DOI:
10.1039/C0MD00227E
(Concise Article)
Med. Chem. Commun., 2011,
2, 726-730
Received
18th November 2010
, Accepted 20th May 2011
First published on 17th June 2011
Abstract
Diabetic retinopathy is the leading cause of blindness to working-age adults. Complications of diabetic retinopathy include pericyte loss, basement membrane thickening of capillaries, microaneurysm formation, and an increase in inflammatory marker levels. We have shown that stimulation of β-adrenergic receptors by β-adrenergic receptor agonists significantly reduces key inflammatory markers in retinal endothelial cells (REC) or Müller cells cultured in high COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundglucose, indicating that restoration of β-adrenergic receptor signaling may be protective to the retina. REC and Müller cells responded to increased glucose concentrations with an increase in inflammatory markers and apoptosis, which is reduced following treatment with β-adrenergic receptor agonists. In our initial studies, we evaluated the ability of optically active R-(−)-isoproterenol (R-1R-1), S-(+)-isoproterenol (S-1S-1S-1), and racemic mixture of (±)-isoproterenol (1), a non-selective β-adrenergic receptor agonist, to reduce the cleavage of caspase 3 and TNFα levels. We observed R-(−)-isoproterenol is more effective than S-(+)-isoproterenol, thus we developed novel optically active analogs of R-(−)-isoproterenol. Of the analogs, we found that compound 12 significantly reduced both caspase 3 and TNFα levels in REC, but did not reduce both markers in Müller cells. Binding data of compound 12 suggest that it is not a β-adrenergic receptor true agonist, but appears to decrease inflammatory levels and apoptosis in REC through an alternate mechanism.
1. Introduction
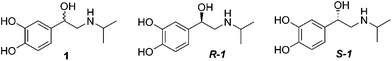
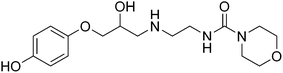
Diabetic retinopathy is the leading cause of blindness in Americans.1 Thickening of the basement membrane of the capillaries in the retina, loss of pericytes, and formation of microaneurysms characterize diabetic retinopathy.2 Although laser photocoagulation surgery is the primary treatment of the disease,3 there is still a need for additional therapeutic options. Müller cells and REC are involved in the pathogenesis of diabetic retinopathy, where Müller cells serve as supporting glial cells of the retina, and REC are decreased as the disease progresses, ultimately leading to blindness.4 We have previously shown that the loss of sympathetic neurotransmission (specifically β-adrenergic receptor signaling) leads to increased inflammatory marker levels and apoptosis of photoreceptors.5,6 We have also demonstrated that retinal Müller cells possess both β1- and β2-adrenergic receptors,7 while retinal endothelial cells possess β1- and β3-adrenergic receptors.8 Our results have shown that treatment with 10 μM (±)-isoproterenol (1) (Fig. 1), a non-selective β-adrenergic receptor agonist (Tocris, Ellisville, MO), or 10 μM (±)-xamoterol (2) (Fig. 2), a selective β1-adrenergic receptor agonist (Tocris, Ellisville, MO) on Müller and REC cells reduces apoptosis in both cell types via a reduction of cleaved caspase 3 levels in vitro.7,8 Because diabetic retinopathy has characteristics of inflammation,9 we have data indicating that TNFα levels are reduced after COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundisoproterenol treatment over an 8-month period.10 However, (±)-isoproterenol (1) produced remodeling of the left ventricle when administered topically, demonstrating negative responses of systemic exposure.11 We proposed to develop novel derivatives of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundisoproterenol, possessing a higher selectivity and more effective (at 1 mM vs. 50 mM for COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundisoproterenol) for retinal β-adrenergic receptors and to evaluate their ability to reduce the cleavage of caspase 3 (a marker of apoptosis) and TNFα levels (a marker of inflammatory marker levels) in the retina.
(±)-Isoproterenol has one asymmetric center and exists as enantiomers. Although two optically active enantiomers have same achiral physical properties in general, discrepancies in physiological activities are quite often observed between the two enantiomers. Therefore, the development of a single enantiomeric compound instead of racemic mixtures is strongly encouraged in new drug development. A number of groups also have reported that β-adrenergic receptor activation is stereoselective.12 Birnbaum et al.13 measured the increase in atrial rate as well as COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcAMP rise after treatment with R-(−)-, or S-(+)-isoproterenol. In rat uteri, maximally simulated levels of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcAMP were 52 ± 3.8 and 40.5 ± 4.5 pmol mg−1 of protein by R-(−)- and S-(+)-isoproterenol, respectively. COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDithiothreitol, which selectively reduces sulfhydryl groups of the receptors presumably altering conformational changes of β-adrenoreceptor, clearly reduces atrial rate after treatment with S-(+)-norepinephrine over that of R-(−)-norepinephrine.14
2. Synthesis
We carried out an enantioselective synthesis of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcatechol amines by using the Sharpless asymmetric dihydroxylation. Kumar et al.15 reported an efficient asymmetric synthesis of enantiomerically single COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound(R)-isoprenaline, (R)-norfluoxetine and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound(R)-fluoxetine using Sharpless asymmetric dihydroxylation. Using this approach, S-(+)-isoproterenol (S-1S-1S-1) was synthesized. We observed S-1S-1S-1 to be significantly less potent than the corresponding R-1R-1. We then synthesized compounds 10, 11 and 12 starting from 3,4-dibenzyloxybenzaldehyde 3 as depicted in Scheme 1. We subjected 3 to Wittig olefination followed by asymmetric dihydroxylation under Sharpless asymmetric dihydroxylation conditions to give diol 5. Tosylation of primary hydroxyl group was carried out with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundtosyl chloride using a catalytic amount of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compounddibutyltin oxide to afford 6. Primary tosyl compound 6 was used as a key intermediate for 10, 11 and 12. The tosylate 6 was treated with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsodium azide followed by reduction using COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundlithium aluminium hydride to incorporate amine functionality and resulting amine was treated with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundmorpholine-4-carbonyl chloride followed by debenzylation to afford 10. Nucleophilic substitution of 6 with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound3-amino propyl morpholine followed by deprotection of benzyl groups using H2/Pd(OH)2 and HCl salt formulations yielded 11. Ethylene diamine was used to treat 6 followed by addition of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundmorpholine-4-carbonyl chloride and the resulting tertiary amine was debenzylated to yield 12.
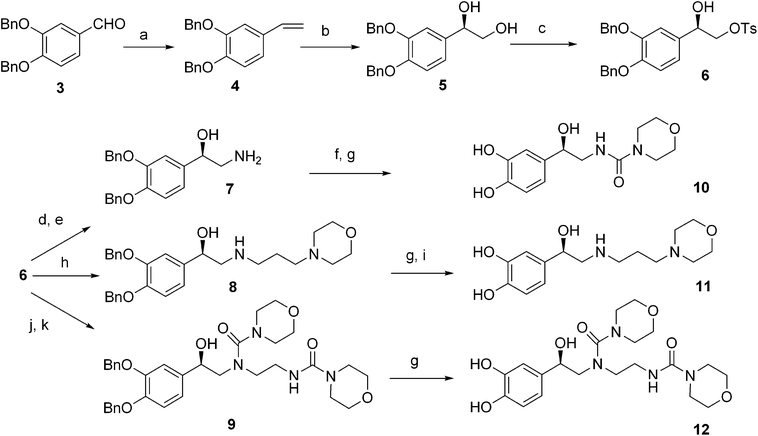 |
| | Scheme 1 (a) PPh3CH3Br, KOtBu, dry THF, 96%; (b) (DHQD)2PHAL, K3Fe(CN)6, K2CO3, t-BuOH–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH2O, OsO4, 0 °C, 48 h, 90%; (c) COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compounddibutyltin oxide (0.2 mol%), TsCl, NEt3, CH2Cl2, rt, 2 h, 95%; (d) NaN3, DMF, reflux, 12 h, 85%; (e) LiAlH4, THF, reflux, 2 h, 75%; (f) COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundmorpholine-4-carbonyl chloride, rt, 12 h; (g) H2/Pd(OH)2, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundMeOH, 12 h; (h) COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound3-amino propyl morpholine, rt, 12 h, 72%; (i) 2.0 M HCl in ether; (j) ethylene diamine, rt, 12 h, 50%; (k) COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundmorpholine-4-carbonyl chloride, rt, 12 h, 66%. | |
2.1 Biology
Retinal
endothelial cells (REC) were purchased from Cell Systems Inc. (Kirkland, WA) and cultured in CSC media grown in high COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundglucose (25 mM). Media was supplemented with 20% FBS and antibiotics. Rat Müller cells (rMC-1, kindly provided by Dr Vijay Sarthy, Northwestern University) were cultured in high COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundglucose (25 mM) in DMEM medium grown and supplemented with 10% FBS and antibiotics. Twenty-four hours before treatment with compounds R-1R-1, S-1S-1S-1, 10, 11, and 12, Müller cells and REC were cultured in high COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundglucose medium with antibiotics and 0% FBS to induce serum starvation. Four dishes of each cell type were cultured in only high COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundglucose to serve as non-treated controls. Four dishes from each cell type for each dosage were cultured in high COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundglucose and treated with 50 nM, 100 nM, and 10 μM dosages of each of the 5 compounds. REC were treated for 30 and 60 minutes, while Müller cells were treated for1 h and 24 h for cells; these times were based on data with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundisoproterenol.7,8Retinal lysates from Müller and REC were assayed using a human TNFα ELISA kit (Thermo Scientific) and cleaved caspase 3 ELISA kit (Cell Signaling).
3. Results
Apoptosis of retinal endothelial cells is a factor in the early phases of diabetic retinopathy. Therefore, we used decreased cleavage of caspase 3 as a marker of reduced apoptosis in cells cultured in high COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundglucose. Compounds R-1R-1, 11, and 12 significantly decreased caspase 3 levels in REC (Fig. 3), while 10 and S-1S-1S-1 did not significantly decrease caspase 3 levels after treatment. Specifically, 12 significantly reduced caspase 3 levels at the 50 nM and 100 nM dosages within the 30 and 60 minute time points, which is lower than (±)-isoproterenol. On the other hand, cleaved caspase 3 levels in Müller cells (Fig. 5) were not significantly decreased after treatment with R-1R-1, S-1S-1S-1, 10, 11, or 12.
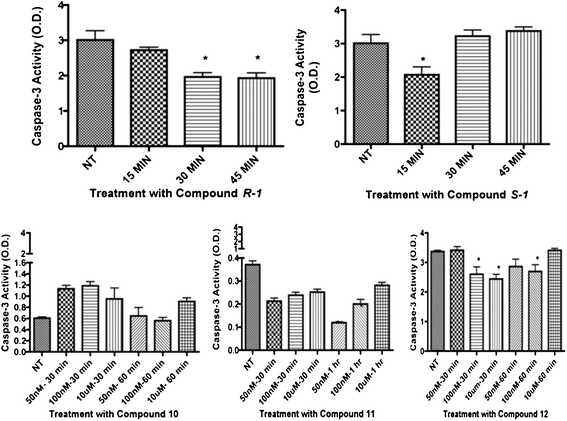 |
| | Fig. 3 Cleaved caspase 3 results in REC for compounds R-1R-1, 10, 11, and 12. Graphs show results of caspase 3 ELISA. For isomers of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundisoproterenol, cells were treated at 10 μM at 15, 30, and 45 minutes. Analogue compounds 11 and 12 were treated at 50 nM, 100 nM, and 10 μM at 30 minutes and 1 hour time points. Treatment groups were compared to not-treated (NT) controls. *P < 0.05 vs. NT. | |
To evaluate inflammatory marker levels in culture, TNFα levels were evaluated. We found that 12 decreased both TNFα and caspase 3 levels in REC while 10 increased TNFα levels (Fig. 4). TNFα levels in REC were unchanged after treatment with R-1R-1, S-1S-1S-1, and 11 (not shown). The non-specific β-adrenergic receptor agonist R-1R-1 and S-1S-1S-1 isomers decreased TNFα inflammatory levels in Müller cells (Fig. 6), consistent with preliminary results from (±)-isoproterenol; however, R-1R-1 decreased TNFα at the 50 nM and 100 nM dosages, which is lower than the effective dose of (±)-isoproterenol. All analogs failed to significantly decrease TNFα in Müller cells. Based upon these results, 12 was shown to significantly decrease both caspase 3 and TNFα in REC but not in Müller cells.
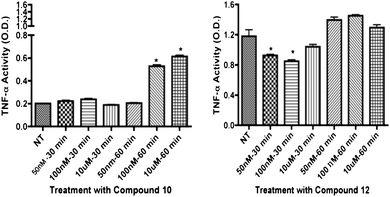 |
| | Fig. 4
TNFα activity results in REC for compounds 10 and 12. Analogue compounds 10 and 12 were treated at 50 nM, 100 nM, and 10 μM at 30 minutes and 1 hour time points. Treatment groups were compared to not-treated (NT) controls. *P < 0.05 vs. NT. | |
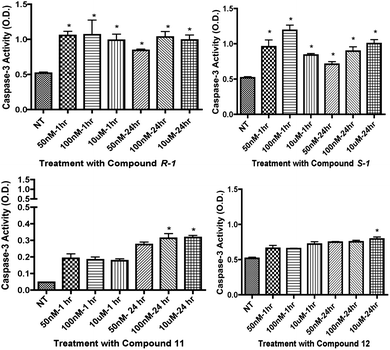 |
| | Fig. 5 Cleaved caspase 3 results in Müller cells for compounds R-1R-1, S-1S-1S-1, 11, and 12. Compounds R-1R-1, S-1S-1S-1, 11 and 12 were treated at 50 nM, 100 nM, and 10 μM at 1 and 24 hour time points. Treatment groups were compared to not-treated (NT) controls. *P < 0.05 vs. NT. | |
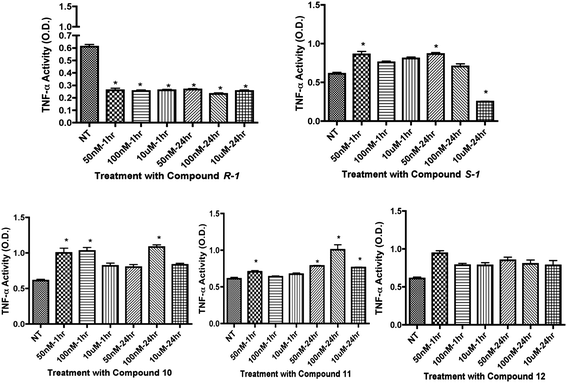 |
| | Fig. 6
TNFα activity results in Müller cells for compounds R-1R-1, S-1S-1S-1, 10, 11 and 12. Analogue compounds R-1R-1, S-1S-1S-1, 10, 11, and 12 were treated at 50 nM, 100 nM, and 10 μM at 1 and 24 hour time points. Treatment groups were compared to not-treated (NT) controls. *P < 0.05 vs. NT. | |
3.1 Discussion
Screening of our novel compounds showed diverse receptor activity between REC and Müller cells. Preliminary experiments used (±)-isoproterenol, and showed it to be effective at reducing caspase 3 in both cell types and TNFα in REC. We observed that R-1R-1 was shown to significantly reduce caspase 3 levels in REC and in Müller cells, while S-1S-1S-1 did not affect caspase 3 or TNFα activity in REC. S-1S-1S-1 did show a decrease in TNFα activity in Müller cells but R-1R-1 was observed to be more effective, as it decreased TNFα levels at lower dosages. Identifying the R-isomer as the more active of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundisoproterenol allowed for formulation of more specific derivatives of the R-isomer. It has been reported that (±)-xamoterol, a selective β1-adrenergic receptor agonist, is able to significantly reduce caspase 3 and TNFα levels in REC.7 It has been reported that some β2-adrenergic receptor selectivity needs to be maintained to stimulate Müller cells.8 We hypothesized that the morpholine moiety accounts for xamoterol's high selectivity for β1-adrenergic receptors, and we therefore designed structural catecholamine derivatives of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundisoproterenol and attached the morpholine moiety to test the ability of these analogues to reduce caspase 3 and TNFα activity. The structural analogues, 10, 11, and 12 had various effects in both cell types. We observed that 10 was not able to significantly decrease caspase 3 or TNFα in REC or Müller cells. Compound 11 was able to decrease caspase 3 levels in REC but was not effective in Müller cells, while 12 was able to decrease both caspase 3 and TNFα in REC. Compound 12 effectively decreased both inflammatory and apoptotic levels in REC and a significant decrease was not observed in Müller cells. Since analogue 12 is more potent on REC pirmary cells, we performed β1-adrenergic receptor binding studies on compound 12, which showed that 12 does not bind to the β1-adrenergic receptor. It is interesting that it must be working by a different mechanism of action. This observation stems from work demonstrating that REC possess only β1- and β3-adrenergic,16 while Müller cells have β1- and β2-adrenergic receptors. These findings suggest that continued synthesis of (−)-isoproterenol (R-1R-1) analogues will present new drug candidates able to reduce apoptotic and inflammatory markers, making them useful therapeutic agents in the treatment of diabetic retinopathy.
4. Conclusion
In this paper we have shown that single enantiomeric R-1R-1 is more potent than S-1S-1S-1 isomer of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundisoproterenol. Due to its effectiveness over S-1S-1S-1, the analogues of R-1R-1 were formulated and 12 was the only analogue to significantly decrease both apoptotic and inflammatory levels in REC cellsin vitro. In addition to the findings on 12, the data using R-1R-1 also suggest that there is a separation of the responsiveness to specific classes of β-adrenergic receptors. REC possess β1- and β3-adrenergic receptors, while Müller cells possess β1- and β2-adrenergic receptors, where the β1- and β2-adrenergic receptor is the dominant type, respectively. These findings suggest that 12 is the most effective analogue in its ability to decrease both inflammatory and apoptotic levels in vitro in REC. This decrease was observed at a lower dosage than the effective dose of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundisoproterenol and it did not bind to β-adrenergic receptors which indicates a different mechanism for 12 as compared to R-1R-1.
Acknowledgements
This work is supported by a Career Development Award from JDRF 2-2006-114 (JJS), JDRF Translational Award 17-2008-1044 (JJS), the William and Mary Greve Special Scholars Award from Research to Prevent Blindness and a departmental award from the Research to Prevent Blindness (Dr Barrett Haik, chair), NEI Vision Core Grant: PHS 3P30 EY013080 (PI: Dianna Johnson). Williams-Guy and Pagadala contributed equally to this work.
References
- R. N. Frank, Diabetic retinopathy, N. Engl. J. Med., 2004, 350, 48 CrossRef CAS.
- L. A. Wiley, G. R. Rupp and J. J. Steinle, Invest. Ophthalmol. Visual Sci., 2005, 46, 744 Search PubMed.
- A. M. El-Asrar, H. S. Al-Mezaine and M. S. Ola, Curr. Opin. Ophthalmol., 2009, 20, 532 CrossRef.
- K. P. Williams and J. J. Steinle, Exp. Eye Res., 2009, 88, 1014 CrossRef.
- J. J. Steinle, N. L. Lindsay and B. L. Lashbrook, Auton. Neurosci., 2005, 120, 46 CrossRef.
- J. J. Steinle, Exp. Eye Res., 2007, 84, 118 CrossRef CAS.
- K. P. Williams and J. J. Steinle, Exp. Eye Res., 2009, 4, 448 CrossRef.
- R. Walker and J. J. Steinle, Invest. Ophthalmol. Visual Sci., 2007, 48, 5276 Search PubMed.
- A. M. Joussen, FASEB J., 2004, 18, 1450 CAS.
- J. Youde, R. J. Walker, T. S. Kern and J. J. Steinle, Exp. Eye Res., 2010, 2, 71 Search PubMed.
- L. C. Heather, A. F. Catchpole, D. J. Stuckey, M. A. Cole, C. A. Carr and K. Clarke, J. Physiol. Pharmacol., 2009, 3, 31 Search PubMed.
- P. N. Patil, C. Li, V. Kumari and J. P. Hieble, Chirality, 2008, 20, 529 CrossRef CAS.
- J. E. Birnbaum, P. W. Abel, G. L. Amidon and C. K. Buckner, J. Pharmacol. Exp. Ther., 1975, 194, 396 CAS.
- L. DeSantis, D. R. Feller and P. N. Patil, Eur. J. Pharmacol., 1974, 28, 302 CrossRef CAS.
- P. Kumar, R. Kumar Upadhyay and R. K. Pandey, Tetrahedron: Asymmetry, 2004, 15, 3955 CrossRef CAS.
- J. J. Steinle, G. W. Booz, C. J. Meininger, J. N. E. Day and H. J. Granger, J. Biol. Chem., 2003, 278, 20681 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c0md00227e |
| ‡ Current address: Department of Biochemistry, Faculty of Pharmacy, Cairo University, Cairo 11562, Egypt |
|
| This journal is © The Royal Society of Chemistry 2011 |
Click here to see how this site uses Cookies. View our privacy policy here.