Phenyldiazenyl benzothiazole derivatives as probes for in vivo imaging of neurofibrillary tangles in Alzheimer's disease brains†
Kenji
Matsumura
a,
Masahiro
Ono
*a,
Shun
Hayashi
a,
Hiroyuki
Kimura
a,
Yoko
Okamoto
b,
Masafumi
Ihara
b,
Ryosuke
Takahashi
b,
Hiroshi
Mori
c and
Hideo
Saji
*a
aGraduate School of Pharmaceutical Sciences, Kyoto University, 46-29 Yoshida Shimoadachi-cho, Sakyo-ku, Kyoto, 606-8501, Japan. E-mail: ono@pharm.kyoto-u.ac.jp; Tel: +81-75-753-4556; Fax: +81-75-753-4568; hsaji@pharm.kyoto-u.ac.jp; Fax: +81-75-753-4568; Tel: +81-75-753-4608
bGraduate School of Medicine, Kyoto University, 54 Shogoin-kawaharacho, Sakyo-ku, Kyoto, 606-8507, Japan
cDepartment of Neuroscience, Osaka City University Medical School, 1-4-3 Asahi-machi, Abeno-ku, Osaka, 545-8585, Japan
First published on 26th April 2011
Abstract
This paper describes the synthesis and biological evaluation of novel phenyldiazenyl benzothiazole (PDB) derivatives as probes for imaging neurofibrillary tangles (NFTs) in patients with Alzheimer's disease (AD). We successfully synthesized three PDB derivatives using a diazo coupling reaction. In binding experiments in vitro, the compounds displayed higher affinity for tau aggregates than for Aβ aggregates. In fluorescent staining experiments using AD brain sections, 9 visualized NFTs clearly. No-carrier-added radioiodinated PDB derivatives were successfully prepared through an iododestannylation reaction from the corresponding tributyltin derivatives. [125I]9 labeled NFTs in sections of brain tissue from a patient with AD, but not a control. In biodistribution experiments using normal mice, the PDB derivatives displayed an uptake into the brain, sufficient for imaging NFTs, ranging from 0.94 to 3.2% ID g−1, but a relatively slow washout. Although further modifications are necessary to improve the pharmacokinetics in the brain, PDB with high affinity for tau aggregates may be useful as a backbone structure to develop agents for imaging NFTs in AD brains.
Introduction
Alzheimer's disease (AD) is a progressive neurodegenerative disorder characterized by cognitive decline, irreversible memory loss, disorientation, and language impairment. Senile plaques (SPs) composed of β-amyloid (Aβ) peptides and neurofibrillary tangles (NFTs) composed of hyperphosphorylated tau protein in the brain are two pathological hallmarks of AD.1 Currently, there is no simple and definitive diagnostic method to detect SPs and NFTs in the brain without postmortem pathological staining of brain tissue. Therefore, techniques to image SPs and NFTs in vivo are strongly desired.Recent success in developing several positron emission tomography (PET)/single photon emission computed tomography (SPECT) imaging agents targeting SPs has provided a window of opportunity to improve the diagnosis of AD. Compounds such as [11C]-4-N-methylamino-4′-hydroxystilbene (SB-13),2,3[11C]-2-(4′-methylaminophenyl)-6-hydroxybenzothiazole (PIB),4–6[11C]-2-(2-[2-dimethylaminothiazol-5-yl]ethenyl)-6-(2-[fluoro]ethoxy)benzoxazole (BF-227),7[11C]-2-[6-(methylamino)pyridin-3-yl]-1,3-benzothiazol-6-ol (AZD2184),8[18F]-2-(1-(2-(N-(2-fluoroethyl)-N-methylamino)naphthalen-6-yl)ethylidene)malononitrile (FDDNP),9,10(E)-4-(N-methylamino)-4′-(2-(2-(2-[18F]-fluoroethoxy)ethoxy)ethoxy)-stilbene (BAY94-9172),11,12 (E)-4-(2-(6-(2-(2-(2-([18F]-fluoroethoxy)ethoxy)ethoxy)pyridyn-3-ylvinyl)-N-methyl benzenamine (AV-45),13–15 and 2-(3-[18F]-fluoro-4-methylamino-phenyl)benzothiazol-6-ol (GE-067)16 have been tested clinically and demonstrated potential utility. Although numerous agents targeting SPs have been reported, there are few radiolabeled agents targeting NFTs.
Previous studies have confirmed that NFTs in the hippocampus and entorhinal cortex are positively correlated with the cognitive decline, whereas SPs are found in the brains of both AD patients and cognitively healthy individuals.17–20 Therefore, NFTs could be a target for imaging by PET/SPECT, enabling a presymptomatic diagnosis and monitoring of the progression and effectiveness of new treatments currently being tested. A previous paper introduced three novel compounds, 4-[2-(2-benzoimidazoyl)ethenyl]-N,N-diethylbenzenamine (BF-126), 2-[(4-methylamino)phenyl]quinoline (BF-158), and 2-(4-aminophenyl)quinoline (BF-170), for the in vivo imaging of NFTs in the AD brain.21 Although these probes displayed affinity for NFTs, they also bound to SPs. Since this report, there has been no publication regarding the development of agents to image NFTs despite their clinical significance. Therefore, PET/SPECT probes with greater affinity for NFTs are urgently required.
Recently, a library containing 72,455 compounds was screened to determine the feasibility of distinguishing tau aggregates from Aβ aggregates with small molecules. Among the compounds which bind to tau aggregates, a phenyldiazenyl benzothiazole (PDB) derivative, 4-[2-(5-methoxy-2-benzothiazolyl)diazenyl]-N,N-dimethyl-benzenamine (Fig. 1), showed the highest affinity for tau aggregates with two-fold selectivity to bind Aβ aggregates.22 An even more recent paper reported that the PDB derivative strongly binds to tau aggregates at sites that at least partially overlap with those bound by ThS.23 These findings prompted us to use the PDB as a core structure in the development of agents to image NFTs. We designed novel radioiodinated PDB derivatives, by substituting a methoxy group with a radioiodine at position 7 and introducing an electron-donating group (dimethylamino, monomethylamino, or amino group) at position 4 of the phenyl group (Fig. 1).
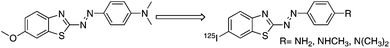 | ||
| Fig. 1 Chemical structure of 125I-labeled PDB derivatives used in the present study. | ||
In the present study, we synthesized three radioiodinated PDB derivatives and evaluated their utility for imaging NFTs in vivo. To the best of our knowledge, this is the first time PDBs have been proposed as NFT imaging agents for detecting AD.
Results and discussion
The PDB derivatives were prepared as outlined in Scheme 1. We used a diazo coupling reaction to obtain compounds 1, 2, and 3. The tributyltin derivatives (4, 5, and 6) were prepared from the corresponding compounds (1, 2, and 3) using a halogen-to-tributyltin exchange reaction catalyzed by Pd(0) in yields of 28.4, 23.7, and 67.3%, respectively. The tributyltin derivatives were used as the starting materials for radioiodination in the preparation of [125I]7, [125I]8, and [125I]9. Novel radioiodinated PDB derivatives were obtained by an iododestannylation reaction using hydrogen peroxide as the oxidant which produced the desired radioiodinated ligands. It was anticipated that the no-carrier-added preparation would result in a final product bearing a theoretical specific activity similar to that of 125I (81.4 TBq mmol−1). The radiochemical identity of the radioiodinated ligands was verified by co-injection with non-radioiodinated compounds from their HPLC profiles. [125I]7, [125I]8, and [125I]9 were each obtained in a radiochemical yield of 30–40% with a radiochemical purity of >99% after HPLC.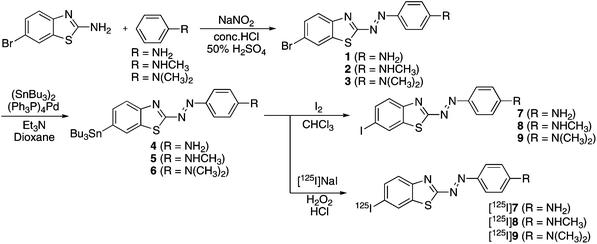 | ||
| Scheme 1 Synthetic route for PDB derivatives. | ||
The affinity of PDB derivatives (7, 8, and 9) for tau aggregates was evaluated based on inhibition of the binding of thioflavin-S (ThS) to tau aggregates. As shown in Fig. 2A, all derivatives competed well with ThS to bind to tau aggregates, indicating that the binding sites of these compounds in tau aggregates partially overlapped with those of ThS. The Ki values for tau aggregates estimated for 7, 8, and 9 were 7.27, 1.37, and 0.48 nM, respectively (Table 1). Several clinical studies found that Aβ imaging agents with Ki values of less than 10 nM successfully detected Aβ plaques in the brain.24,25 Furthermore, the concentration of tau aggregates (∼150 to 300 pmol mg−1 of wet tissue) was reported to be higher than that of Aβ aggregates (∼9 pmol mg−1 of wet tissue) in the frontal and temporal cortices in late-stage AD.26,27 These findings indicate that 7, 8, and 9 have sufficiently high affinity for tau aggregates to image NFTs in vivo. Since these compounds inhibited the binding of ThS to tau aggregates, they may have affinity for the β-sheet structure common to both Aβ and tau aggregates. To confirm the selectivity for tau aggregates relative to Aβ aggregates, we evaluated the binding to Aβ1–42 aggregates using a competitive assay with ThS. The three derivatives inhibited the binding of ThS to Aβ1–42 aggregates and tau aggregates similarly (Fig. 2B). The Ki values for Aβ1–42 aggregates estimated for 7, 8, and 9 were 6.40, 5.08, and 8.24 nM, respectively (Table 1). The ratio of the Ki values for tau and Aβ1–42 aggregates was 0.88 for 7, 3.71 for 8, and 17.2 for 9, indicating the electronegativity of the nitrogen atom of the aminophenyl group to play an important role in determining the selectivity. We also evaluated the affinity of SB-13,2,3 a well known Aβ imaging probe, for tau and Aβ1–42 aggregates according to the same procedure. The Ki values of SB-13 for tau and Aβ1–42 aggregates were 82.2 and 81.2 nM, respectively, and the ratio of the Ki value for tau and Aβ1–42 aggregates was 0.99. This result indicated that PDB derivatives, especially 9, have higher selectivity for tau than SB-13. Then, we carried out further binding experiments with AD brain sections using 9.
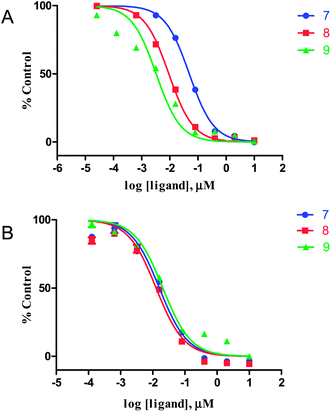 | ||
| Fig. 2 Inhibition curves for the binding of thioflavin-S to tau (A) and Aβ1–42 (B) aggregates. | ||
Next, [125I]9 was investigated for its affinity for NFTs by in vitroautoradiography in human AD brain sections (Fig. 3). Autoradiographic images of [125I]9 showed high levels of radioactivity in the brain tissue (Fig. 3A). Furthermore, we confirmed that the hot spots of [125I]9 corresponded with those of in vitro immunohistochemical staining against phosphorylated tau in sections of the same AD brain (Fig. 3B). Conversely, the normal human brain displayed no remarkable accumulation of [125I]9 (Fig. 3D). In addition, little radioactivity accumulated in the white matter of the brain (Fig. 3A), indicating that nonspecific signals would be reduced in vivo. The hot spots in autoradiography also corresponded with those of in vitro immunohistochemical staining against Aβ1–42 (Fig. 3C), indicating that PDB derivatives do not have enough selectivity to show high contrast between NFTs and SPs in an autoradiographic study. This result was thought to reflect the higher affinity for both tau and Aβ1–42 aggregates of 9 (Ki = 0.48 and 8.24 nM, respectively) than SB-13 (Ki = 82.2 and 81.2 nM, respectively). Therefore, further structural modification of the PDB scaffold is needed to improve the selectivity for tau aggregates.
![Autoradiogram of [125I]9 (A) and immunohistochemical staining with an antibody against phosphorylated tau (B) and Aβ1–42 (C) in brain sections from the same patient. Autoradiogram of [125I]9 in a control brain section (D).](/image/article/2011/MD/c1md00034a/c1md00034a-f3.gif) | ||
| Fig. 3 Autoradiogram of [125I]9 (A) and immunohistochemical staining with an antibody against phosphorylated tau (B) and Aβ1–42 (C) in brain sections from the same patient. Autoradiogram of [125I]9 in a control brain section (D). | ||
To further confirm the affinity of 9 for NFTs in the AD brain, fluorescent staining was carried out in sections of brain tissue (Fig. 4). Numerous fluorescent spots were detected in the entorhinal cortex of AD brain sections (Fig. 4A). The fluorescent labeling pattern corresponded to that obtained with ThS (Fig. 4B). These results, from in vitroautoradiography and fluorescent staining, demonstrate the feasibility of using [125I]9 as a probe for detecting NFTs in the brains of AD patients.
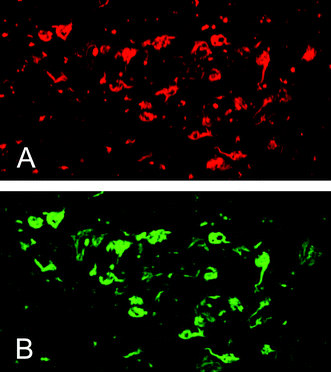 | ||
| Fig. 4 Fluorescent staining with 9 (A) or thioflavin-S (B) in the entorhinal cortex of an AD brain section. | ||
One important requirement for an imaging agent of the brain is to penetrate the blood–brain barrier after an intravenous injection.28 To evaluate the uptake of PDB derivatives in the brain, biodistribution experiments in normal mice were performed with radioiodinated forms ([125I]7, [125I]8, and [125I]9) (Table 2). The initial uptake of [125I]7, [125I]8, and [125I]9 2 min after the injection was 0.96, 1.03, and 0.94% ID g−1, respectively. The radioactivity then showed a persistent increase, reaching 3.23, 3.13, and 2.89% ID g−1. However, an imaging agent should also be rapidly washed out from the normal brain, in which there is no trapping mechanism for PDB derivatives.25 Nevertheless, the retention of these probes in the normal mouse brain suggested extensive non-specific binding which would contribute to a high level of background noise in vivo. The results suggest that PDB derivatives had unfavorable pharmacokinetics in normal mice, despite their good affinity for tau aggregates. Although factors such as molecular size, ionic charge, and lipophilicity affect a compound's uptake into and washout from the brain, previous studies suggest that lipophilicity is important to improving pharmacokinetics.2,29,30 Additional structural changes, that is, reducing lipophilicity by introducing a hydrophilic group, are necessary to improve the properties of the PDB derivatives. Improvements to the pharmacokinetics of radioactivity in the brain with high specific binding to tau aggregates should lead to the development of more useful PET/SPECT imaging agents based on the benzothiazole scaffold.
| Tissue | Time after injection/min | |||
|---|---|---|---|---|
| 2 | 10 | 30 | 60 | |
| a Each value represents the mean (SD) for 5 animals. b Expressed as % injected dose per organ. | ||||
| [ 125 I]7 | ||||
| Blood | 8.44 (1.10) | 4.05 (0.41) | 3.27 (0.15) | 2.56 (0.26) |
| Liver | 28.5 (2.51) | 27.7 (0.51) | 19.1 (1.69) | 15.5 (1.32) |
| Kidney | 9.54 (0.68) | 10.4 (0.75) | 9.70 (0.80) | 8.88 (0.50) |
| Intestine | 1.18 (0.41) | 1.96 (0.60) | 7.49 (1.35) | 13.6 (3.53) |
| Spleen | 5.78 (1.80) | 8.54 (0.43) | 8.07 (1.37) | 7.84 (1.29) |
| Pancreas | 3.36 (0.37) | 5.25 (0.34) | 5.22 (0.49) | 4.04 (0.40) |
| Heart | 12.8 (1.29) | 7.28 (1.53) | 5.32 (0.86) | 3.58 (0.62) |
| Lung | 18.8 (2.63) | 10.0 (0.59) | 6.56 (0.27) | 5.23 (0.71) |
| Stomachb | 0.76 (0.51) | 1.28 (0.30) | 2.28 (0.16) | 3.69 (0.43) |
| Brain | 0.96 (0.07) | 1.56 (0.15) | 2.50 (0.25) | 3.23 (0.39) |
| [ 125 I]8 | ||||
| Blood | 6.04 (0.75) | 3.64 (0.36) | 2.40 (0.31) | 1.83 (0.14) |
| Liver | 27.2 (2.66) | 23.6 (2.24) | 18.6 (2.71) | 13.2 (1.36) |
| Kidney | 17.4 (2.68) | 17.4 (1.95) | 10.8 (1.34) | 7.67 (1.37) |
| Intestine | 2.11 (0.41) | 6.27 (0.56) | 17.1 (0.86) | 22.9 (5.47) |
| Spleen | 13.8 (5.09) | 17.8 (1.25) | 12.2 (1.51) | 9.74 (1.26) |
| Pancreas | 4.90 (2.33) | 10.7 (2.32) | 9.80 (1.64) | 7.20 (0.67) |
| Heart | 21.2 (1.28) | 12.3 (1.31) | 7.2 (2.71) | 5.24 (1.36) |
| Lung | 45.7 (4.89) | 17.9 (1.56) | 8.96 (3.57) | 8.96 (1.03) |
| Stomachb | 0.81 (0.12) | 1.47 (0.20) | 2.03 (0.51) | 2.28 (0.41) |
| Brain | 1.03 (0.13) | 2.02 (0.13) | 2.90 (0.23) | 3.13 (0.10) |
| [ 125 I]9 | ||||
| Blood | 7.12 (1.11) | 3.13 (2.20) | 2.24 (1.88) | 1.55 (1.88) |
| Liver | 25.2 (3.23) | 15.2 (4.31) | 11.3 (4.85) | 6.43 (1.43) |
| Kidney | 11.1 (2.03) | 11.3 (1.86) | 8.04 (1.44) | 9.53 (1.43) |
| Intestine | 1.46 (0.33) | 2.80 (0.72) | 6.72 (0.76) | 8.10 (0.90) |
| Spleen | 5.70 (1.37) | 5.92 (1.24) | 4.93 (0.75) | 3.15 (0.61) |
| Pancreas | 3.04 (0.52) | 4.96 (1.12) | 5.15 (1.17) | 8.11 (1.24) |
| Heart | 12.6 (1.84) | 7.15 (2.60) | 4.25 (2.52) | 4.13 (2.30) |
| Lung | 29.3 (6.05) | 11.7(7.63) | 7.78 (7.64) | 6.55 (8.09) |
| Stomachb | 0.71 (0.09) | 1.25 (0.11) | 2.21 (0.70) | 6.34 (6.61) |
| Brain | 0.94 (0.18) | 1.56 (0.35) | 2.54 (0.40) | 2.89 (0.42) |
In conclusion, we successfully designed and synthesized three radioiodinated PDB derivatives as probes for the imaging of NFTs in the brain. In binding experiments in vitro using tau aggregates, the derivatives, especially 9, displayed high affinity for tau aggregates. 9 clearly stained NFTs in experiments using autoradiography and fluorescent staining with AD brain sections, reflecting the results of the assays. In biodistribution experiments using normal mice, the PDB derivatives displayed good brain uptake, but persistent radioactivity in the brain made them unsuitable for imaging NFTs in vivo. Appropriate structural changes to the PDB scaffold may lead to useful NFT imaging agents.
Acknowledgements
This study was supported by the Funding Program for Next Generation World-Leading Researchers, and a Grant-in-aid for Young Scientists (A) and Exploratory Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan.References
- D. J. Selkoe, Physiol. Rev., 2001, 81, 741 CAS.
- M. Ono, A. Wilson, J. Nobrega, D. Westaway, P. Verhoeff, Z. P. Zhuang, M. P. Kung and H. F. Kung, Nucl. Med. Biol., 2003, 30, 565 Search PubMed.
- N. P. Verhoeff, A. A. Wilson, S. Takeshita, L. Trop, D. Hussey, K. Singh, H. F. Kung, M. P. Kung and S. Houle, Am. J. Geriatr. Psychiatr., 2004, 12, 584 Search PubMed.
- C. A. Mathis, Y. Wang, D. P. Holt, G. F. Huang, M. L. Debnath and W. E. Klunk, J. Med. Chem., 2003, 46, 2740 CrossRef CAS.
- W. E. Klunk, H. Engler, A. Nordberg, Y. Wang, G. Blomqvist, D. P. Holt, M. Bergstrom, I. Savitcheva, G. F. Huang, S. Estrada, B. Ausen, M. L. Debnath, J. Barletta, J. C. Price, J. Sandell, B. J. Lopresti, A. Wall, P. Koivisto, G. Antoni, C. A. Mathis and B. Langstrom, Ann. Neurol., 2004, 55, 306 CrossRef CAS.
- A. Lockhart, J. R. Lamb, T. Osredkar, L. I. Sue, J. N. Joyce, L. Ye, V. Libri, D. Leppert and T. G. Beach, Brain, 2007, 130, 2607 Search PubMed.
- Y. Kudo, N. Okamura, S. Furumoto, M. Tashiro, K. Furukawa, M. Maruyama, M. Itoh, R. Iwata, K. Yanai and H. Arai, J. Nucl. Med., 2007, 48, 553 Search PubMed.
- A. E. Johnson, F. Jeppsson, J. Sandell, D. Wensbo, J. A. Neelissen, A. Jureus, P. Strom, H. Norman, L. Farde and S. P. Svensson, J. Neurochem., 2009, 108, 1177 Search PubMed.
- E. D. Agdeppa, V. Kepe, J. Liu, S. Flores-Torres, N. Satyamurthy, A. Petric, G. M. Cole, G. W. Small, S. C. Huang and J. R. Barrio, J. Neurosci., 2001, 21, RC189 Search PubMed.
- K. Shoghi-Jadid, G. W. Small, E. D. Agdeppa, V. Kepe, L. M. Ercoli, P. Siddarth, S. Read, N. Satyamurthy, A. Petric, S. C. Huang and J. R. Barrio, Am. J. Geriatr. Psychiatr., 2002, 10, 24 Search PubMed.
- W. Zhang, S. Oya, M. P. Kung, C. Hou, D. L. Maier and H. F. Kung, Nucl. Med. Biol., 2005, 32, 799 Search PubMed.
- C. C. Rowe, U. Ackerman, W. Browne, R. Mulligan, K. L. Pike, G. O'Keefe, H. Tochon-Danguy, G. Chan, S. U. Berlangieri, G. Jones, K. L. Dickinson-Rowe, H. P. Kung, W. Zhang, M. P. Kung, D. Skovronsky, T. Dyrks, G. Holl, S. Krause, M. Friebe, L. Lehman, S. Lindemann, L. M. Dinkelborg, C. L. Masters and V. L. Villemagne, Lancet Neurol., 2008, 7, 129 CrossRef CAS.
- W. Zhang, M. P. Kung, S. Oya, C. Hou and H. F. Kung, Nucl. Med. Biol., 2007, 34, 89 Search PubMed.
- S. R. Choi, G. Golding, Z. Zhuang, W. Zhang, N. Lim, F. Hefti, T. E. Benedum, M. R. Kilbourn, D. Skovronsky and H. F. Kung, J. Nucl. Med., 2009, 50, 1887 Search PubMed.
- D. F. Wong, P. B. Rosenberg, Y. Zhou, A. Kumar, V. Raymont, H. T. Ravert, R. F. Dannals, A. Nandi, J. R. Brasic, W. Ye, J. Hilton, C. Lyketsos, H. F. Kung, A. D. Joshi, D. M. Skovronsky and M. J. Pontecorvo, J. Nucl. Med., 2010, 51, 913 Search PubMed.
- M. Koole, D. M. Lewis, C. Buckley, N. Nelissen, M. Vandenbulcke, D. J. Brooks, R. Vandenberghe and K. Van Laere, J. Nucl. Med., 2009, 50, 818 Search PubMed.
- P. V. Arriagada, J. H. Growdon, E. T. Hedley-Whyte and B. T. Hyman, Neurology, 1992, 42, 631 Search PubMed.
- H. Braak and E. Braak, Hippocampus, 1993, 3, 239 Search PubMed.
- T. Gomez-Isla, R. Hollister, H. West, S. Mui, J. H. Growdon, R. C. Petersen, J. E. Parisi and B. T. Hyman, Ann. Neurol., 1997, 41, 17 Search PubMed.
- T. Gomez-Isla, W. Wasco, W. P. Pettingell, S. Gurubhagavatula, S. D. Schmidt, P. D. Jondro, M. McNamara, L. A. Rodes, T. DiBlasi, W. B. Growdon, P. Seubert, D. Schenk, J. H. Growdon, B. T. Hyman and R. E. Tanzi, Ann. Neurol., 1997, 41, 809 Search PubMed.
- N. Okamura, T. Suemoto, S. Furumoto, M. Suzuki, H. Shimadzu, H. Akatsu, T. Yamamoto, H. Fujiwara, M. Nemoto, M. Maruyama, H. Arai, K. Yanai, T. Sawada and Y. Kudo, J. Neurosci., 2005, 25, 10857 Search PubMed.
- N. S. Honson, R. L. Johnson, W. Huang, J. Inglese, C. P. Austin and J. Kuret, Neurobiol. Dis., 2007, 28, 251 Search PubMed.
- N. S. Honson, J. R. Jensen, A. Abraha, G. F. Hall and J. Kuret, Neurotoxic. Res., 2009, 15, 274 Search PubMed.
- M. P. Kung, C. Hou, Z. P. Zhuang, A. J. Cross, D. L. Maier and H. F. Kung, Eur. J. Nucl. Med. Mol. Imaging, 2004, 31, 1136 CAS.
- H. F. Kung, S. R. Choi, W. Qu, W. Zhang and D. Skovronsky, J. Med. Chem., 2010, 53, 933 CrossRef CAS.
- S. Khatoon, I. Grundke-Iqbal and K. Iqbal, J. Neurochem., 1992, 59, 750 CrossRef CAS.
- J. Wang, D. W. Dickson, J. Q. Trojanowski and V. M. Lee, Exp. Neurol., 1999, 158, 328 CrossRef CAS.
- C. A. Mathis, Y. Wang and W. E. Klunk, Curr. Pharm. Des., 2004, 10, 1469 CrossRef CAS.
- M. Ono, H. Kawashima, A. Nonaka, T. Kawai, M. Haratake, H. Mori, M. P. Kung, H. F. Kung, H. Saji and M. Nakayama, J. Med. Chem., 2006, 49, 2725 CrossRef CAS.
- Y. Cheng, M. Ono, H. Kimura, S. Kagawa, R. Nishii and H. Saji, Bioorg. Med. Chem. Lett., 2010, 20, 6141 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c1md00034a |
| This journal is © The Royal Society of Chemistry 2011 |
