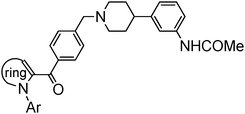
Synthesis and SAR studies of benzimidazole derivatives as melanin concentrating hormone receptor 1 (MCHR1) antagonists: Focus to detune hERG inhibition†
Pradip K.
Sasmal
*ac,
Sanjita
Sasmal
a,
Chandrasekhar
Abbineni
a,
B.
Venkatesham
a,
P. Tirumala
Rao
a,
M.
Roshaiah
a,
Ish
Khanna
a,
V. J.
Sebastian
a,
J.
Suresh
a,
Manvendra P.
Singh
a,
Rashmi
Talwar
a,
Dhanya
Shashikumar
a,
K. Harinder
Reddy
a,
Thomas M.
Frimurer
b,
Øystein
Rist
b,
Lisbeth
Elster
b and
Thomas
Högberg
*bd
aDiscovery Research, Dr Reddy's Laboratories Ltd., Bollaram Road, Miyapur, Hyderabad, 500049, India
b7TM Pharma A/S, Fremtidsvej 3, DK-2970, Hørsholm, Denmark
cDr Reddy's Laboratories Ltd., Innovation Plaza, Bachupally, Hyderabad, 500072, India. E-mail: sasmalpk@yahoo.co.in; Fax: +91 40 4434 6125; Tel: +91 40 4434 6865
dLEO Pharma, Chemical Research, Industriparken 55, DK-2750, Ballerup, Denmark. E-mail: thomas.hogberg@leo-pharma.com; Tel: +45 44 94 58 88
First published on 7th March 2011
Abstract
Melanin concentrating hormone receptor 1 (MCHR1) antagonists are potentially useful in the treatment of several CNS disorders such as obesity, stress, depression and anxiety. In a previous article, we have described a novel series of benzimidazoles as MCHR1 antagonists. These compounds showed good efficacy in obesity models but the lead compound also showed potent inhibition of hERG potassium channel. Described herein the medicinal chemistry attempts to reduce hERG inhibition while retaining MCHR1 antagonistic profile.
Melanin-concentrating hormone (MCH) is a cyclic 19-amino acid neuropeptide that is expressed predominantly in the lateral hypothalamus and zona incerta,1 though it is found throughout the central nervous system (CNS), including in the striatum and cortex. Accumulated evidence has demonstrated that MCH is an important mediator of energy homeostasis, plays a variety of physiological roles and provides therapeutic opportunities for several disorders such as obesity, stress, depression and anxiety.2 Regarding the roles of MCH in obesity, a single injection of MCH into the CNS stimulates food intake in rats3 and the chronic administration leads to an increase in body weight.4 Transgenic mice over expressing MCH gene are susceptible to insulin resistance and obesity.5 Mice lacking the gene encoding MCH are hypophagic, lean, and maintain elevated metabolic rates.6 Consistent with this phenotype, genetically altered mice that lack the gene encoding the MCH receptor maintain elevated metabolic rates and thus remain lean despite hyperphagia on a normal diet.7 These data indicate that successful antagonism of the MCHR1 system could lead to weight loss. A number of MCHR1 antagonists with anti-obesity effects in animals have been reviewed recently.8 The pharmaceutical industry has spent significant efforts to identify safe and efficacious MCHR1 antagonists but the association with inhibition of the hERG potassium channel has been a major hurdle to overcome for medicinal chemists.9 We have previously described10 compounds 1 and 2 to be potent MCHR1 antagonists but both of them were found to be potent hERG channel blockers as well as briefed in Fig. 1.
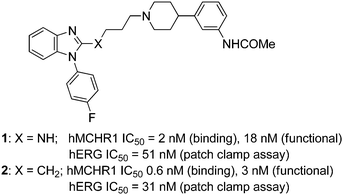 | ||
| Fig. 1 Potent MCHR1 antagonists. | ||
We describe herein our efforts on structural modifications of this chemotype to improve upon the safety window with respect to hERG inhition.11 The approaches explored to improve upon hERG inhibition included (i) modulation of basicity (pKa) of piperidine, (ii) increased polar surface area and lowered cLogP, and (iii) reduced flexibility of connector chains in 1 and 2.12
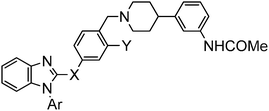
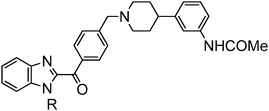
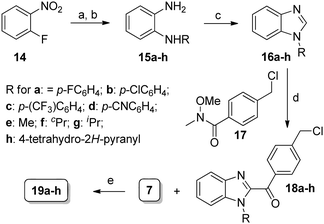
Synthesis of compounds presented herein (Table 1–4) is outlined in Schemes 1–4. The synthesis of compound 8 was commenced with the nucleophilic displacement reaction of 2-chlorobenzimidazole (3) with 4-hydroxybenzaldehyde (4) to afford 5 which after reduction, chlorination with SOCl2 and coupling with arylpiperidine moiety 713 furnished the desired compounds (Scheme 1). The synthesis of compound 13a was begun with the benzylic bromination14 of commercially available 10a with NaBrO3 followed by LiCl and SOCl2 treatment to furnish the acid chloride intermediate 11a which after coupling with diamine 15a (Scheme 2) afforded 12a. The N-alkylation of arylpiperidine 7 with 12a furnished the desired compound. For the synthesis of 13b, first 3-cyano-4-methylbenzoic acid (9) was subjected to Arndt–Eistert homologation15 to make 2-(3-cyano-4-methylphenyl)acetic acid (10b) which was then subsequently manipulated to 13b as mentioned in Scheme 1.
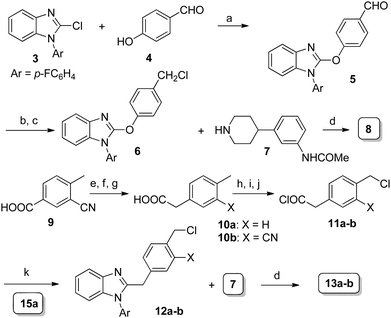 | ||
Scheme 1 Reagents and conditions: (a) K2CO3, DMF, 100 °C, 14 h; (b) NaBH4, MeOH, 0 °C to rt, 2–4 h; (c) SOCl2, CHCl3, rt, 2–6 h; (d) DMF, 70 °C, 1–3 h; (e) (i) SOCl2, EtOAc, reflux, 2 h (ii) CH2N2, diethyl ether, 0 °C, 30 min; (f) AgOBz, Et3N, MeOH, reflux, 4 h; (g) LiOH, THF–H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), rt, 2 h; (h) NaBrO3, NaHSO3, EtOAc, H2O, rt, 36 h; (i) LiCl, acetone, reflux, 24 h; (j) SOCl2, EtOAc, reflux, 1 h, (k) (i) Et3N, CH2Cl2, rt, 1 h; (ii) AcOH, 65 °C, 6 h. 1), rt, 2 h; (h) NaBrO3, NaHSO3, EtOAc, H2O, rt, 36 h; (i) LiCl, acetone, reflux, 24 h; (j) SOCl2, EtOAc, reflux, 1 h, (k) (i) Et3N, CH2Cl2, rt, 1 h; (ii) AcOH, 65 °C, 6 h. | ||
 | ||
| Scheme 2 Reagents and conditions: (a) RNH2, K2CO3, neat, reflux or 160 °C, 6–12 h; (b) NiCl2·6H2O, NaBH4, MeOH, 0 °C to rt, 30 min or 10% Pd/C, H2, MeOH, 6–14 h; (c) HCO2H, reflux, 4 h; (d) LDA, 17, THF, −78 °C to rt, 3 h; (e) DMF, 60 °C, 4–6 h. | ||
 | ||
| Scheme 3 Reagents and conditions: (a) NaBrO3, NaHSO3, EtOAc, H2O, rt, 5 h; (b) LiCl, acetone, reflux, 24 h; (c) (i) SOCl2, EtOAc, reflux, 1 h; (ii) N,O-dimethylhydroxylamine hydrochloride, pyridine, CH2Cl2, rt, 2 h; (d) 16a, LDA, THF, −78 °C to rt, 4 h; (e) NBS, (PhCO)2O2, 1,2-dichloroethane, hν, rt, 24 h; (f) 7, DMF, 60 °C, 4–6 h; (g) N,O-dimethylhydroxylamine hydrochloride, EDC·HCl, HOBt, Et3N, CH2Cl2, rt, 6–10 h. | ||
 | ||
| Scheme 4 Reagents and conditions: (a) LDA, 17, THF, −78 °C to rt, 3 h; (b) 7, DMF, 70 °C, 4–6 h; (c) 4-fluoroaniline, K2CO3, neat, 140 °C, 6 h; (d) 10% Pd/C, H2 (balloon), MeOH, 14 h; (e) HC(OEt)3, reflux, 4 h; (f) 1-fluoro-4-iodobenzene, CuI, K2CO3, L-proline, DMSO, 80 °C, 16 h. | ||
The synthesis of compounds 19a–h is outlined in Scheme 2. The SN2Ar substitution on 2-fluoronitrobenzene (14) with various amines followed by reduction led to ortho-phenylene diamine derivatives 15a–h. Conversion to N-alkyl(aryl) benzimidazoles 16a–h was accomplished by refluxing with formic acid. The crucial C2 acylation on 16a–h with the Weinreb amide 17 in the presence of LDA afforded compounds 18a–h16 which were coupled with 7 to furnish the desired compounds.
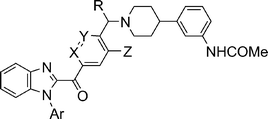
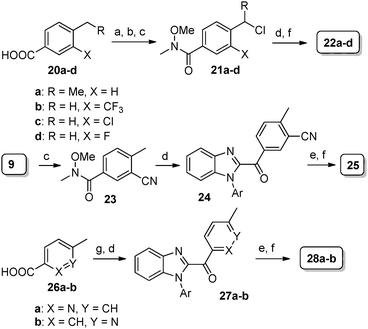
The molecules with modifications in central the benzylic core of 19a are outlined in Scheme 3. Commercially available benzoic acid derivatives 20a–d were converted to the corresponding Weinreb amides 21a–d which were converted to desired products after C2 acylation onto 16a followed by coupling with amine counterpart 7. The corresponding cyano derivative 25 was synthesized by LDA mediated acylation on 16a with 23 (prepared from 9) followed by benzylic bromination with NBS under irradiation conditions and coupling with amine 7. In a similar fashion, pyridyl derivatives 28a–b were synthesized from 5-methylpicolinic acid (26a) and 6-methylnicotinic acid (26b) as outlined in Scheme 3.
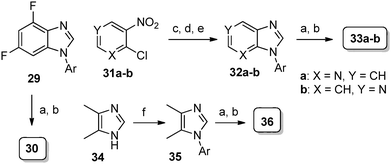
Compound 30 was prepared from 29 following the standard protocol. The syntheses of imidazopyridine derivatives 33a–b were achieved by performing set of reactions as outlined in Scheme 4. The synthesis of 36 was accomplished through sequence involving N-arylation17 of 4,5-dimethylimidazole (34), LDA mediated acylation and N-alkylation with 7.
This series of benzimidazole derivatives were tested in a SPA bead based [125I]MCH binding assay10 using Chinese hamster ovary (CHO-K1) cell membranes expressing human recombinant MCHR1 receptors for their MCHR1 potency. The functional antagonism was measured in an IP3 SPA-YSI assay.10 The select set of molecules showing potent MCHR1 antagonism were tested in patch clamp assay18 to measure their potential to block hERG potassium channel (Table 5).
| Compd | hERG IC50 (nM) or %inhibition at 1uM | IC50 IP3 (nM) | Ratio IC50's hERG/IP3 |
|---|---|---|---|
| a Values are mean of at least two experiments. | |||
| 1 | 51 | 18 | 3 |
| 2 | 31 | 3 | 10 |
| 8 | 80% | 53 | — |
| 13a | 98% | 3 | — |
| 13b | 42% | 3 | 131 |
| 19a | 1970 | 15 | 8 |
| 19b | 1700 | 217 | 26 |
| 19h | 1200 | 55 | 24 |
| 22d | 1900 | 80 | — |
| 28a | 37% | 37 | — |
| 28b | 27% | 66 | — |
| 30 | 70% | 43 | — |
| 33a | 58% | 53 | — |
| 33b | 36% | 59 | — |
| 36 | 1700 | 96 | 18 |
As indicated in Fig. 1, initial hits 1 and 2 blocked hERG channels potently with an IC50 of 51 and 31 nM respectively. In attempts to seek an acceptable balance between pKa, cLogP and connector chain flexibility, the connector chain in 1 and 2 was replaced by aryl linked molecules listed in Table 1. Both benzyl and phenoxy analogs showed potent MCHR1 antagonism. The compound 13 that demonstrated excellent hMCHR1 potency also gave potent inhibition of hERG channel (98% inhibition at 1 μM, Table 5). The cyano substituted analog 13b showed comparable MCHR1 antagonism to 13a in the functional assay along with reduced hERG inhibition at 1 μM (42% vs. 98% in case of 13a; Table 5). The benzoyl analog 19a that tweaks pKa of benzimidazole and piperidine showed excellent potency in both binding (IC50 = 11 nM) and functional assays (IC50 = 15 nM). The compound also demonstrated markedly improved safety window with 131-fold separation of functional MCHR1 antagonism over hERG inhibition (Table 5).
Encouraged by results on 19a, isosteric variants of benzyl were synthesized and evaluated (Table 2). Attempted steric crowding as in 22a to influence hERG channel accessibility led to significant loss in MCHR1 antagonism potency. Unlike compound 13b (Table 1), introduction of cyano or other groups such as Cl, CF3 at ortho-position of the benzyl moiety led to drop in MCHR1 antagonism profile. The compound 22d showed better potency (IC50 = 46 nM) in binding assay and hERG inhibition profile comparable to 19a. The pyridyl molecules 28a and 28b showed good MCHR1 antagonism and relatively improved hERG inhibition profile but no improvement over 19a.
To optimize compound 19a for MCHR1 and hERG profile, a number of N-substitued benzimidazoles were also explored (Table 3). The introduction of other larger electron withdrawing groups such as para Cl, CF3 or CN (19b–d) in the southern phenyl ring was detrimental to MCHR1 potency. To reduce lipophilicity in this class of molecules, the N-aryl part was replaced with smaller N-alkyl groups (19e–g). A gradual improvement in MCHR1 potency in the functional assay was observed in moving from a smaller methyl group to the iso-propyl group but the potency was still insufficient for 19g. The pyranyl derivative 19h showed encouraging potency with IC50 of about 50 nM in binding and functional assays, but no improvement with respect to lowered hERG inhibition.
In continuation of our studies to improve the hERG profile we also explored alternative polar heterocycles and substituted benzimidazol derivatives (Table 4). The fluoro substituted analog 30 showed good MCHR1 binding affinity and functional activity, but was surprisingly potent in the hERG assay (70% inhibition at 1 μM). The imidazopyridines 33a and 33b were fairly potent MCHR1 antagonists and 33b also demonstrated better hERG inhibition profile. The most promising of these modifications seems to be 4,5-dimethylimidazole derivative 36 with good MCHR1 antagonism and hERG inhibition activity.
Conclusions
In summary, we have identified few potent MCHR1 antagonists with excellent potency in both binding and functional assays. The detailed structure activity studies undertaken led to better insights on the structural requirements for MCHR1 antagonism and hERG inhibition. Compound 19a has emerged as lead showing good potency (IC50 of 15 nM) and selectivity over hERG (131 fold separation from functional hERG inhibition). Additional experimentation with 19a is required to understand the full potential of this compound. Besides obesity, the disclosed MCHR1 ligands might find their application in pharmacological intervention of other diseases involving MCHR1 signaling pathways.Acknowledgements
The authors thank Ann Christensen, Helle Zancho Andresen and Lisbeth Delorang Andersen for excellent technical assistance.Notes and references
- J. L. Nahon, Biol., 2006, 329, 623 CAS.
- (a) G. Hervieu, Expert Opin. Ther. Targets, 2003, 7, 495 CrossRef CAS; (b) G. Hervieu, Expert Opin. Ther. Targets, 2006, 10, 211 CrossRef CAS; (c) T. Shimazaki, T. Yoshimizu and S. Chaki, CNS Drugs, 2006, 20, 801 CrossRef CAS; (d) G. Rivera, V. Bocanegra-Garcia, S. Galiano, N. Cirauqui, J. Ceras, S. Perez, I. Aldana and A. Monge, Curr. Med. Chem., 2008, 15, 1025 CrossRef CAS; (e) T. J. Kowalski and T. Sasikumar, BioDrugs, 2007, 21, 311 CrossRef CAS; (f) S. Chaki, T. Funakoshi, S. Hirota-Okuno, M. Nishiguchi, T. Shimazaki, M. Iijima, A. J. Grottick, K. Kanuma, K. Omodera, Y. Sekiguchi, S. Okuyama, T. A. Tran, G. Semple and W. Thomsen, J. Pharmacol. Exp. Ther., 2005, 313, 831 CAS.
- M. Rossi, S. A. Beak, S.-J. Choi, C. J. Small, D. G. A. Morgan, M. A. Ghatei, D. M. Smith and S. R. Bloom, Brain Res., 1999, 846, 164 CrossRef CAS.
- A. Gomori, A. Ishihara, M. Ito, S. Mashiko, H. Matsushita, M. Yumoto, M. Ito, T. Tanaka, S. Tokita, M. Moriya, H. Iwaasa and A. Kanatani, Am. J. Physiol.: Endocrinol. Metab., 2003, 284, E583–E588 CAS.
- D. S. Ludwig, N. A. Tritos, J. W. Mastaitis, R. Kulkarni, E. Kokkotou, J. Elmquist, B. Lowell, J. S. Flier and E. Maratos-Flier, J. Clin. Invest., 2001, 107, 379 CrossRef CAS.
- M. Shimada, N. A. Tritos, B. B. Lowell, J. S. Flier and E. Maratos-Flier, Nature, 1998, 396, 670 CrossRef CAS.
- (a) Y. Chen, C. Hu, C.-K. Hsu, Q. Zhang, C. Bi, M. Asnicar, H. M. Hsiung, N. Fox, L. J. Slieker, D. D. Yang, M. L. Heiman and Y. Shi, Endocrinology, 2002, 143, 2469 CrossRef CAS; (b) D. J. Marsh, D. T. Weingarth, D. E. Novi, H. Y. Chen, M. E. Trumbauer, A. S. Chen, X.-M. Guan, M. M. Jiang, Y. Feng, R. E. Camacho, Z. Shen, E. G. Frazier, H. Yu, J. M. Metzger, S. J. Kuca, L. P. Shearman, S. Gopal-Truter, D. J. MacNeil, A. M. Strack, D. E. MacIntyre, L. H. T. Van der Ploeg and S. Qian, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 3240 CrossRef CAS.
- (a) B. Borowsky, M. M. Durkin, K. Ogozalek, M. R. Marzabadi, J. DeLeon, B. Lagu, R. Heurich, H. Lichtabau, Z. Shaposhnik, I. Daniewska, T. P. Blackburn, T. A. Branchek, C. Gerald, P. J. Vaysse and C. Forray, Nat. Med., 2002, 8, 825 CAS; (b) S. Takekawa, A. Asami, Y. Ishihara, J. Terauchi, K. Kato, Y. Shimomura, M. Mori, H. Murakoshi, K. Kato, N. Suzuki, O. Nishimura and M. Fujino, Eur. J. Pharmacol., 2002, 438, 129 CrossRef CAS; (c) M. D. McBriar and T. J. Kowalski, Annu. Rep. Med. Chem., 40, 119 Search PubMed; (d) H. Dyke and N. C. Ray, Expert Opin. Ther. Pat., 2005, 15, 1303 Search PubMed; (e) Y. Shi, Peptides, 2004, 25, 1605 CrossRef CAS; (f) A. Browning, Expert Opin. Ther. Pat., 2004, 14, 313 Search PubMed; (g) T. J. Kowalski and M. D. McBriar, Expert Opin. Invest. Drugs, 2004, 13, 1113 CrossRef CAS; (h) J. L. Mendez-Andino and J. A. Wos, Drug Discovery Today, 2007, 12, 972 CrossRef CAS; (i) M. D. McBriar, H. Guzik, S. Shapiro, J. Paruchova, R. Xu, A. Palani, J. W. Clader, K. Cox, W. J. Greenlee, B. E. Hawes, T. J. Kowalski, K. O'Neill, B. D. Spar, B. Weig, D. J. Weston, C. Farley and J. Cook, J. Med. Chem., 2006, 49, 2294 CrossRef CAS; (j) L. L. Rokosz, Expert Opin. Drug Discovery, 2007, 2, 1301 Search PubMed; (k) Y. Liu, K. Sprenger, G. Maynard, H. Friedman, L. Anciro, L. Rajachandran and A. Changchit, J. Clin. Pharmacol., 2009, 49, 1101.
- (a) J. L. Mendez-Andino and J. A. Wos, Drug Discovery Today, 2007, 12, 972 CrossRef CAS; (b) L. L. Rokosz and D. W. Hobbs, Drug News Perspect., 2006, 19, 273 CrossRef CAS.
- P. K. Sasmal, S. Sasmal, P. T. Rao, B. Venkatesham, M. Roshaiah, C. Abbineni, I. Khanna, V. P. Jadhav, J. Suresh, R. Talwar, S. Muzeeb, J.-M. Receveur, T. M. Frimurer, Ø. Rist, L. Elster and T. Högberg, Bioorg. Med. Chem. Lett., 2010, 20, 5443 CrossRef CAS.
- For recent reviews on hERG, see: (a) I. Staudacher, P. Schweizer, H. A. Katus and D. Thomas, Curr. Opin. Drug. Discov. Devel., 2010, 13, 23 Search PubMed; (b) H. Wulff, N. A. Castle and L. A. Pardo, Nat. Rev. Drug Discovery, 2009, 8, 982 CrossRef CAS; (c) C. E. Pollard, J. P. Valentin and T. G. Hammond, Br. J. Pharmacol., 2008, 154, 1538 CrossRef CAS; (d) K. Finlayson, H. J. Witchel, J. McCulloch and J. Sharkey, Eur. J. Pharmacol., 2004, 500, 129 CrossRef CAS.
- (a) U. Zachariae, F. Giordanetto and A. G. Leach, J. Med. Chem., 2009, 52, 4266 CrossRef CAS; (b) C. Jamieson, E. M. Moir, Z. Rankovic and G. Wishart, J. Med. Chem., 2006, 49, 5029 CrossRef CAS.
- J. M. Goss and S. E. Schaus, J. Org. Chem., 2008, 73, 7651 CrossRef CAS.
- D. Kikuchi, S. S. Sakaguchi and Y. Ishii, J. Org. Chem., 1998, 63, 6023 CrossRef CAS.
- S. M. Newman and P. F. Beal, J. Am. Chem. Soc., 1950, 72, 5163 CrossRef CAS.
- Synthetic protocol for C2-acylation of benzimidazole to prepare 18a: To a solution of diisopropyl amine (0.92 mL, 6.53 mmol) in THF (3 mL) at −78 °C was added nBuLi (1.6 M in THF, 3.5 mL, 5.6 mmol) drop wise and stirred at 0 °C for 30 min. The reaction mixture was recooled to −78 °C and a solution of 16a (565 mg, 3.26 mmol) in THF (6 mL) was added dropwise. After stirring for 10 min, a solution of 17 (697 mg, 3.26 mmol) in THF (4 mL) was added drop wise. The reaction mixture was allowed to warm to room temperature over a period of 3 h and then quenched by the addition of saturated aqueous ammonium chloride solution. The mixture was diluted with ethyl acetate and the organic layer was washed successively with water, brine and dried over anhydrous Na2SO4. Concentration and purification of the crude over silica gel (100–200 mesh) using 10% ethyl acetate in petroleum ether as eluent afforded compound 18a (717 mg, 60% yield) as a viscous liquid.
- H. Zhang, Q. Cai and D. Ma, J. Org. Chem., 2005, 70, 5164 CrossRef CAS.
- HEK293 cells stably expressing hERG potassium channels were voltage clamped using automated Port-A-Patch electrophysiology system. Test items were dissolved in DMSO and diluted with external recording buffer. Cells were exposed to test concentration for approximately 5 min or till a steady state block was reached at 20–35 °C. Each cell acted as its own control. Cisapride (1 μM) was used as an internal positive control to confirm the sensitivity of the test system to hERG inhibition. The extent of inhibition of channel was expressed as a percentage of the control response (minus the test compound).
Footnote |
| † DRL Publication No. 720. |
| This journal is © The Royal Society of Chemistry 2011 |