An approach to enzyme inhibition employing reversible boronate ester formation†
Ivanhoe K. H.
Leung‡
,
Tom
Brown Jr‡
,
Christopher J.
Schofield
* and
Timothy D. W.
Claridge
*
Department of Chemistry, University of Oxford, Chemistry Research Laboratory, 12 Mansfield Road, Oxford, OX1 3TA, United Kingdom. E-mail: christopher.schofield@chem.ox.ac.uk; tim.claridge@chem.ox.ac.uk; Fax: +44 (0)1865 285 002; Tel: +44 (0)1865 285 000
First published on 7th March 2011
Abstract
Dynamic combinatorial chemistry (DCC) is a potentially useful method for the identification of biomacromolecule ligands; however, the number of reactions applicable to DCC in aqueous solution is limited. We report studies that investigate the reversible reaction of boronic acids with alcohols as an approach to enzyme inhibition, employing α-chymotrypsin as a model system. NMR techniques (11B NMR and 1H waterLOGSY) were used to observe ternary complexes of boronic acids, sugars and α-chymotrypsin, and were useful for distinguishing preferentially binding combinations of boronic acids and sugars. The results reveal that both the propensity of boronate ester formation in solution and affinity of the boronate ester for the target enzyme determine whether ternary complex formation is observed. The results also provide proof of principle for the boronate ester approach to DCC versusprotein targets.
Introduction
The electrophilic nature of boronic acids has been exploited for the inhibition of enzymes containing nucleophilic active site residues, including serine proteases, proteasomes and serine β-lactamases.1,2Boronic acids can react reversibly with an active site nucleophile to form a tetrahedral complex which can act as an analogue of the tetrahedral intermediates in catalysis.2Boronic acids, and the tetrahedral ions formed by their reaction with water, also react with alcohols to form boronate esters. In aqueous solution at neutral pH the reactions of boronic acids with alcohols, including diols, are reversible (ESI Figure S1†),3 with the equilibrium position dependent upon on the pKa values of the boronic acid and the alcohol.4 The structure of the alcohol can also be important, with diols that are conformationally locked in a syn-periplanar arrangement, as in some furanoses, forming more stable complexes.4The generation of libraries of new molecules formed by the reversible reaction of relatively simple components is an emerging tool for the generation of novel protein ligands that may, for example, be used as leads for the generation of potent and selective enzyme inhibitors. When large numbers of compounds are generated and screened, this method is referred to as dynamic combinatorial chemistry (DCC). Various reversible reactions have been used for generating protein ligands in aqueous solution including thiol-disulfide exchange and addition-elimination reactions including carbonyl groups (for reviews, see ref. 5). However, the development of appropriate reversible reactions in the context of pharmaceutical applications can be challenging because they must occur in aqueous solution under conditions that remain compatible with biomacromolecules. The analysis of ligand binding in DCC experiments involving biomacromolecules is also not always straightforward. Although indirect methods involving library analysis have been developed,5 the reversible nature of DCC reactions means that direct analyses on the target biomacromolecule are attractive. Biophysical techniques including mass spectrometry6 and X-ray crystallography7 have been applied to study such interactions with target enzymes. NMR spectroscopy may also be used to monitor such interactions8 and has been applied to study binding interactions in systems based on hemithioacetal formation.9,10
We reasoned that reversible boronate ester formation may represent a suitable class of reaction for the development of protein ligands or enzyme inhibitors. Evidence for this proposal comes from reports that the addition of specific diols/sugars, such as D-fructose (ESI Figure S2†), can improve the potency of inhibition of nucleophilic enzymes by boronic acids.11 Here we report on NMR-based investigations into reversible boronate ester formation and its application to enzyme inhibition using the serine protease α-chymotrypsin (αCT) as a model system.
Results and discussion
Initial experiments were undertaken to observe ternary enzyme-boronic acid-sugar complex formation by NMR spectroscopy. 1-Benzothiophen-2-ylboronic acid (1) (Fig. 1) is an inhibitor of some serine proteases (IC50 for αCT = 5 μM) and serine β-lactamases (IC50 for the AmpC serine β-lactamase = 27 nM),12 and was therefore used as a model boronic acid. Boronic acids form covalent complexes with serine proteases, and 11B NMR is a useful technique for the direct observation of the free and protein bound boron-species.1311B is quadrupolar (I = 3/2) and this property can be exploited to probe the hybridisation state of the boron atom.14 In NMR analyses, broad peaks are often observed if the 11B nucleus is in a sp2-hybridised trigonal planar environment, whereas relatively sharper peaks are often observed for the 11B nucleus in the more highly symmetrical sp3-hybridised tetrahedral environment;14 The slow rotational correlation time associated with protein-boronic acid/boronate ester complexes may further contribute to line-narrowing.15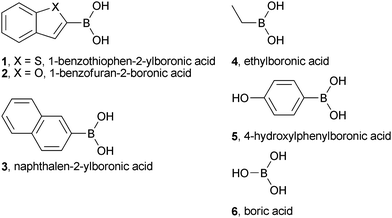 | ||
| Fig. 1 Structures of the boronic acids used in this study. | ||
The pKa of 1 (corresponding to RB(OH)2 + H2O ⇌ RB(OH)3− + H+) was found to be ∼7.4 (ESI Figure S3†), therefore initial experiments were conducted at pH 5.8 to ensure the free boronic acid was predominantly in the sp2-hybridised form (ESI Figure S4a†: peak A; 26.4 ppm; linewidth >300 Hz). Upon addition of D-fructose to 1, the 11B signal was sharpened and was shifted to lower ppm, indicating the formation of a tetrahedral, sp3 hybridised boronate ester species (ESI Figure S4b†: peak B; 6.72 ppm; linewidth ∼190 Hz). A similar peak sharpening and shift was observed when αCT was added to 1 at pH 5.8 to form a binary αCT-1 complex (ESI Figure S4c†: peak D; 2.35 ppm; linewidth ∼90 Hz), suggesting that the boronic acid is in a tetrahedral boronate form when bound to αCT. The addition of D-fructose to the αCT-1 binary complex further sharpened and shifted the 11B signal (ESI Figure S4d†: peak C; 6.47 ppm; linewidth ∼60 Hz), consistent with the formation of a ternary αCT-1-D-fructose complex in which the αCT-bound 11B nucleus is in an sp3-hydribised tetrahedral environment. These results support previous studies employing kinetic and X-ray crystallographic analyses11,16 providing evidence for the formation of ternary boronic acid-sugar complexes with nucleophilic enzymes.
In screening experiments employing reversible boronate ester formation, the distribution of boronate esters reflects their respective thermodynamic stabilities. When a mixture of boronic acids and diols is exposed to an external influence, such as the presence of a protein, the equilibrium shifts and those components (i.e.boronate esters) that interact with the template are stabilized. Using 11B NMR, we observed that the presence of αCT appeared to move the equilibrium towards the formation of the 1-D-fructose boronate ester. Thus, in the absence of αCT, ∼140 mM D-fructose was required to observe the formation of 1-D-fructose boronate ester, but in the presence of 1 mM αCT, only ∼2 mM D-fructose was required (Fig. 2). Analogous changes to the equilibrium position have been observed by NMR in a dynamic compound library system involving hemithioacetal formation with Mycobacterium tuberculosispantothenate synthetase.9
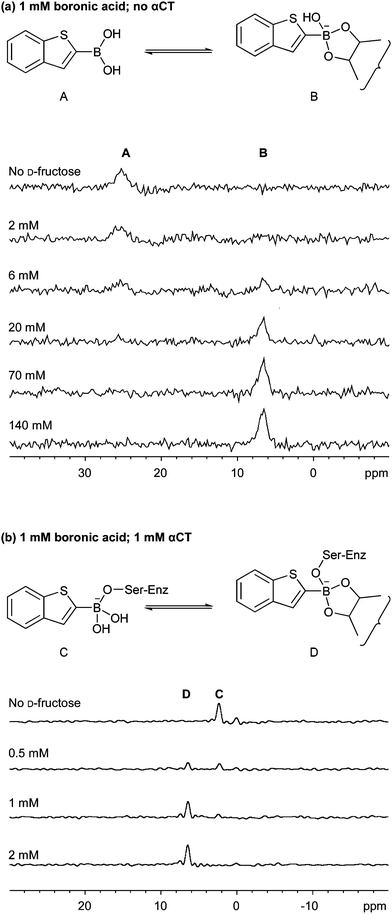 | ||
| Fig. 2 The presence of αCT moves the equilibrium position towards the formation of the 1-D-fructose boronate ester. 11B NMR spectra of (a) the addition of D-fructose to boronic acid 1 and (b) the addition of D-fructose to boronic acid 1 in the presence of one equivalent of α-CT. | ||
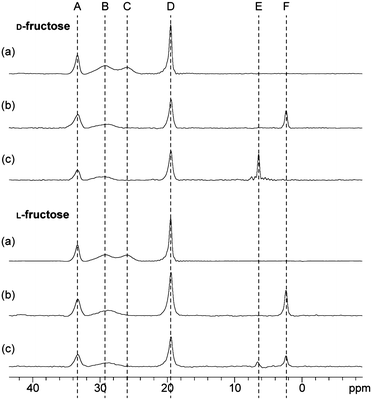
In subsequent experiments we investigated the correlation between αCT inhibition and boronate ester binding. Sugars, including D-fructose, on their own do not bind to αCT to any significant extent as observed by ligand based NMR analyses (data not shown). It has been proposed11 that the efficacy of sugars in increasing the inhibitory effect of boronic acids depends on both: (i) the propensity of the boronate ester to form in solution and (ii) the affinity of the boronate ester complex for the enzyme. We found that only D-fructose improved substantially the inhibitory effect of 1 (78 ± 2.4% activity, relative to that in the absence of sugar), with D-glucose (97 ± 4.8%), L-glucose (94 ± 3.5%) and L-fructose (97 ± 0.6%) having little effect. These results imply selectivity in the formation of ternary αCT-boronic acid-sugar complexes. The affinities of these same sugars for 1 were determined by conducting a series of titrations monitored by 1H and 11B NMR. The affinities of D-fructose and L-fructose for 1 were similar (KA = ∼52 M), but those of D-glucose and L-glucose were weaker (KA = <2 M) (ESI Figure S5†), consistent with previous studies on the reaction of phenylboronic acid with polyols.17 These data further suggest that the more potent inhibition observed in the presence of D-fructose relative to L-fructose results from more efficient recognition by αCT of either D-fructose or of the D-fructose-boronate ester complex. In accordance with this, we found that D-fructose readily forms a ternary αCT-1-fructose complex, whereas use of L-fructose resulted in the formation of only a small amount of a ternary complex under similar conditions (Fig. 3). Titrations revealed that at least 30 times more L-fructose (than D-fructose) was required to fully form the ternary αCT-1-fructose complex (Fig. 3). We also observed stereoselectivity for boronate ester formation in the case of αCT-1 and L-/D-sorbose (ESI Figure S2†), in which case the αCT-1-L-sorbose complex is preferentially formed over the αCT-1-D-sorbose complex (ESI Figure S6†). The relative increase in potency observed for D-fructose over (both D- and L-) glucose may reflect, at least in part, the higher affinity of D-fructose for boronic acids in solution. This follows from the increased population (∼25% of the total) of the β-furanose form in which the C-1 and C-2 hydroxyl groups are in a syn-periplanar arrangement for D- (and L-) fructose, compared to the ∼0.1% of the α-furanose form present in (both D- and L-) glucose solution.18
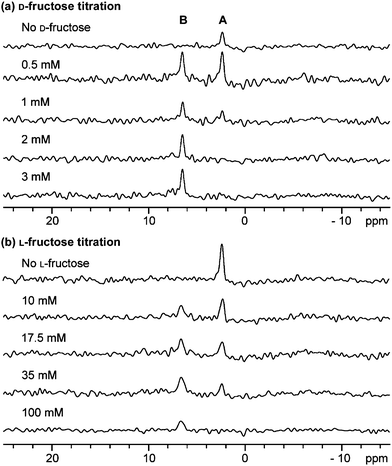 | ||
| Fig. 3 Formation of an αCT-1-fructose ternary complex as monitored by 11B NMR. The formation of a ternary enzyme–boronic acid–sugar complex on titration of (a) D-fructose and (b) L-fructose (at the concentrations shown) into a mixture of 1 (1 mM) and αCT (1.3 mM). Peak A is the αCT-1 binary complex and peak B is the αCT-1-fructose ternary complex. | ||
Further investigations revealed the selectivity of the αCT active site for particular boronic acids. 1-Benzofuran-2-boronic acid (2) (Fig. 1), the oxygen analogue of boronic acid 1, is also an inhibitor of serine proteases (IC50 for αCT = 14 μM) and serine β-lactamases (IC50 for AmpC β-lactamase = 220 nM).12 It has a similar pKa value to 1 (pKa ∼ 7.3, ESI Figure S3†) and both 1 and 2 are equally reactive to boronate ester formation in solution as demonstrated by NMR analyses (ESI S5†). 11B NMR was used to monitor the interaction of αCT with boronic acids 1 and 2 in the presence and absence of sugars (Fig. 4a and ESI Figure S7†). Addition of D-fructose revealed the preferential formation of the αCT-1-D-fructose over the αCT-2-D-fructose ternary complex, demonstrating subtle active site selectivity and illustrating the potential of this technique to analyse equilibrium mixtures.
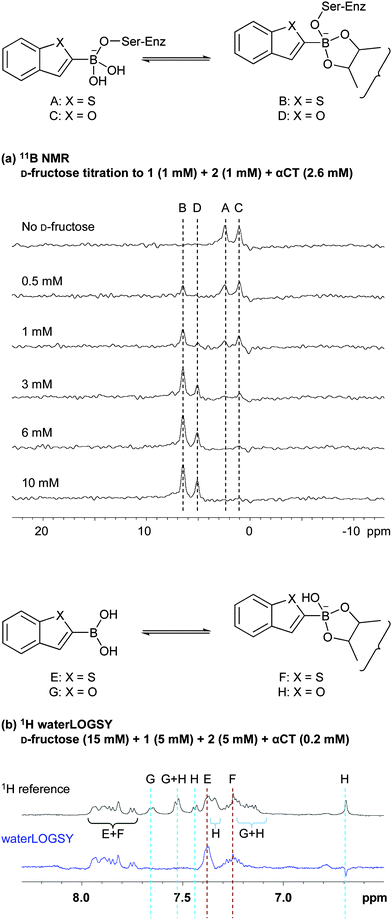 | ||
| Fig. 4 αCT preferentially binds to 1 and 1-D-fructose boronate ester, as shown by (a) 11B NMR: (Peak A: αCT-1 binary complex; Peak B: αCT-1-D-fructose ternary complex; Peak C: αCT-2 binary complex; Peak D: αCT-2-D-fructose ternary complex), and (b) 1H waterLOGSY analyses: (Peak E: 1; Peak F: 1-D-fructose boronate ester; Peak G: 2; Peak H: 2-D-fructose boronate ester). | ||
A particular requirement for the use of boronic acids as templates in the development of protein ligands or enzyme inhibitors is that they readily form boronate esters under the solution conditions appropriate to the target enzyme. The dynamics of boronate ester formation depends upon the structure and pKa of the boronic acid, and the solution pH (ESI Figure S1†). For instance, the pKa of naphthalen-2-ylboronic acid (3) (Fig. 1) is ∼8.8 (ESI Figure S3†) and at pH 5.8, a ∼1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 3/D-fructose ratio was required to form 50% of the 3-D-fructose boronate ester complex (compared to 1
500 3/D-fructose ratio was required to form 50% of the 3-D-fructose boronate ester complex (compared to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 for 1); at pH 7.0 this was reduced to ∼1
6 for 1); at pH 7.0 this was reduced to ∼1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, and at pH 8.0, ∼1
50, and at pH 8.0, ∼1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (as determined by 1H and 11B NMR, ESI Figure S8†). Thus, in addition to the selection of suitable chelating alcohols, the properties of the boronic acid should be appropriate for boronate ester formation to enable assessment of any enhanced inhibitory potency. To demonstrate the dependencies on pKas and to investigate the utility of NMR for the screening of a set of simple boronic acids, we selected four structurally different boronic acids including 1, ethylboronic acid (4), 4-hydroxyphenylboronic acid (5), and boric acid (6) (Fig. 1), and monitored the formation of ternary enzyme-boronic acid-sugar complexes by 11B NMR at pH 5.8 in the presence and absence of sugars (Fig. 5). Upon addition of αCT to the mixture of bor(on)ic acids (without sugar), boronic acid 1 was identified as the only compound leading to an observable complex with αCT. Addition of D-fructose revealed the formation of a αCT-1-D-fructose ternary complex. These results suggest that αCT binds preferentially with 1 out of the tested mixture of boronic acids, and, in support of the previous inhibition analyses, further demonstrate the preferential binding of 1 with D-fructose. In part this selectivity likely reflects the low efficacy of 4, 5 and 6 in forming boronate esters at pH 5.8 due to their pKa values (typically 10–12 for alkylboronic acids) and, in the case of 5, the electron-donating nature of its 4-hydroxyphenyl substituent.
10 (as determined by 1H and 11B NMR, ESI Figure S8†). Thus, in addition to the selection of suitable chelating alcohols, the properties of the boronic acid should be appropriate for boronate ester formation to enable assessment of any enhanced inhibitory potency. To demonstrate the dependencies on pKas and to investigate the utility of NMR for the screening of a set of simple boronic acids, we selected four structurally different boronic acids including 1, ethylboronic acid (4), 4-hydroxyphenylboronic acid (5), and boric acid (6) (Fig. 1), and monitored the formation of ternary enzyme-boronic acid-sugar complexes by 11B NMR at pH 5.8 in the presence and absence of sugars (Fig. 5). Upon addition of αCT to the mixture of bor(on)ic acids (without sugar), boronic acid 1 was identified as the only compound leading to an observable complex with αCT. Addition of D-fructose revealed the formation of a αCT-1-D-fructose ternary complex. These results suggest that αCT binds preferentially with 1 out of the tested mixture of boronic acids, and, in support of the previous inhibition analyses, further demonstrate the preferential binding of 1 with D-fructose. In part this selectivity likely reflects the low efficacy of 4, 5 and 6 in forming boronate esters at pH 5.8 due to their pKa values (typically 10–12 for alkylboronic acids) and, in the case of 5, the electron-donating nature of its 4-hydroxyphenyl substituent.
 | ||
| Fig. 5 Equilibrium mixture analysis by 11B NMR: (a) boronic acids only; (b) boronic acids + αCT; (c) boronic acids + αCT + D/L-fructose. Assays included the following bor(on)ic acids (at 2 mM concentration each): 1 (peak C), 4 (peak A), 5 (peak B), 6 (peak D). Addition of αCT (∼2 mM) led to the selective formation of a binary enzyme-boronic acid complex with 1 (peak F). Addition of fructose (12 mM) leads to the selective formation of a ternary αCT-boronic acid-fructose complex with 1 (peak E). Addition of D-fructose gives full conversion to the ternary αCT-1-D-fructose complex whilst <50% conversion is observed for L-fructose. | ||
Whilst the use of reversible boronate ester formation shows potential for the development of inhibitors of greater affinity, it is apparent that the use of 11B NMR spectroscopy is likely not an optimal general method for screening in most cases. Whilst enabling the direct observation of the ternary boronate-enzyme complexes, 11B NMR resonances are broad, owing to the quadrupolar 11B nucleus,14 meaning boronic acids with similar structures often cannot be distinguished owing to signal overlap. In addition, high concentrations of boronic acids and protein are necessary to compensate for the relatively low sensitivity of 11B NMR. Alternatively, 1H-based screening techniques such as waterLOGSY (water-ligand observed via gradient spectroscopy)19 offer superior resolution and sensitivity and may represent a more attractive NMR methodology. Furthermore, waterLOGSY employs observation of the free ligand, and has the further benefit of requiring relatively small amounts of protein. We therefore investigated the use of waterLOGSY for detection of binding of boronate esters/boronic acids to αCT. As in the previous 11B experiment, the boronic acid pair 1 and 2 was used to investigate waterLOGSY screening at pH 5.8. A slight excess of D-fructose was used to ensure the formation of some boronate ester species. In agreement with the 11B NMR experiments, the waterLOGSY analyses revealed that αCT preferentially selects 1-D-fructose boronate ester over the 2-D-fructose boronate ester (Fig. 4b and ESI Figure S9†). In contrast, no selectivity was observed in the equivalent waterLOGSY experiment with L-fructose; Presumably all four components (1, 1-L-fructose, 2 and 2-L-fructose) bind weakly to αCT (ESI Figure S10†).
Conclusions
Overall, the results of our work validate the potential of the reversible reaction of boronic acids with alcohols to produce boronate esters for exploring active site ligand interactions. In the presence of a target protein, the most favourable combination of boronic acids and sugars that combine to give a ternary enzyme-bound complex can be identified by NMR analyses. The observation of selective complex formation provides leads for further biophysical analysis involving, for example, protein crystallography and/or the development of stable analogues, as employed in DCC approaches.5,20 The model enzyme that we used, αCT, possess a nucleophilic serine residue at its active site, enabling reversible formation of a ternary protein-ligand complex that has been monitored by NMR. It is important to note, however, that the observation of a ternary complex depends not only on the affinity of the boronate ester for the target protein, but also on the propensity of the boronate ester to form in solution, which in turn is dependent upon the pKa of the boronic acid and on solution pH.The development of efficient methods for the screening of members of a DCC library that bind to a target macromolecule can be challenging. We have shown that 11B NMR and 1H waterLOGSY can be used to monitor the formation of ternary enzyme-boronate ester complexes. 11B NMR allows direct detection of the protein-bound boronic acids and, despite suffering from low intrinsic sensitivity and broad resonances (ESI Figures S1 and S4†), 11B NMR is sensitive to the local 11B environment, is free from protein background signals and is useful for distinguishing free and bound species. 1H based techniques such as waterLOGSY have superior sensitivity but may be limited by the exchange kinetics of the reversibly forming protein-ligand complex and remain prone to signal overlap from large compound libraries.
In our study the enhanced inhibition of αCT by boronic acids in the presence of D-fructose in particular was shown to correlate with greater stability of the enzyme-boronate ester ternary complex. The relative lack of enhanced inhibition in the presence of L-fructose likewise correlates with a significantly lower propensity for the ternary complex to form with this stereoisomer, despite similar affinities for the formation of the binary boronate ester complexes for both D- and L- fructose in the absence of αCT.
Finally we note that the dynamic nature of the boronic acid-ester interconversions suggests that reversible reactions including boronic acids may be useful for the development of dynamic combinatorial libraries of potential inhibitors. Various reversible reactions have been used in the context of dynamic combinatorial chemistry and related approaches to enzyme inhibition, including thiol-disulfide exchange and addition-elimination reactions involving carbonyl groups.5 Provided suitable methods for library screening can be developed, the boronic acid template may become another scaffold for protein-ligand investigations utilising dynamic combinatorial chemistry.
Experimental
Materials
Chemicals were purchased from Sigma-Aldrich or Alfa Aesar, with the exceptions of Tris-D11 (Cambridge Isotope Laboratories), and D2O (Apollo Scientific). αCT was purchased as lyophilized powder (from bovine pancreas, Type II, ≥40 units/mg protein).Inhibition assay
The hydrolysis of succinyl-Ala-Ala-Pro-Phe-para-nitroanilide was monitored by measuring the increase in absorbance at 405 nm as a function of time. The temperature was kept constant at 25 °C. Measurements were performed using an Envision microplate reader (Perkin-Elmer, Waltham, MA, USA) and using 96-well plates (Corning, Lowell, MA, USA). Assays were started by adding substrate to either enzyme or a mixture of enzyme and inhibitor (in either case incubated at 25 °C for 10 min). The contents of each well were mixed manually using a multi-channel pipette (Eppendorf, Hamburg, Germany) immediately after adding substrate. Measurements were performed in triplicate. Initial rates were taken by measuring the slope of a line of best fit through the first six data points, which were recorded at 10–20 s intervals. Residual activities were calculated by dividing the initial rate of the reaction in the presence of inhibitor by that in the absence of inhibitor. Activities relative to the activity in the absence of sugar were calculated by dividing the rate of the reaction in the presence of sugar by that in the absence of sugar. Concentrations were as follows: enzyme (5 nM), substrate (100 μM), boronic acid (25 μM), sugar (2.5 mM).11B NMR experiments
Experiments were conducted at 160 MHz (for 11B) using a Bruker DRX 500 MHz spectrometer equipped with a standard 5 mm broadband probe. Typical experimental parameters were as follows: acquisition time 16 ms, relaxation delay 400 ms, number of scans 4000–16000. The baselines were corrected by spline fit processing or backward linear prediction (28–30 points back predicted and 256 linear prediction coefficients).1H WaterLOGSY experiments
Experiments were conducted using a Bruker Avance II 500 MHz spectrometer equipped with a 5 mm TXI probe. Typical experimental parameters were as follows: mixing time 1 s, relaxation delay 5 s, number of scans 64. Samples were prepared in 90% H2O/10% D2O and solvent excitation was achieved using a 16 ms, 180 degree selective rectangular shape pulse (Squa100.1000) set at the H2O frequency. Protein background suppression was achieved by a 30 ms spin-lock and water suppression was achieved by the excitation sculpting method using a 2 ms 180 degree selective Squa100.1000 pulse at the H2O frequency.Abbreviations
| αCT | α-chymotrypsin |
| DCC | Dynamic Combinatorial Chemistry |
| waterLOGSY | water-ligand observed via gradient spectroscopy. |
Acknowledgements
The authors gratefully acknowledge support of this project by the EPSRC and the BBSRC.Bibliographic references and notes
- P. C. Trippier and C. McGuigan, Med. Chem. Commun., 2010, 1, 183–198 RSC; W. Yang, X. Gao and B. Wang, Med. Res. Rev., 2003, 23, 346–368 CrossRef CAS.
- L. Hedstrom, Chem. Rev., 2002, 102, 4501–4524 CrossRef CAS; D. A. Matthews, R. A. Alden, J. J. Birktoft, S. T. Freer and J. Kraut, J. Biol. Chem., 1975, 250, 7120–7126 CAS; C. A. Kettner, R. Bone, D. A. Agard and W. W. Bachovchin, Biochemistry, 1988, 27, 7682–7688 CrossRef CAS; W. W. Bachovchin, W. Y. L. Wong, S. Farr-Jones, A. B. Shenvi and C. A. Kettner, Biochemistry, 1988, 27, 7689–7697 CrossRef CAS; V. K. Antonov, T. V. Ivanina, I. V. Berezin and K. Martinek, FEBS Lett., 1970, 7, 23–25 CrossRef CAS; M. Philipp and M. L. Bender, Proc. Natl. Acad. Sci. U. S. A., 1971, 68, 478–480 CAS; K. A. Koehler and G. E. Lienhard, Biochemistry, 1971, 10, 2477–2483 CrossRef CAS.
- G. Springsteen and B. H. Wang, Tetrahedron, 2002, 58, 5291–5300 CrossRef CAS; T. D. James, M. D. Phillips and S. Shinkai, in Boronic Acids in Saccharide Recognition; RSC Publishing: 2006 Search PubMed.
- J. Yan, G. Springsteen, S. Deeter and B. Wang, Tetrahedron, 2004, 60, 11205–11209 CAS.
- P. T. Corbett, J. Leclaire, L. Vial, K. R. West, J. L. Wietor, J. K. Sanders and S. Otto, Chem. Rev., 2006, 106, 3652–3711 CrossRef CAS; O. Ramström and J.-M. Lehn, Nat. Rev. Drug Discovery, 2002, 1, 26–36 CrossRef CAS; S. J. Rowan, S. J. Cantrill, G. R. L. Cousins and J. K. M. Sanders, Angew. Chem., Int. Ed., 2002, 41, 898–952 CrossRef; J.-M. Lehn and A. V. Eliseev, Science, 2001, 291, 2331–2332 CrossRef CAS; G. R. L. Cousins, S. A. Poulsen and J. K. M. Sanders, Curr. Opin. Chem. Biol., 2000, 4, 270–279 CrossRef CAS; J.-M. Lehn, Chem.–Eur. J., 1999, 5, 2455–2463 CrossRef CAS; B. Klekota and B. L. Miller, Trends Biotechnol., 1999, 17, 205–209 CrossRef CAS; M. F. Greaney and V. T. Bhat, in Dynamic Combinatorial Chemistry: In Drug Discovery, Bioinorganic Chemistry, and Materials Sciences, ed. B. L. Miller, Wiley, New Jersey, 2010. ch. 2, pp. 43–82 Search PubMed.
- S. A. Poulsen and L. F. Bornaghi, Bioorg. Med. Chem., 2006, 14, 3275–3284 CrossRef CAS; S. A. Poulsen, J. Am. Soc. Mass Spectrom., 2006, 17, 1074–1080 CrossRef CAS; R. D. Sussmuth and G. Jung, J. Chromatogr., Biomed. Appl., 1999, 725, 49–65 CrossRef.
- M. S. Congreve, D. J. Davis, L. Devine, C. Granata, M. O'Reilly, P. G. Wyatt and H. Jhoti, Angew. Chem., 2003, 115, 4617–4620 CrossRef; A. M. Davis, S. J. Teague and G. J. Kleywegt, Angew. Chem., Int. Ed., 2003, 42, 2718–2736 CrossRef CAS.
- B. Meyer and T. Peters, Angew. Chem., Int. Ed., 2003, 42, 864–890 CrossRef CAS; M. J. Shapiro and J. R. Wareing, Curr. Opin. Chem. Biol., 1998, 2, 372–375 CrossRef CAS; H. Kessler, Angew. Chem., Int. Ed. Engl., 1997, 36, 829–831 CAS.
- D. E. Scott, G. J. Dawes, M. Ando, C. Abell and A. Ciulli, ChemBioChem, 2009, 10, 2772–2779 CrossRef CAS.
- R. Caraballo, H. Dong, J. P. Ribeiro, J. Jiménez-Barbero and O. Ramström, Angew. Chem. Int. Ed., 2010, 49, 589–593.
- H. Suenaga, K. Nakashima and S. Shinkai, J. Chem. Soc., Chem. Commun., 1995, 29–30 RSC; H. Suenaga, M. Mikami, H. Yamamoto, T. Harada and S. Shinkai, J. Chem. Soc., Perkin Trans. 1, 1995, 1733–1738 RSC.
- G. S. Weston, J. Blázquez, F. Baquero and B. K. Shoichet, J. Med. Chem., 1998, 41, 4577–4586 CrossRef CAS.
- S. Zhong, F. Jordan, C. Kettner and L. Polgar, J. Am. Chem. Soc., 1991, 113, 9429–9435 CrossRef CAS; J. E. Baldwin, T. D. W. Claridge, A. E. Derome, B. D. Smith, M. Twyman and S. G. Waley, J. Chem. Soc., Chem. Commun., 1991, 573–574 RSC; J. E. Baldwin, T. D. W. Claridge, A. E. Derome, C. J. Schofield and B. D. Smith, Bioorg. Med. Chem. Lett., 1991, 1, 9–12 CrossRef CAS.
- R. R. Gupta and M. D. Lechner, in Chemical Shifts and Coupling Constants for Boron-11 and Phosphorus-31, Springer-Verlag: 1997 Search PubMed.
- P. S. Hubbard, J. Chem. Phys., 1970, 53, 985–987 CrossRef CAS; T. E. Bull, J. Magn. Reson., 1972, 8, 344–353 CAS.
- T. R. Transue, J. M. Krahn, S. A. Gabel, E. F. DeRose and R. E. London, Biochemistry, 2004, 43, 2829–2839 CrossRef CAS.
- J. P. Lorand and J. O. Edwards, J. Org. Chem., 1959, 24, 769–774 CrossRef CAS.
- P. M. Collins and R. J. Ferrier, in Monosaccharides: Their Chemistry and their Roles in Natural Products; Wiley: Chichester, 1995 Search PubMed; S. J. Angyal, Adv. Carbohydr. Chem. Biochem., 1984, 42, 15–68 Search PubMed.
- C. Dalvit, P. Pevarello, M. Tatò, M. Veronesi, A. Vulpetti and M. Sundström, J. Biomol. NMR, 2000, 18, 65–68 CrossRef CAS.
- R. Caraballo, M. Sakulsombat and O. Ramström, ChemBioChem, 2010, 11, 1600–1606 CrossRef CAS; B. M. R. Liénard, R. Hüting, P. Lassaux, M. Galleni, J.-M. Frère and C. J. Schofield, J. Med. Chem., 2008, 51, 684–688 CrossRef CAS; B. M. R. Liénard, N. Selevsek, N. J. Oldham and C. J. Schofield, ChemMedChem, 2007, 2, 175–179 CrossRef CAS; N. R. Rose, E. C. Y. Woon, G. L. Kingham, O. N. F. King, J. Mecinović, I. J. Clifton, S. S. Ng, J. Talib-Hardy, U. Oppermann, M. A. McDonough and C. J. Schofield, J. Med. Chem., 2010, 53, 1810–1818 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c1md00011j |
| ‡ These authors made equal contributions to the work. |
| This journal is © The Royal Society of Chemistry 2011 |
