitution of Zn(II) by Co(II) has become an essential technique to address the structural basis of catalytic properties in Zn(II) enzymes.15 From this standpoint, the preparation of an apo-enzyme is a crucial step.For metallo-β-lactamases, apo-enzymes of BcII and CcrA have been prepared and spectroscopic properties of their Co(II)-substituted enzymes were investigated.9–13 In IMP-1, however, apo-enzymes prepared by the dialysis method were not recovered perfectly after the addition of excess Zn(NO3)2,16,17 and the quest for the preparation of an apo-enzyme and metal substitution is ongoing.
Recently, we established a preparation method for apo-IMP-1 with a combination of the chelating agent, EDTA, and a desalting technique with a PD-10 column.14 As a result, upon addition of Zn(II) ions to the prepared apo-IMP-1, the enzyme activity could be recovered comparable to that observed with the native enzyme. Thus, this preparation allowed us to investigate the spectroscopic properties of Co(II)-substituted IMP-1.
Binding of Co(II) to apo-IMP-1
We previously carried out the spectrophotometric titration of apo-IMP-1 with Co(II) in 50 mM MOPS–NaOH (pH 7.0), 1.0 M NaCl, and 30% glycerol.14 The UV-visible spectral changes in 50 mM Tris–HCl (pH 7.4), 1.0 M NaCl, and 25% glycerol are shown in Fig. 2A. Note that the spectra were almost identical even when taken in the different buffer solutions. Successive addition of Co(II) to apo-IMP-1 exhibited an intense S− of Cys221 → Co(II) ligand-to-metal charge transfer (LMCT) band at 350 nm and d–d absorptions in the range of 500–650 nm. The assignment of the LMCT band at 350 nm is supported by disappearance of this band in the UV-visible spectra of the Co(II)-substituted mutants of IMP-1 and other metallo-β-lactamases,9,11,14 where the Cys221 ligand is replaced by Ala or Ser. Absorbances in both the LMCT and d–d bands continued to increase as Co(II) was added, reaching a plateau at 2 equiv. per apo-IMP-1. The spectrum of apo-IMP-1 containing 1.0 equiv. of Co(II) exhibited absorption maxima at 350, 520, 552, 612, and 635 nm (Fig. 2A). Judging from the increase in the absorbance of the LMCT band at 350 nm, Co(II) distributes between the two sites in the ratio of 2 in the Zn1 site to 1 in the Zn2 site. This result agrees with that for BcII reported by de Seny et al.11 Macroscopic dissociation constants for apo-IMP-1 with Co(II) were derived from spectral changes of the enzyme upon Co(II) binding, using the program DynaFit,18 and found to be KD1 < 60 nM and KD2 = 0.3 μM.14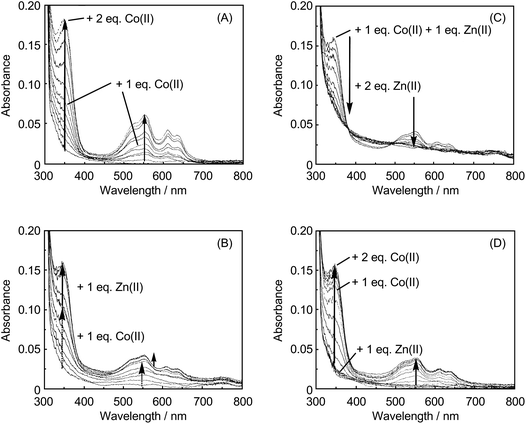 | ||
| Fig. 2 UV-visible spectral changes of apo-IMP-1 with varying concentrations of Co(II) or Zn(II). (A) Solid arrow: apo-IMP-1 (150 μM) in 50 mM Tris–HCl (pH 7.4), 1.0 M NaCl and 25% glycerol was titrated by 0.2 equiv. of Co(II) to a total of 2 equiv. (B) 1 equiv. of Co(II) in increments of 0.2 equiv. was added to apo-IMP-1 (178 μM) in 50 mM Tris–HCl (pH 7.4), 1.0 M NaCl, and 25% glycerol (dashed arrow), followed by the addition of Zn(II) (0.2 equiv. increments) up to 1 equiv. (solid arrow). (C) 2 equiv. of Zn(II) in increments of 0.2 equiv. was added to apo-IMP-1 containing 1 equiv. of Co(II) and 1 equiv. of Zn(II) (see final spectrum in (B)). (D) 1 equiv. of Zn(II) in increments of 0.2 equiv. was added to apo-IMP-1 (178 μM) in 50 mM Tris–HCl (pH 7.4), 1.0 M NaCl, and 25% glycerol, followed by the addition of Co(II) (0.2 equiv. increment) up to 2 equiv. (solid arrow). | ||
Binding selectivity of Co(II) and Zn(II) to the Zn1 and Zn2 sites in apo-IMP-1
Next, we investigated the preference of Co(II) and Zn(II) for two metal binding sites. Spectral changes of apo-IMP-1 upon successive addition of 1 equiv. of Co(II), followed by the addition of Zn(II) (by 0.2 equiv. increments for each metal) in 50 mM Tris–HCl (pH 7.4), 1.0 M NaCl, and 25% glycerol are shown in Fig. 2B. As described above, the spectrum of apo-IMP-1 containing 1 equiv. of Co(II) exhibited absorption maxima at 350, 520, 552, 612, and 635 nm. Addition of 1 equiv. of Zn(II) to apo-IMP-1 containing 1 equiv. of Co(II) resulted in the increase in the absorbance of the LMCT band at 350 nm, whereas those of the d–d bands in the visible range did not increase significantly with 1 equiv. of Zn(II) added. The increase in the LMCT band at 350 nm is indicative of Co(II) binding at the Zn2 site.9,11,13,14,19,20 Therefore, this spectral behaviour seems to indicate that Co(II) is pushed out from the Zn1 site with an occupation of Zn(II) at the Zn1 site and shifted to the Zn2 site. This result suggests that Zn(II) preferentially binds to the Zn1 site over the Zn2 site.When 2 equiv. of Zn(II) was added to apo-IMP-1 containing 1 equiv. of Co(II) and 1 equiv. of Zn(II), the absorption bands at 350 nm and 500–650 nm disappeared as the concentration of Zn(II) increased (Fig. 2C), suggesting that the replacement of Co(II) by Zn(II) takes place at both the Zn1 and the Zn2 sites, suggesting a higher preference of Zn(II) for both the Zn2 and Zn1 sites than Co(II).
Fig. 2D shows spectral changes of apo-IMP-1 upon successive addition of 1 equiv. of Zn(II), followed by the addition of 2 equiv. of Co(II) (by 0.2 equiv. increment for each metal) in 50 mM Tris–HCl (pH 7.4), 1.0 M NaCl, and 25% glycerol. No change in the spectrum of apo-IMP-1 was observed in the presence of 1 equiv. of Zn(II). The addition of Co(II) resulted in the increase in both the LMCT and d–d bands, where the final spectrum is consistent with that in Fig. 2B, suggesting that Co(II) binds to the Zn2 site. Interestingly, the extinction coefficients (ε) of the LMCT band at 350 nm are ca. 730 and ca. 900 M−1 cm−1, respectively, when 1 and 2 equiv. of Co(II) were added after addition of 1 equiv. of Zn(II) to apo-IMP-1. These values are smaller than that of apo-IMP-1 in the presence of 2 equiv. of Co(II) [Fig. 2A, ε = ca. 1200 M−1 cm−1, that is, Co(II) totally occupies the Zn2 site], suggesting that Zn(II) was already distributed between the Zn1 and Zn2 sites before addition of Co(II).
Preliminary differential scanning calorimetry measurements also showed enhancement in the denaturation temperature (Td) from 56.4 °C for apo-IMP-1 or 56.5 °C for mono-Zn(II)-IMP-1 [apo-IMP-1 plus 1 equiv. of Zn(II)] to 76.9 °C for native IMP-1.14 These analyses revealed that Zn1 contributes to the enzyme activity, whereas Zn2 plays an important role both in stabilizing the protein structure and in increasing catalytic efficiency of the enzyme.
Interaction of mercaptoacetic acid with Co(II)-substituted IMP-1
The coordination of the thiolate group in mercaptoacetic acid to a metal ion in the active site was followed by UV-visible spectroscopy of Co(II)-substituted IMP-1 prepared from apo-IMP-1 and 2 equiv. of Co(II) with mercaptoacetic acid. The spectral changes of Co(II)-substituted IMP-1 with mercaptoacetic acid in 50 mM Tris–HCl (pH 7.4), 1.0 M NaCl, and 25% glycerol are shown in Fig. 3. The addition of mercaptoacetic acid to Co(II)-substituted IMP-1 up to 2 equiv. resulted in a marked increase in the absorbance of the S−-to-Co(II) LMCT band at 350 nm and a shift of the d–d bands (555, 597, and 635 nm) with an increase in the absorbance (Fig. 3). The former feature is characteristic of the S− of mercaptoacetic acid → Co(II) LMCT band, implicating coordination of the thiolate group of mercaptoacetic acid to Co(II). Bicknell et al. prepared Co(II)-substituted angiotensin converting enzyme [Co(II)-ACE] and characterized the catalytic metal binding site both in Co(II)-ACE and in its inhibitor, captopril, by UV-visible spectroscopy.21 The visible spectrum of Co(II)-ACE exhibits a single broad maximum at 525 nm (ε = 75 M−1 cm−1).21 Upon addition of captopril to Co(II)-ACE, the spectrum of Co(II)-ACE–captopril complex displays peaks at 540 (ε = 350 M−1 cm−1), 618 (520), and 637 (560) nm, indicating that maxima at longer wavelength and the increase in the absorbance are changes of the d–d transition of Co(II) by inhibitor binding to the active-site metal. In IMP-1, the spectra of Co(II)-substituted IMP-1–mercaptoacetic acid have two features: (i) enhanced absorbance without change in wavelength and (ii) a change in absorption that appears to be a new absorption rather than a shift in an absorption band. This spectral change is similar to that of Co(II)-BcII–D-captopril complex.22![Spectrophotometric titration of Co(ii)-substituted IMP-1 with mercaptoacetic acid. Solid arrow: apo-IMP-1 (171 μM) in 50 mM Tris–HCl (pH 7.4), 1.0 M NaCl, and 25% glycerol was titrated by 0.2 equiv. of Co(ii) to a total of 2 equiv. Dashed arrow: 2 equiv. of mercaptoacetic acid in increments of 0.2 equiv. was added to Co(ii)-substituted IMP-1 [apo-IMP-1 containing 2 equiv. of Co(ii)]. Inset: plot of the absorbances at 350 nm (squares) and 600 nm (circles) as a function of the concentration of added mercaptoacetic acid. The absorbances of apo-IMP-1 and Co(ii)-substituted IMP-1 were subtracted from the absorbance of Co(ii)-substituted IMP-1 added mercaptoacetic acid. The solid lines represent fits obtained from numerical simulation of a one-step binding model to the data using the program Dynafit (BioKin, Ltd.).18 The apparent dissociation constant obtained was 14 μM.](/image/article/2011/MD/c1md00062d/c1md00062d-f3.gif) | ||
| Fig. 3 Spectrophotometric titration of Co(II)-substituted IMP-1 with mercaptoacetic acid. Solid arrow: apo-IMP-1 (171 μM) in 50 mM Tris–HCl (pH 7.4), 1.0 M NaCl, and 25% glycerol was titrated by 0.2 equiv. of Co(II) to a total of 2 equiv. Dashed arrow: 2 equiv. of mercaptoacetic acid in increments of 0.2 equiv. was added to Co(II)-substituted IMP-1 [apo-IMP-1 containing 2 equiv. of Co(II)]. Inset: plot of the absorbances at 350 nm (squares) and 600 nm (circles) as a function of the concentration of added mercaptoacetic acid. The absorbances of apo-IMP-1 and Co(II)-substituted IMP-1 were subtracted from the absorbance of Co(II)-substituted IMP-1 added mercaptoacetic acid. The solid lines represent fits obtained from numerical simulation of a one-step binding model to the data using the program Dynafit (BioKin, Ltd.).18 The apparent dissociation constant obtained was 14 μM. | ||
When changes in the absorbances at 350 and 650 nm were plotted as a function of the concentration of added mercaptoacetic acid, a plateau was observed for the [mercaptoacetic acid]/[Co(II)-substituted IMP-1] ratio above 1 (Fig. 3, inset), indicating that mercaptoacetic acid binds to Co(II)-substituted IMP-1 to form a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex. The apparent dissociation constant of mercaptoacetic acid with Co(II)-substituted IMP-1 was estimated by nonlinear least-squares fitting of the spectrophotometric titration data using the program DynaFit,18 and found to be KD = 14 μM. In control experiments, it could be ruled out that mercaptoacetic acid binds to a free Co(II) ion because the spectra of Co(II)SO4 in 50 mM Tris–HCl (pH 7.4), 1.0 M NaCl, and 25% glycerol are quite different from those of Co(II)-IMP-1–mercaptoacetic acid complex when mercaptoacetic acid is added from 0 to 2 equiv. (Fig. S1, ESI†).
1 complex. The apparent dissociation constant of mercaptoacetic acid with Co(II)-substituted IMP-1 was estimated by nonlinear least-squares fitting of the spectrophotometric titration data using the program DynaFit,18 and found to be KD = 14 μM. In control experiments, it could be ruled out that mercaptoacetic acid binds to a free Co(II) ion because the spectra of Co(II)SO4 in 50 mM Tris–HCl (pH 7.4), 1.0 M NaCl, and 25% glycerol are quite different from those of Co(II)-IMP-1–mercaptoacetic acid complex when mercaptoacetic acid is added from 0 to 2 equiv. (Fig. S1, ESI†).
Antony et al. applied the polarizable molecular mechanics method SIBFA to search for the most stable binding modes of captopril and thiomandelate inhibitors to a 104-residue model of CcrA metallo-β-lactamase.23,24 The most stably bound complex is a monodentate complex, in which S− bridges the two Zn(II) ions, with the carboxylate and carbonyl groups in captopril or the carboxylate group in thiomandelate interacting with the nearest residues around the dinuclear metal binding site. These are consistent with the X-ray crystal structures of BlaB metallo-β-lactamase complexed with D-captopril25 and IMP-1 or VIM-2 metallo-β-lactamase complexed with mercaptocarboxylate inhibitors.7,26
Considering the results of UV-visible spectroscopy of Co(II)-substituted IMP-1 with mercaptoacetic acid, molecular mechanics calculations, and X-ray crystallography, it was concluded that the thiolate group in mercaptoacetic acid bridges the two Co(II) ions in the active site (Fig. 4).
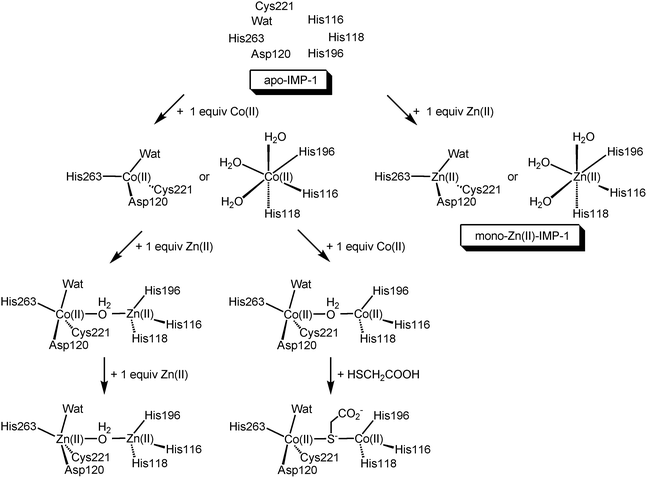 | ||
| Fig. 4 Proposed modes of reconstruction of IMP-1 from apo-IMP-1 by Zn(II) and Co(II) and the interaction of Co(II)-substituted IMP-1 with mercaptoacetic acid. | ||
Conclusions
In summary, we have investigated the metal preference of Co(II) and Zn(II) for dinuclear metal binding sites in apo-IMP-1 and the interaction of Co(II)-substituted IMP-1 with mercaptoacetic acid. We proposed models for the reconstruction of IMP-1 from apo-IMP-1 by Zn(II) and Co(II) and for the interaction of Co(II)-substituted IMP-1 with mercaptoacetic acid (Fig. 4). One equiv. of Co(II) or Zn(II) distributes between both the Zn1 and Zn2 sites. Metal binding modes for Co(II) and Zn(II) to apo-IMP-1 are similar to those found in BcII metallo-β-lactamase.11,13 Unlike IMP-1 and BcII metallo-β-lactamases, CcrA and Bla2 metallo-β-lactamases showed that Zn(II) binding to apo-enzyme was sequential.12,19Based on the results of UV-visible spectroscopy of Co(II)-substituted IMP-1 with mercaptoacetic acid, the inhibitory effect of mercaptoacetic acid using the double-disk method for detection of metallo-β-lactamase-producing Gram-negative bacteria is thought to arise from the coordination of the thiolate group in mercaptoacetic acid to two Zn(II) ions present in the active site of the metallo-β-lactamase.
Experimental
Chemicals
Antibiotics, cephaloridine from Shionogi & Co., Ltd. (Osaka, Japan) and ampicillin from Meiji Seika Co., Ltd. (Tokyo, Japan), were kindly donated. Cephalothin was purchased from Sigma-Aldrich Co., Ltd. (St Louis, USA). Disodium ethylenediaminetetraacetate (Na2EDTA), glycerol, and tris(hydroxymethyl)aminomethane (Tris) were purchased from Nacalai Tesque, Inc. (Kyoto, Japan). Mercaptoacetic acid was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). CoSO4·7H2O, ultrapure water, and 3-morpholinopropanesulfonic acid (MOPS) were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Zn(NO3)2·6H2O was purchased from Katayama Chemical Industries Co., Ltd. (Osaka, Japan). The other reagents were obtained commercially and were of the highest quality available.Preparation of enzyme
IMP-1 metallo-β-lactamase from Serratia marcescens was prepared from the extracts of Escherichia coliJM109, which harbored plasmid pSMBNU24 containing the gene, blaIMP-1, of S. marcescensTN9106, according to the published method.5,27E. coliJM109 cells were cultured in the Luria–Bertani medium containing 50 μg mL−1ampicillin at 37 °C for 13 h. The cells were collected by centrifugation (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g) at 4 °C for 15 min and resuspended in 50 mM phosphate buffer (pH 7.0) containing 2 μM Zn(NO3)2. A crude enzyme solution was obtained from the suspension by sonication followed by centrifugation (150
000 × g) at 4 °C for 15 min and resuspended in 50 mM phosphate buffer (pH 7.0) containing 2 μM Zn(NO3)2. A crude enzyme solution was obtained from the suspension by sonication followed by centrifugation (150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g) at 4 °C for 1 h. IMP-1 was purified by a SP Sepharose Fast Flow column (ϕ 26 mm × 10 cm; flow rate, 60 mL h−1; GE Healthcare UK Ltd., UK) using an eluent of 50 mM phosphate buffer containing 2 μM Zn(NO3)2 with a gradient of 0 to 0.4 M NaCl in 10 mL fractions and a Sephadex G-75 (ϕ 16 mm × 90 cm; flow rate, 12 mL h−1; GE Healthcare UK Ltd, Buckinghamshire, UK) with 50 mM Tris–HCl buffer (pH 7.4) containing 2 μM Zn(NO3)2. The fractions (3 mL each) that showed enzymatic activity were collected and concentrated by ultrafiltration (YM-10, Millipore Co., MA, USA). The purity of the enzyme was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; PAGEL, SPU-15S, ATTO, Tokyo, Japan) by comparison with protein molecular markers (Oriental Industries Co., Ltd., Tokyo, Japan). The concentration of the purified IMP-1 enzyme was determined by measuring the absorbance at 280 nm of enzyme preparation using an extinction coefficient of 4.9 × 104 M−1 cm−1.
000 × g) at 4 °C for 1 h. IMP-1 was purified by a SP Sepharose Fast Flow column (ϕ 26 mm × 10 cm; flow rate, 60 mL h−1; GE Healthcare UK Ltd., UK) using an eluent of 50 mM phosphate buffer containing 2 μM Zn(NO3)2 with a gradient of 0 to 0.4 M NaCl in 10 mL fractions and a Sephadex G-75 (ϕ 16 mm × 90 cm; flow rate, 12 mL h−1; GE Healthcare UK Ltd, Buckinghamshire, UK) with 50 mM Tris–HCl buffer (pH 7.4) containing 2 μM Zn(NO3)2. The fractions (3 mL each) that showed enzymatic activity were collected and concentrated by ultrafiltration (YM-10, Millipore Co., MA, USA). The purity of the enzyme was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; PAGEL, SPU-15S, ATTO, Tokyo, Japan) by comparison with protein molecular markers (Oriental Industries Co., Ltd., Tokyo, Japan). The concentration of the purified IMP-1 enzyme was determined by measuring the absorbance at 280 nm of enzyme preparation using an extinction coefficient of 4.9 × 104 M−1 cm−1.
β-Lactamase activity
Unless stated otherwise, a spectrophotometric method was used to measure the initial rate of consumption of a substrate, cephalothin or cephaloridine, by the enzyme.Preparation of apo-IMP-1
Apo-IMP-1 was prepared by a combination of EDTA and desalting column chromatography (PD-10, GE Healthcare UK Ltd., UK) according to our previously reported procedure.14In the spectrophotometric titration of Co(II)-substituted IMP-1 with mercaptoacetic acid, Apo-IMP-1 (171 μM) in 50 mM Tris–HCl (pH 7.4), 1.0 M NaCl, and 25% glycerol was titrated by the sequential addition of 0.2 equiv. of a mercaptoacetic acid stock solution prepared in 50 mM Tris–HCl (pH 7.4), 1.0 M NaCl, and 25% glycerol. UV-visible spectra were recorded at room temperature on a Shimadzu UV-2200 spectrophotometer (Kyoto, Japan), using 1 cm microcells. The interval of each titration was 5 min.
The apparent dissociation constant for mercaptoacetic acid with Co(II)-substituted IMP-1 was derived from spectral changes in absorbances at 350 and 650 nm with increasing concentration of mercaptoacetic acid, using the program DynaFit (BioKin, Ltd.).18
The following equilibrium was used for fitting.
Acknowledgements
Work related to the preparation and purification of enzyme and UV-visible spectroscopy was supported by the Ministry of Health, Labor, and Welfare of Japan (grant no. H21-Shinkou-Ippan-008). The DSC measurements were supported in part by a Grant-in-Aid for Scientific Research (B) (no. 183902038) and a Grant-in Aid for Scientific Research (C) (no. 21590116) from the Japanese Society for the Promotion of Science.References
- T. R. Walsh, M. A. Toleman, L. Poirel and P. Nordmann, Clin. Microbiol. Rev., 2005, 18, 306–325 CrossRef CAS.
- Y. Arakawa, M. Murakami, K. Suzuki, H. Ito, R. Wacharotayankun, S. Ohsuka, N. Kato and M. Ohta, Antimicrob. Agents Chemother., 1995, 39, 1612–1615 CAS.
- K. K. Kumarasamy, M. A. Toleman, T. R. Walsh, J. Bagaria, F. Butt, R. Balakrishnan, U. Chaudhary, M. Doumith, C. G. Giske, S. Irfan, P. Krishnan, A. V. Kumar, S. Maharjan, S. Mushtaq, T. Noorie, D. L. Paterson, A. Pearson, C. Perry, R. Pike, B. Rao, U. Ray, J. B. Sarma, M. Sharma, E. Sheridan, M. A. Thirunarayan, J. Turton, S. Upadhyay, M. Warner, W. Welfare, D. M. Livermore and N. Woodford, Lancet Infect. Dis., 2010, 10, 597–602 CrossRef CAS.
- R. C. Moellering, Jr, N. Engl. J. Med., 2010, 363, 2377–2379 Search PubMed.
- M. Goto, T. Takahashi, F. Yamashita, A. Koreeda, H. Mori, M. Ohta and Y. Arakawa, Biol. Pharm. Bull., 1997, 20, 1136–1140 CAS.
- Y. Arakawa, N. Shibata, K. Shibayama, H. Kurokawa, T. Yagi, H. Fujiwara and M. Goto, J. Clin. Microbiol., 2000, 38, 40–43 CAS.
- N. O. Concha, C. A. Janson, P. Rowling, S. Pearson, C. A. Cheever, B. P. Clarke, C. Lewis, M. Galleni, J.-M. Frère, D. J. Payne, J. H. Bateson and S. S. Abdel-Meguid, Biochemistry, 2000, 39, 4288–4298 CrossRef CAS.
- M. Galleni, J. Lamotte-Brasseur, G. M. Rossolini, J. Spencer, O. Dideberg, J.-M. Frère and The metallo-β-lactamase working group., Antimicrob. Agents Chemother., 2001, 45, 660–663 Search PubMed.
- M. W. Crowder, Z. Wang, S. L. Franklin, E. P. Zovinka and S. J. Benkovic, Biochemistry, 1996, 35, 12126–12132 CrossRef CAS.
- L. I. Llarrull, M. F. Tioni and A. J. Vila, J. Am. Chem. Soc., 2008, 130, 15842–15851 Search PubMed.
- D. de Seny, U. Heinz, S. Wommer, M. Kiefer, W. Meyer-Klaucke, M. Galleni, J.-M. Frère, R. Bauer and H. W. Adolph, J. Biol. Chem., 2001, 276, 45065–45078 Search PubMed.
- G. R. Periyannan, A. L. Costello, D. L. Tierney, K. W. Yang, B. Bennett and M. W. Crowder, Biochemistry, 2006, 45, 1313–1320 Search PubMed.
- L. I. Llarrull, M. F. Tioni, J. Kowalski, B. Bennett and A. J. Vila, J. Biol. Chem., 2007, 282, 30586–30595 Search PubMed.
- Y. Yamaguchi, S. Ding, E. Murakami, K. Imamura, S. Fuchigami, R. Hashiguchi, H. Mori, S. Suzuki, J. Wachino, Y. Arakawa and H. Kurosaki, 2011, submitted.
- W. Maret and B. L. Vallee, Methods Enzymol., 1993, 226, 52–71 CAS.
- S. Siemann, D. Brewer, A. J. Clarke, G. I. Dmitrienko, G. Lajoie and T. Viswanatha, Biochim. Biophys. Acta, 2002, 1571, 190–200 CrossRef CAS.
- S. Haruta, H. Yamaguchi, E. T. Yamamoto, Y. Eriguchi, M. Nukaga, K. O'Hara and T. Sawai, Antimicrob. Agents Chemother., 2000, 44, 2304–2309 Search PubMed.
- P. Kuzmič, Anal. Biochem., 1996, 237, 260–273 CrossRef CAS.
- M. J. Hawk, R. M. Breece, C. E. Hajdin, K. M. Bender, Z. Hu, A. L. Costello, B. Bennett, D. L. Tierney and M. W. Crowder, J. Am. Chem. Soc., 2009, 131, 10753–10762 Search PubMed.
- E. G. Orellano, J. E. Girardini, J. A. Cricco, E. A. Ceccarelli and A. J. Vila, Biochemistry, 1998, 37, 10173–10180 CrossRef CAS.
- R. Bicknell, B. Holmquist, F. S. Lee, M. T. Martin and J. F. Riordan, Biochemistry, 1987, 26, 7291–7297 CrossRef CAS.
- U. Heinz, R. Bauer, S. Wommer, W. Meyer-Klaucke, C. Papamichaels, J. Bateson and H. W. Adolph, J. Biol. Chem., 2003, 278, 20659–20666 CrossRef CAS.
- J. Antony, J. P. Piquemal and N. Gresh, J. Comput. Chem., 2005, 26, 1131–1147 Search PubMed.
- J. Antony, N. Gresh, L. Olsen, L. Hemmingsen, C. J. Schofield and R. Bauer, J. Comput. Chem., 2002, 23, 1281–1296 Search PubMed.
- I. García-Sáez, J. Hopkins, C. Papamicael, N. Franceschini, G. Amicosante, G. M. Rossolini, M. Galleni, J.-M. Frère and O. Dideberg, J. Biol. Chem., 2003, 278, 23868–23873 CrossRef CAS.
- Y. Yamaguchi, W. Jin, K. Matsunaga, S. Ikemizu, Y. Yamagata, J. Wachino, N. Shibata, Y. Arakawa and H. Kurosaki, J. Med. Chem., 2007, 50, 6647–6653 CrossRef CAS.
- E. Osano, Y. Arakawa, R. Wacharotayankun, M. Ohta, T. Horii, H. Ito, F. Yoshimura and N. Kato, Antimicrob. Agents Chemother., 1994, 38, 71–78 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: UV-visible spectral changes of Co(II) with varying concentrations of mercaptoacetic acid (Fig. S1). See DOI: 10.1039/c1md00062d |
| ‡ These authors contributed equally to the work. |
| § Present address: Department of Bacteriology, Nagoya University Graduate School of Medicine, 65 Tsurumai-cho, Showa-ku, Nagoya, Aichi 466–8550, Japan |
| This journal is © The Royal Society of Chemistry 2011 |