Synthesis and biological evaluation of novel 1-aryl, 5-(phenoxy-substituted)aryl-1,4-pentadien-3-one derivatives†
Kai
Yuan
,
Baoan
Song
*,
Linhong
Jin
,
Shuai
Xu
,
Deyu
Hu
,
Xiaoqing
Xu
and
Song
Yang
*
State Key Laboratory Breeding Base of Green Pesticide and Agricultural Bioengineering, Key Laboratory of Green Pesticide and Agricultural Bioengineering, Ministry of Education, Guizhou University, Guiyang, 550025, China. E-mail: songbaoan22@yahoo.com
First published on 14th April 2011
Abstract
A series of 1-aryl, 5-(phenoxy-substituted)aryl-1,4-pentadien-3-one derivatives were synthesized and evaluated for anticancer activity. Amongst the synthesized ethers, 4A and 4Y exhibited substantial antiproliferative activity with IC50 values ranging from 6.6–8.6 μmol L−1 against a variety of human cancer cell lines. Preliminarily mechanism of antitumor action of 4A by DNA Ladder, Annexin V/PI double staining and related studies indicated growth inhibition of PC3, BGC-823 and Bcap-37 cells by induction of tumor cells apoptosis.
Introduction
Curcumin is the principal curcuminoid of the popular Indian spice turmeric, which is a member of the ginger family. Curcumin and its derivatives have extensive bioactivities such as bactericidal,1 antioxidative,2 anti-inflammatary3 and anti-HIV4 (Fig. 1). They are particularly known for their ability to resist mutation and raise anticancer activity.5 The research in this area indicates that curcumin derivatives can induce apoptosis, e.g. carcinoma of colorectal, renal and hepatocarcinoma cancer cells.6 Great interest has been devoted to the synthesis of new curcumin analogues exhibiting enhanced biological properties, e.g. the novel ferrocenyl curcuminoids were synthesized by covalent anchorage of three different ferrocenyl ligands. They were more effective in inhibiting B16 melanoma cells proliferation in vitro (Fig. 1).7 Structurally, the new analogues are symmetrical 1,5-diarylpentadienones whose aromatic rings possess alkoxy substituents at 3 and 5 positions. Analysis of the effects of the analogues on the expression of cancer-related genes indicated that some of them could induce down-regulation of β-catenin, Ki-ras, cyclin D1, c-Myc, and ErbB-2 at as low as one eighth of the concentration at which curcumin normally exerts an effect.8 Shibata's group reported some newly synthesized curcumin analogs containing methoxymethyl group (compound A) having improved potential to prevent colorectal carcinogenesis in vivo,9 whereas Hiroyuki's group obtained similar class of compounds by studying structure–activity relationship of C5-curcuminoids Ai-ii.10 | ||
| Fig. 1 Organic curcumin derivatives and the target compounds 4A and 4Y. | ||
In an attempt to obtain more potent compounds without disturbing the core structure of curcumin to keep the medicinal properties and safety profile intact, minute structural variations were made by omitting the active methylene group and one carbonyl group leading to 1,4-pentadiene-3-ones to yield new structures which still displayed antioxidative properties. A series of diphenyl-1,4-pentadiene-3-ones, together with cyclopentanone and cyclohexanone analogues were prepared and tested for inhibition of lipid peroxidation by Sardjiman's group.11 Similarly, Adams's group have reported 3,5-bis(2-flurobenzylidene)piperidin-4-one C with better antimour activity but lower toxicity than curcumin.12 Furthermore, W. M. Weber, et al. obtained curcumin and related enones which inhibited TNFα-induced activation of NFκB thereby contributing to the development of pro-survival and anti-apoptotic state. Among these compounds, 1,5-bis(3-pyridyl) -1, 4-pentadien-3-one has been proved to be the most effective one with an IC50 value of 3.4 ± 0.2 μM.13 Nevertheless, to the best of our knowledge, there has been no report on the inhibition of PC3, BGC-823 and Bcap-37 cells with 1,5-diarylpentadien-3-one derivatives bearing a phenolic ether pharmacophore. This prompted us to study the new class of compounds 4 and investigate their preliminarily mechanism of action as potent anticancer agents.
Results and discussion
Chemistry
The key intermediate 3 was synthesized starting from the 4-(2-hydroxyphenyl)-3-butene-2-ones or 4-(4-hydroxyphenyl)-3-butene-2-one 2 with substituted benzaldehyde, water and ethanol in water at 5–10 °C, followed by dropwise addition of NaOH solution. The mixture was stirred at 5–10 °C for 3 h, then stirred at room temperature for 7 h, with carbon dioxide in solution about 45 min. The resulting precipitate was filtered and washed with water, dried. The solid was purified by thin layer chromatography with silica gel (ethyl acetate/dichloromethane/petroleum ether, V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V
V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V = 2
V = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford intermediates 3. The target compounds 4A–4AF were synthesized by reaction of halogenated hydrocarbons with intermediates 3, in the presence of K2CO3 and KI in refluxing acetone for 2 h, as can be seen in Scheme 1. The solvent was then removed under reduced pressure and the crude yellow products were purified by recrystallization using acetone/petroleum ether (V
1) to afford intermediates 3. The target compounds 4A–4AF were synthesized by reaction of halogenated hydrocarbons with intermediates 3, in the presence of K2CO3 and KI in refluxing acetone for 2 h, as can be seen in Scheme 1. The solvent was then removed under reduced pressure and the crude yellow products were purified by recrystallization using acetone/petroleum ether (V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V = 1
V = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to give the target compounds 4A–4AF.
2) to give the target compounds 4A–4AF.
 | ||
| Scheme 1 Synthetic route to the title compounds. Reagents and conditions: (a) NaOH, EtOH–H2O, r.t. 11 h, 76%; (b) gently bubbling CO2, r.t., 45 min, 74%; (c) substituted benzaldehyde, NaOH, EtOH–H2O, 5–10 °C for 3h, r.t. for 7 h; (d) gently bubbling CO2, r.t., 45 min, 51–58% for two steps; (e) RX, KI/K2CO3/acetone, 58 °C, 2 h, 61–81%. | ||
Biological evaluation
| Compda | IC50 (μM)b | ||
|---|---|---|---|
| PC3c | Bcap37d | BGC823e | |
| a These compounds were tested as the free base. b IC50 concentrations needed to inhibit cell growth by 50% as determined from the dose response curve. Determination was done in three separate experiments and each was performed in triplicate. c Prostate cancer. d Breast cancer. e Stomach cancer. f ADM (adriamycin) was used as positive control. g The value was determined through MTT assay. | |||
| 4A | 7.1 ± 0.5 | 7.3 ± 0.1 | 7.2 ± 0.1 |
| 4Y | 8.0 ± 0.3 | 8.6 ± 0.3 | 6.6 ± 0.2 |
| ADMf | 1.4g ± 0.1 | 2.0 ± 0.3 | 2.6 ± 0.4 |
 | ||
| Fig. 2 The AO/EB staining of compound 4A on PC3 cells for 72 h. | ||
It can be seen from Fig. 2 that the cells treated with 4A changed little, the nucleus was green and the morphous was in the form of pycnosis. The cells presented apoptotic morphous. Little red cells indicated that 4a was associated with low cytotoxicity. Therefore, we concluded that 4A could induce apoptosis without any significant cytotoxicity.
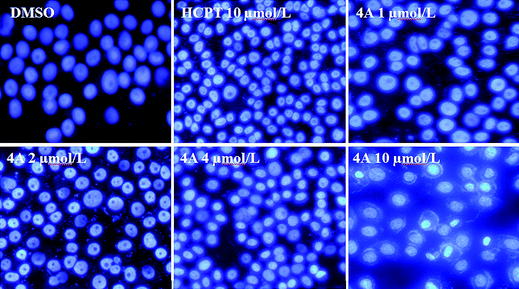 | ||
| Fig. 3 The Hoechst 33258 staining of compound 4A on PC3 cells for 72 h. | ||
It can be seen from Fig. 3 that cells of the negative group were normal blue. Nucleus of cells at high concentration group and HCPT group appeared compact and condensed. The cells exhibited strong blue fluorescence and revealed typical apoptotic morphology. Therefore, it appears that 4A induced apoptosis against PC3 cells. The results were identical with the previous AO/EB double staining.
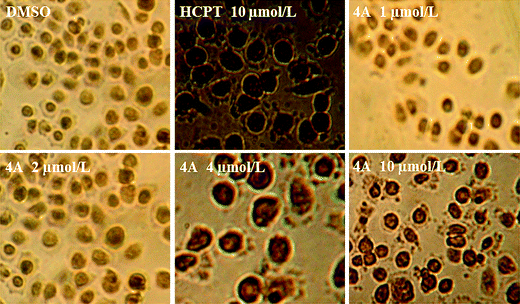 | ||
| Fig. 4 The TUNEL staining of compound 4A on PC3 cells for 48 h. | ||
It can be seen from Fig. 4 that cells of the negative group do not appear as brown precipitate. The cells at high concentration group and HCPT group appeared as brown precipitate. Therefore, we further concluded that 4A induced apoptosis against PC3 cells. The results were identical with the previous experiment.
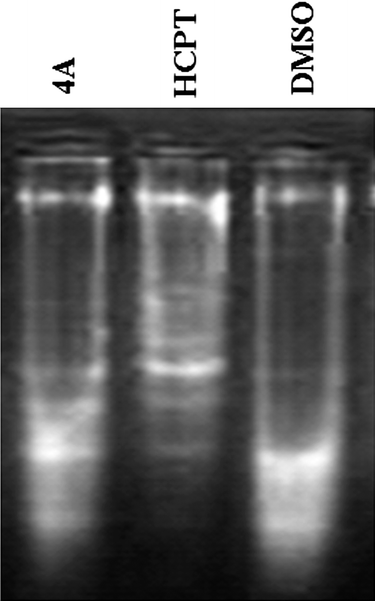
It can be seen from Fig. 5 that cells of the negative group did not appear as DNA Ladder. The cells in compound 4A and HCPT group appeared as typical DNA Ladder. Therefore, it again leads to the fact that 4A induced apoptosis against PC3 cells. The results are similar to the previous experiments.
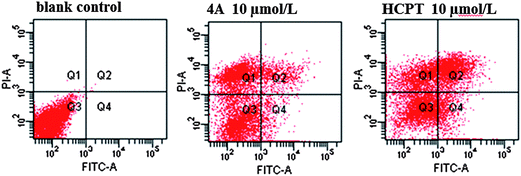 | ||
| Fig. 6 The scatter diagram of FCM tested apoptosis. | ||
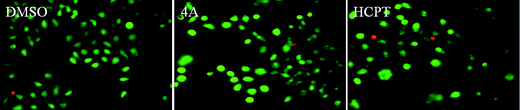
It is evident from Fig. 6 that the second and forth quadrant of 4A and HCPT group presented positive results. These observations demonstrated that 4A and HCPT group exhibited simultaneously early and late apoptotic cells against PC3 cells for 72 h after being treated with the compound. This is in line with our assumption that 4A induced apoptosis against PC3 cells. The results were identical with the previous finding. The effects of action time and concentration of 4A against PC3 cells are provided in Fig. 7.
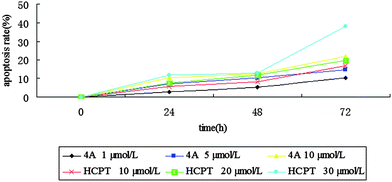 | ||
| Fig. 7 Effects of concentration of 4A on apoptosis rate. | ||
It can be seen from Fig. 7 that the apoptosis rate increased with the action time and concentration of 4A. Moreover, the apoptosis rate changed in a dose-dependent manner. The highest rate of apoptosis was 21.9% when the cells were treated with 4A at the concentration of 10 μmol L−1 for 72 h. Thus, 4A induced apoptosis in a dose-dependent manner.
Conclusion
In summary, we prepared a series of novel 1, 5-diaryl-1, 4-pentadien-3-one derivatives containing phenolic ether moieties as potential anticancer agents. The results show that in vitro inhibitory activities IC50 of 4A and 4Y were 7.1 μmol L−1 and 8.0μmol L−1, 7.3 μmol L−1 and 8.6μmol L−1, 7.2μmol L−1 and 6.6 μmol L−1 against PC3, BGC-823 and Bcap-37 cells, respectively. It shows that 4A and 4Y has high inhibitory activity and obvious dose dependent relationship on the three cancer cells.The results also show that 4A induces apoptosis against PC3 cells as detected by AO/BO staining, Agarose Gel Electrophoresis and FCM. To conclude, we found that 4A possesses high antitumor activity and can inhibit the growth of tumor cells by inducing tumor cells apoptosis, the effect of which largely depends on the concentration of 4A. Further studies on the mechanism of interaction need to be conducted in the future.
Acknowledgements
The authors wish to thank the National Key Program for Basic Research (Nos. 2010CB126105, 2010CB134504), and the National Natural Science Foundation of China (Nos. 20872021, 20662004) for the financial support.References
- S. Mishra, U. Narain, R. Mishra and K. Misra, Bioorg. Med. Chem., 2005, 13, 1477 Search PubMed.
- S. Venkateswarlu, M. S. Ramachandra and G. V. Subbaraju, Bioorg. Med. Chem., 2005, 13, 6374 CrossRef CAS.
- C. Selvam, S. M. Jachak, T. R. Thilagavathi and A. K. Chakraborti, Bioorg. Med. Chem. Lett., 2005, 15, 1793 CrossRef CAS.
- R. Costi, R. D. Santo, M. Artico, S. Massa, R. Ragno, R. Loddo, M. La Colla, E. Tramontano, P. La Colla and A. Pani, Bioorg. Med. Chem., 2004, 12, 199 CrossRef CAS.
- L. Lin, Q. Shi, C. Y. Su, C. C. Shih and K. H. Lee, Bioorg. Med. Chem., 2006, 14, 2527 CrossRef CAS.
- M. C. Jiang, H. F. Yang-Yen, J. J. Yen and J. K. Lin, Nutr. Cancer, 1996, 26, 111 Search PubMed.
- A. Arezki, G. G. Chabot, L. Quentin, D. Scherman, G. Jaouen and E. Brul, Med. Chem. Commun., 2011, 2, 190, 10.1039/c0md00231c , in press.
- H. Ohori, H. Yamakoshi, M. Tomizawa, M. Shibuya, Y. Kakudo, A. Takahashi, S. Takahashi, S. Kato, T. Suzuki, C. Ishioka, Y. Iwabuchi and H. Shibata, Mol. Cancer Ther., 2006, 5, 2563 CrossRef CAS.
- H. Shibata, H. Yamakoshi, A. Sato, H. Ohori, Y. Kakudo, C. Kudo, Y. Takahashi, M. Watanabe, H. Takano, C. Ishioka, T. Noda and Y. Iwabuchi, Cancer Sci., 2009, 100, 956 Search PubMed.
- Y. Hiroyuki, O. Hisatsugu, K. Chieko and S. Atsuko, Bioorg. Med. Chem., 2010, 18, 1083 Search PubMed.
- S. S. Sardjiman, M. S. Reksohadiprodjo, H. Hakim, H. V. D. Goot and H. Timmerman, Eur. J. Med. Chem., 1997, 32, 625 Search PubMed.
- B. K. Adams, E. M. Ferstl, M. C. Davis, M. Herold, S. Kurtkaya, R. F. Camalier, M. G. Hollingshead, G. Kaur, E. A. Sausville, F. R. Rickles, J. P. Snyder, D. C. Liotta and M. Shoji, Bioorg. Med. Chem., 2004, 12, 3871 Search PubMed.
- W. M. Weber, L. A. Hunsaker, C. N. Roybal, E. V. Bobrovnikova-Marjon, S. F. Abcouwer, R. E. Royer, L. M. Decka and D. L. Vander Jagtb, Bioorg. Med. Chem., 2006, 14, 2450 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c1md00038a |
| This journal is © The Royal Society of Chemistry 2011 |